Practice Essentials
The liver is the second most commonly transplanted major organ, after the kidney. In 2018, 8,250 patients received a liver transplant and 12,975 patients were on the waiting list for a liver transplant. As of June 30, 2017, nearly 83,925 liver transplant recepients were living with a functioning liver graft. [1, 2] Imaging is an important part of the liver transplantation workup and identification of postsurgical complications [3] . An increasing number of orthotopic and living-donor liver transplantations are being performed to salvage patients with otherwise incurable end-stage liver disease (ESLD). Meticulous preoperative evaluation can decrease the frequency of postoperative complications.
Similarly, imaging plays an important role in detecting postsurgical complications. Post-transplant complications can be categorized into vascular, non-vascular and biliary. Ultrasonography (US), CT, MRI, cholangiography, angiography and scintigraphy are the most common radiological modalities used to evaluate the hepatic graft. US is excellent for screening for biliary, arterial, and venous problems. [4] Doppler ultrasonography plays an important role in the postoperative management of liver transplantation. [5, 6, 7] Contrast-enhanced, dual-phase CT scanning and nonenhanced CT scanning can help to detect or confirm these postoperative complications. CT scanning is especially useful in depicting fluid collections and is most commonly used to guide percutaneous aspirations and abscess drainages. [8, 9, 10, 11, 12, 13, 14] With the availability of advanced scanners, magnetic resonance imaging (MRI), MR angiography (MRA), MR venography (MRV), and MR cholangiopancreatography (MRCP) are increasingly used to better define the preoperative and postoperative biliary tree and to evaluate hepatic vascular anatomy and patency. Many complications can be temporized or corrected by using interventional radiologic techniques. Some can be treated by using endoscopic retrograde cholangiopancreatography (ERCP). With a combination of these tools, many patients can be spared the morbidity and potential mortality associated with repeat operation or retransplantation. [15, 16, 17, 9]
(See the images below of transplantation complications.)
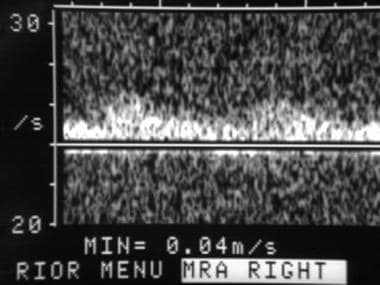 Spectral Doppler ultrasonographic waveform of the right hepatic artery in a 60-year-old man, 8 years after orthotopic liver transplantation. The image demonstrates the typical rounded tardus parvus waveform morphology, which is indicative of upstream arterial thrombosis or severe stenosis. Subsequent angiography confirmed occlusion at the hepatic arterial anastomosis.
Spectral Doppler ultrasonographic waveform of the right hepatic artery in a 60-year-old man, 8 years after orthotopic liver transplantation. The image demonstrates the typical rounded tardus parvus waveform morphology, which is indicative of upstream arterial thrombosis or severe stenosis. Subsequent angiography confirmed occlusion at the hepatic arterial anastomosis.
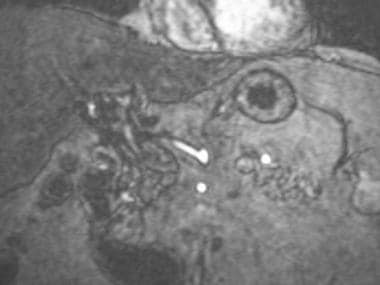 A-1: Magnetic resonance angiogram in a transplantation patient with hepatic artery thrombosis. Magnetic resonance angiogