Practice Essentials
Cerebral aneurysm is a cerebrovascular disorder in which weakness in the wall of an intracranial artery causes a localized dilation or ballooning of the blood vessel. If an aneurysm ruptures, blood leaks into the space around the brain and causes a subarachnoid hemorrhage (SAH). Unruptured intracranial aneurysms (UIAs) are relatively common in the general population, found in and estimated 1-5% of the general population. [1] They are being discovered incidentally with increasing frequency because of the widespread use of high-resolution magnetic resonance imaging (MRI) scanning. Only a very small percentage (1 in 200 to 400) will ruputure. [2, 3, 4]
Cerebral aneurysms involve both the anterior circulation and the posterior, or vertebrobasilar, circulation. Anterior circulation aneurysms arise from the internal carotid artery or any of its branches, whereas posterior circulation aneurysms arise from the vertebral artery, basilar artery, or any of their branches. (An internal carotid artery aneurysm is shown in the image below.)
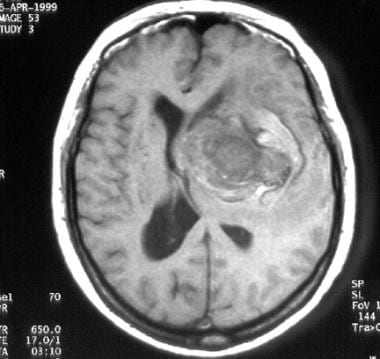 T1-weighted magnetic resonance image (MRI) of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. A large intracerebral mass with a significant amount of surrounding edema is depicted. The lesion is a giant internal carotid artery aneurysm.
T1-weighted magnetic resonance image (MRI) of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. A large intracerebral mass with a significant amount of surrounding edema is depicted. The lesion is a giant internal carotid artery aneurysm.
Intracranial aneurysms are named according to the artery, the segment of origin, or both; for example, anterior communicating aneurysms arise from the anterior communicating artery, and posterior communicating artery aneurysms arise from the internal carotid artery near the origin of the posterior communicating artery. Intracranial aneurysms are classified into saccular and nonsaccular types on the basis their shape and etiology. Nonsaccular aneurysms include atherosclerotic, fusiform, traumatic, and mycotic types. Saccular, or berry, aneurysms have several anatomic characteristics that distinguish them from other types of intracranial aneurysms. Typically, saccular aneurysms arise at a bifurcation or along a curve of the parent vessel, or they point in the direction in which flow would proceed if the curve were not present.
The methods of imaging of aneurysms have expanded greatly, including advanced magnetic resonance angiography (MRA), CT angiography (CTA), and digital subtraction angiography (DSA). Each modality has advantages and limitations, and they are used variably at various stages in the evaluation of cerebral aneurysms.
After the diagnosis, the specific anatomic details of the aneurysm must be reported to categorize the lesion, select appropriate management, and assess treatment outcomes. For treatment selection, the following are required: accurate measurement of the neck size; a neck-to-dome ratio descriptor; measures of the aneurysm in 3 dimensions; and the relationship of the aneurysm to the surrounding vessels. For treated aneurysms, the presence, measurements, and descriptors of residuals and any parent vessel changes are needed, along with identification of any new aneurysm development. [2]
Preferred examination
A strong clinical suspicion of an aneurysm may be validated by the use of several diagnostic studies, including CT scanning, lumbar puncture, MRI, and cerebral angiography. Noncontrast CT remains the initial imaging test of choice to evaluate for suspected SAH because of its high sensitivity for acute hemorrhage, wide availability on a 24-hour basis, lack of absolute contraindications, speed of image acquisition, and ease of patient monitoring. However, its very high initial sensitivity for SAH declines progressively with time, and in these situations, MRI is useful. Noncontrast MRI with susceptibility-weighted sequences is likely to be as sensitive as noncontrast head CT scans in the detection of intracranial hemorrhage. [5]
Diffuse, severe SAH is seldom helpful in identifying the specific site of the aneurysm. Localized SAH, however, may be highly indicative of the site of aneurysm rupture, as in cases in which blood is present in the sylvian fissure as a result of a rupture of a middle cerebral artery (MCA) trifurcation aneurysm or in cases in which interhemispheric blood is present between the anterior part of the frontal lobes as a result of the rupture of an aneurysm of the anterior communicating artery. [6, 7, 8, 9, 10, 11, 12]
Digital substraction angiography (DSA) remains the gold standard imaging test to evaluate cerebral artery aneurysms. However, DSA is an invasive imaging test with potential complications and is therefore usually not a first-line imaging test, except perhaps in patients with acute SAH. [5]
The use of high-resolution CT angiography combined with the use of DSA with dynamic rotational views provides the best possible visualization of the flow pattern and characteristics of any intracranial aneurysm. [6, 7, 8, 9] . Computational fluid dynamics using 4D-CTA models can show a reliable geometric and hemodynamic information in the intracranial circulation. [13]
Limitations of techniques
In patients with diffuse SAH, CT scans may not depict the precise site of aneurysm rupture. In severely anemic patients with a small hemorrhage, false-negative CT findings do occur, although rarely. Small amounts of SAH may be cleared from the cerebrospinal fluid (CSF) and may not be visible as areas of increased attenuation on CT scans as soon as 1 or 2 days after the initial severe headache; therefore, a nonenhanced CT scan of the head obtained after this time may show false-negative findings of SAH. [14]
Guidelines
The American Heart Association/American Stroke Association (AHA/ASA) guidelines for the management of unruptured intracranial aneurysms (UIA) includes the following imaging recommendations [2] :
-
Digital subtraction angiography (DSA) can be useful compared with noninvasive imaging for identification and evaluation of cerebral aneurysms if surgical or endovascular treatment is being considered.
-
DSA is the most sensitive imaging for follow-up of treated aneurysms.
-
CTA and MRA are useful for detection of UIA.
-
MRA is a reasonable alternative for follow-up for treated aneurysms, with DSA used as necessary when deciding on therapy.
-
For patients with UIAs that are managed noninvasively without either surgical or endovascular intervention, radiographic follow-up with MRA or CTA at regular intervals is indicated. An initial follow-up study at 6 to 12 months after discovery, followed by subsequent yearly or every other year follow-up, may be reasonable.
-
For patients with UIAs that are managed noninvasively and in whom there are no contraindications to MRI, it may be reasonable to consider time-of-flight (TOF) MRA rather than CTA for repeated long-term follow-up.
-
Coiled aneurysms, especially those with wider neck or dome diameters or those that have residual filling, should have follow-up evaluation, but the timing and duration are uncertain.
-
Imaging after surgical intervention, to document aneurysm obliteration, is recommended given the differential risk of growth and hemorrhage for completely versus incompletely obliterated aneurysms.
-
Long-term follow-up imaging may be considered after surgical clipping given the combined risk of aneurysm recurrence and de novo aneurysm formation. Long-term follow-up may be particularly important for those aneurysms that are incompletely obliterated during initial treatment.
-
Surveillance imaging after endovascular treatment of UIAs lacking high-risk features for recurrence is probably indicated.
-
Patients with ≥2 family members with IA or SAH should be offered aneurysmal screening by CTA or MRA. Risk factors that predict a particularly high risk of aneurysm occurrence in such families include history of hypertension, smoking, and female sex.
-
Patients with a history of autosomal dominant polycystic kidney disease, particularly those with a family history of IA, should be offered screening by CTA or MRA.
-
It is reasonable to offer CTA or MRA screening to patients with coarctation of the aorta and patients with microcephalic osteodysplastic primordial dwarfism.
American College of Radiology (ACR) Appropriateness Criteria for imaging of cerebrovascular disease includes the following key recommendations [5] :
-
DSA is the gold standard for carotid artery evaluation and should be performed if noninvasive imaging is inconclusive or contradictory.
-
Patients with risk factors for cerebral aneurysms can undergo noninvasive screening with TOF-MRA or CTA. TOF-MRA does not require IV contrast and lacks ionizing radiation exposure to the patient. However, it may not be feasible in claustrophobic or morbidly obese patients and patients with cardiac devices or metal shrapnel. CTA has higher spatial resolution but requires IV contrast and exposes the patient to radiation.
-
The initial imaging study in patients presenting with suspected nontruamatic SAH should be noncontrast head CT.
-
Initial evaluation in patients with acute nontraumatic SAH could start with DSA or noninvasive imaging with CTA or MRA. If the initial DSA is negative, then CTA or MRA should subsequently be performed. If CTA or MRA was performed as the initial imaging test and was negative, then DSA should be performed for further evaluation. If both initial DSA and noninvasive studies are negative, DSA should be repeated in 1 to 2 weeks.
-
Definitive imaging follow-up of treated aneurysms is performed with DSA. However, MRA has shown promise in the follow-up of previously treated aneurysms, whether contrast-enhanced MRA or noncontrast TOF-MRA. CTA is a noninvasive imaging alternative. However, CTA is severely limited in the evaluation of previously coiled aneurysms by metal artifact.
-
Unruptured aneurysms that are incidentally discovered on noninvasive imaging are best followed up using the same noninvasive imaging modality on which the initial diagnosis was made.
Computed Tomography
CT is usually the initial diagnostic procedure when SAH is suspected (see the image below). A good-quality nonenhanced CT scan may depict SAH in more than 90% of patients who undergo scanning within 48 hours, depending on the location and extent of the subarachnoid blood and the time elapsed since ictus. The location of the subarachnoid blood identifies the presumed location of the ruptured aneurysm, a finding often supported by the demonstration of an aneurysm in the area of maximum clot localization or the area of the maximum amount of subarachnoid blood. [12]
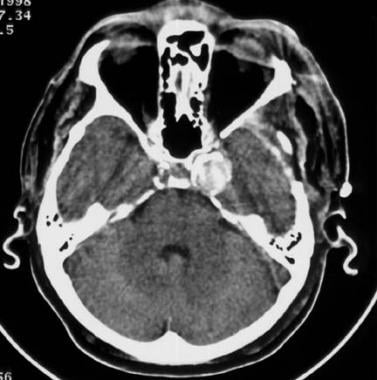 Nonenhanced CT scan of a middle-aged man with headaches. The patient had a giant aneurysm of the left internal carotid artery in its intracavernous segment. This aneurysm is densely calcified and is easily depicted.
Nonenhanced CT scan of a middle-aged man with headaches. The patient had a giant aneurysm of the left internal carotid artery in its intracavernous segment. This aneurysm is densely calcified and is easily depicted.
In particular, CT is useful in patients with multiple aneurysms. In addition to indicating the location of the vascular lesion, a CT scan may show unsuspected anomalies, such as a related arteriovenous malformation, intraparenchymal hematoma, or hydrocephalus. Finally, by providing a quantitative measure of the amount of blood in the subarachnoid cisterns and ventricles, the initial CT scan provides a reliable predictive index that may be used to identify patients who are likely to have a vasospasm. Most often, the Fisher grading system is used to classify SAH; this system is based on the amount of blood visible on the CT scan. The Fisher grading system is as follows:
-
Grade 1 - No subarachnoid blood detected
-
Grade 2 - Diffuse vertical layers thicker than 1 mm
-
Grade 3 - Localized clot and/or vertical layer thicker than 1 mm
-
Grade 4 - Intracerebral or intraventricular clot with diffuse or no subarachnoid blood
The advent of high-resolution CT angiography with the implementation of dynamic 3D reconstructions allows for a superb visualization of the intracranial vessels, as well as the anatomy and characteristics of the aneurysm; images with a similar resolution enable the visualization of intracranial vessels. However, it does not show progression on the pattern of flow. [15, 16] Although some authors have suggested that high-resolution CT angiography may replace cerebral angiography, in the author's opinion, high-resolution CT angiography complements digital substraction angiography.
Newer techniques using spectral CT imaging with rapid kV-switching in subtraction angiography can provide better bone removal with significantly reduced radiation and contrast dose, when compared to the conventional subtraction method. [17, 18]
The introduction of virtual-reality technology in medical procedures has opened new frontiers when planning the surgical treatment of intracranial aneurysms. [19]
Computational fluid dynamics using 4D-CTA models can provide reliable geometric and hemodynamic information of the intracranial circulation. [20]
Degree of confidence
Subarachnoid bleeding is demonstrated in more than 90% of patients, depending on the location and extent of the subarachnoid blood and the time elapsed since ictus. [6, 8] Occasionally, extravasation of contrast can be seen in patients with subarachnoid hemorrhage caused by ruptured intracranial aneurysms. [9]
Lumbar puncture is usually reserved for the screening of patients with potential sentinel bleeding or for confirming the presence of bleeding in patients whose clinical history is suggestive but whose CT findings are negative.
Magnetic Resonance Imaging
MRI can provide additional details about the regional anatomy and the size, shape, and content of an aneurysm (see the images below). [11] Compared to 1.5T MRA, the use of high-field strength (3T) and, even more so, ultra-high-field strength (7T) enables the visualization of the lumina of much smaller intracranial vessels while adding a contrast agent. [21, 22, 23, 24]
 T1-weighted magnetic resonance image (MRI) of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. A large intracerebral mass with a significant amount of surrounding edema is depicted. The lesion is a giant internal carotid artery aneurysm.
T1-weighted magnetic resonance image (MRI) of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. A large intracerebral mass with a significant amount of surrounding edema is depicted. The lesion is a giant internal carotid artery aneurysm.
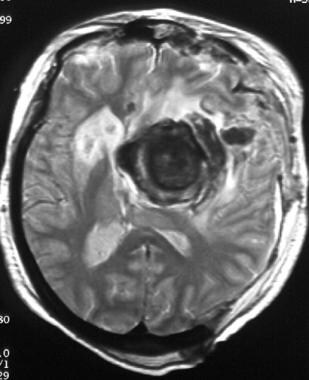 T2-weighted MRI of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. The lesion is a giant internal carotid artery aneurysm. Note the flow void, the blood breakdown products within the layers of mural thrombus, and calcification within the aneurysm that produces a marked hypointense signal. Significant surrounding edema is depicted.
T2-weighted MRI of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. The lesion is a giant internal carotid artery aneurysm. Note the flow void, the blood breakdown products within the layers of mural thrombus, and calcification within the aneurysm that produces a marked hypointense signal. Significant surrounding edema is depicted.
Most intracranial aneurysms appear as an area of flow void larger than the healthy vessels in that region. Their interior usually enhances significantly after the intravenous administration of gadolinium–diethylenetriamine penta-acetic acid. Most giant aneurysms have calcifications and an intraluminal clot, but their residual lumen may be depicted as a region of flow void. The thrombosed areas may have variable signal intensity, which represents blood products at different stages. MRIs may also depict small amounts of parenchymal blood surrounding the aneurysms; this finding indicates which of the multiple aneurysms have bled. [25, 26]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MR angiography (MRA) scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
MRA is useful in detecting intracranial aneurysms in both symptomatic patients and asymptomatic patients. MRA is a noninvasive and sensitive method of testing for an aneurysm in symptomatic patients who are at high risk (ie, patients with polycystic renal disease and those with 1 or more first-order family members with documented cerebral aneurysms). [8]
In symptomatic patients, MRA can reveal the site of aneurysmal dilation. Two main types of MRA—the time-of-flight (TOF) technique and the in-flow technique—are used more often than the phase-sensitive or the phase-contrast technique. For both time-of-flight and in-flow sequences, a large set of axial source images are acquired; these are then reformatted into images that appear similar to conventional angiograms. The most common method used is the maximum intensity projection (MIP) method. The more widespread use of 3T imaging and the new 7T MRIs have improved the ability to diagnose intracranial aneurysms. Time-of-flight angiography is a technique allowing the visualization of blood flow in vessels. [27, 28, 29] The 7T TOF MRA is able to visualize the entire Circle of Willis, including small perforating branches.
It is possible to follow up aneurysms with MRI even after endovascular coiling. [25, 26]
Advances in MRI technology, including the use of newer 3T units, enable excellent visualization of the intracranial vessels. The use of high-resolution magnetic resonance angiography is appropriate for the follow-up of patients who have undergone treatment with endovascular coiling; it accurately delineates residual aneurysm necks and parent vessel patency (in the absence of a stent) and enables excellent visualization of contrast filling within the coil mass. However, when a stent has been placed in the parent artery, MRI may not be appropriate because of the presence of artifacts. [26]
Degree of confidence
MRI alone is sensitive in the evaluation of subarachnoid and intraparenchymal hemorrhage. Small aneurysms may be missed. MRA is more sensitive to small aneurysms, and it can reliably depict lesions as small as 3-4 mm; however, for optimal sensitivity, MIP images should always be viewed in conjunction with the source images; small aneurysms may be missed if only the MIP images are reviewed. Angiography should still be considered the criterion standard for the detection of small aneurysms.
A well-known challenge is the interpretation of MRI in patients who have undergone surgical clipping or endovascular coiling of an intracranial aneurysm. The metallic artifact caused by the titanium clips or the coil mass precludes proper visualization of the vessels or residual aneurysm sac. Brain magnetic resonance angiography with non-contrast-enhanced ultra-short echo time (UTE) sequencing and embedding the clip in homemade phantom is a novel technique for patients who undergo cerebral aneurysm clipping. [27]
Ultrasonography
In patients with subarachnoid hemorrhage (SAH), transcranial Doppler (TCD) ultrasonography is a noninvasive technique that is useful in detecting vasospasm of the intracranial arteries. Most measurements are obtained by using particular cranial windows of relatively thin bone. The most common cranial window is the transtemporal one, which is located above the zygoma. This window is used to measure velocities of the middle cerebral artery, the anterior cerebral artery, the distal internal carotid artery, and the proximal posterior cerebral artery. The transorbital window enables measurement of the ophthalmic artery and the internal carotid artery; the suboccipital window enables measurement of the vertebral arteries and the basilar artery. [30]
In addition, TCD ultrasonography provides repeated serial measurements that may show a pattern of increasing flow velocities in patients with subarachnoid hemorrhage (SAH), which can lead to clinical deterioration. Clinical decision making, such as decisions concerning the initiation and duration of hypervolemic, hypertensive therapy, may be aided by TCD. [31, 32]
Nuclear Imaging
Some giant aneurysms in the cavernous segment of the internal carotid artery may be treated with occlusion of the artery. Treatment of these lesions depends heavily on the demonstration of cerebrovascular reserve, which is the ability to tolerate temporary or permanent carotid artery occlusion. This reserve is assessed by means of an endovascular balloon occlusion test, with qualitative or quantitative cerebral blood flow measurements at single-photon emission CT (SPECT) scanning. Patients who can tolerate balloon occlusion and have no significant areas of hypoperfusion on SPECT scans are candidates for carotid occlusion.
Angiography
Cerebral angiography remains the definitive preoperative diagnostic tool in patients with intracranial aneurysms (see the images below). Angiography may also be used to detect and evaluate aneurysmal multiplicity or other associated vascular diseases, assess collateral circulation, identify congenital anomalies, and diagnose cerebral vasospasm and aid in their treatment. [11]
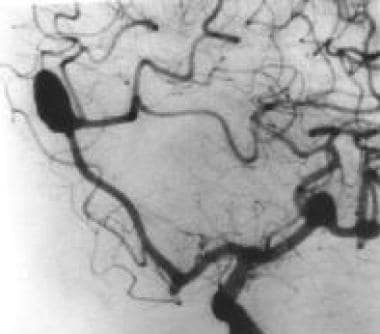 Left oblique cerebral angiogram in a patient with multiple intracranial aneurysms shows an anterior communicating aneurysm and a middle cerebral artery aneurysm. The patient underwent a frontotemporoparietal craniotomy, during which surgical clips were placed in both lesions in one setting.
Left oblique cerebral angiogram in a patient with multiple intracranial aneurysms shows an anterior communicating aneurysm and a middle cerebral artery aneurysm. The patient underwent a frontotemporoparietal craniotomy, during which surgical clips were placed in both lesions in one setting.
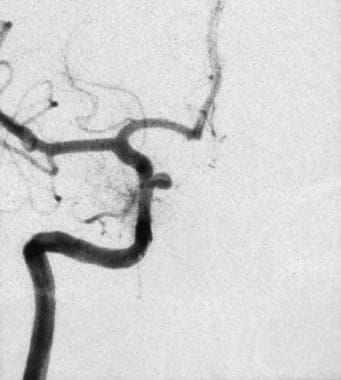 Left oblique cerebral angiogram in a patient with a proximal intracranial internal carotid artery aneurysm. The surgical approach to this aneurysm requires a craniotomy with an orbitotomy and drilling of the anterior clinoid process; however, this aneurysm has a favorable neck-to-fundus ratio for endovascular coil placement.
Left oblique cerebral angiogram in a patient with a proximal intracranial internal carotid artery aneurysm. The surgical approach to this aneurysm requires a craniotomy with an orbitotomy and drilling of the anterior clinoid process; however, this aneurysm has a favorable neck-to-fundus ratio for endovascular coil placement.
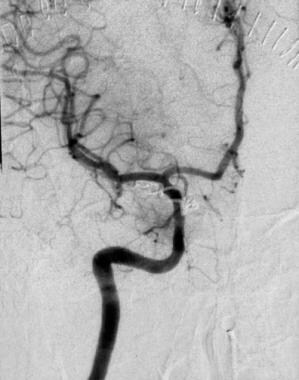 Image obtained after the placement of a Guglielmi detachable coil in the aneurysm. The patency of the internal carotid artery and all its branches is preserved. Contrast material does not fill the aneurysm.
Image obtained after the placement of a Guglielmi detachable coil in the aneurysm. The patency of the internal carotid artery and all its branches is preserved. Contrast material does not fill the aneurysm.
A routine angiographic examination consists of a selective 4-vessel study, including both internal carotid and both vertebral arteries. This study enables the evaluation of the cerebral circulation to determine the source of subarachnoid hemorrhage (SAH) and to identify other concomitant lesions that may influence the surgical plan.
Multiple views are often necessary to delineate the origin of vessels overlapping the aneurysms and the configuration of the aneurysm neck. In the anterior circulation, carotid ophthalmic aneurysms are often best seen on the lateral or 45° oblique projection. Posterior communicating and anterior choroidal aneurysms are usually well profiled on the lateral and oblique projections.
Carotid bifurcation aneurysms and some middle cerebral aneurysms may warrant the use of a straight anteroposterior, or Caldwell, projection. The basal, or submental vertex (SMV), projection may help define the anatomy of middle cerebral aneurysms. In the posterior circulation, oblique projections are useful in showing the basilar bifurcation, posterior inferior cerebellar aneurysms, or aneurysms of the vertebrobasilar junction. Occasionally, a straight anteroposterior view may be required to depict an aneurysm of the posterior inferior cerebellar artery.
Possible findings that may affect the patient's treatment include the presence of multiple intracranial aneurysms; areas of intracranial arterial stenoses; associated arteriovenous malformations; and anatomic variations, such as a fetal origin of a posterior cerebral artery or a persistent primitive trigeminal artery. In patients with multiple aneurysms and SAH, angiographic clues to the bleeding source include the size of the aneurysm, vessel displacements from adjacent hematomas, local vasospasm, and an irregular aneurysm contour or nipplelike protrusion on the aneurysm. The escape of contrast agent during the study is an ominous sign that indicates a repeat rupture of the aneurysm.
Hwang et al compared angiographic outcomes at 2 years postembolization for stent- and nonstent-assisted coiled unruptured aneurysms and found that the additional hemodynamic and biologic effects of stents designed for neck remodeling did not appear to enhance progressive occlusion and prevent recanalization. [14] They concluded that stent placement may not contribute to a positive long-term angiographic outcome for unruptured aneurysms with an unfavorable configuration for coiling.
Possible complications
Aneurysmal rupture during cerebral angiography may occur as a result of traction, the performance of maneuvers near the aneurysmal neck, or catheter misplacement in the neck of an aneurysm that increases dynamic pressures in the aneurysm. One should always be prepared to perform open craniotomy promptly if the aneurysm ruptures. If a thrombus obstructs the vessel, a fibrinolytic such as urokinase or tissue plasminogen should be used immediately.
-
T1-weighted magnetic resonance image (MRI) of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. A large intracerebral mass with a significant amount of surrounding edema is depicted. The lesion is a giant internal carotid artery aneurysm.
-
T2-weighted MRI of a middle-aged woman with progressive headaches, aphasia, and right-sided hemiparesis. The lesion is a giant internal carotid artery aneurysm. Note the flow void, the blood breakdown products within the layers of mural thrombus, and calcification within the aneurysm that produces a marked hypointense signal. Significant surrounding edema is depicted.
-
Left oblique cerebral angiogram in a patient with multiple intracranial aneurysms shows an anterior communicating aneurysm and a middle cerebral artery aneurysm. The patient underwent a frontotemporoparietal craniotomy, during which surgical clips were placed in both lesions in one setting.
-
Left oblique cerebral angiogram in a patient with a proximal intracranial internal carotid artery aneurysm. The surgical approach to this aneurysm requires a craniotomy with an orbitotomy and drilling of the anterior clinoid process; however, this aneurysm has a favorable neck-to-fundus ratio for endovascular coil placement.
-
Image obtained after the placement of a Guglielmi detachable coil in the aneurysm. The patency of the internal carotid artery and all its branches is preserved. Contrast material does not fill the aneurysm.
-
Nonenhanced CT scan of a middle-aged man with headaches. The patient had a giant aneurysm of the left internal carotid artery in its intracavernous segment. This aneurysm is densely calcified and is easily depicted.
-
Substraction digital angiography of a patient with a ruptured anterior communicating artery aneurysm.
-
3D CTA of a patient with a ruptured anterior communicating artery aneurysm.
-
3D CTA of a patient with a ruptured anterior communicating artery aneurysm treated with endovascular coiling.
-
Digital angiography (AP view) of a patient with a basilar artery bifurcation intracranial aneurysm.
-
Post coiling digital angiography (AP view) of a patient with a basilar artery bifurcation intracranial aneurysm. The use of substraction technique allows for a better definition of the patient's anatomy.
-
3D CTA of an internal carotid aneurysm treated with flow diversion technique at our institution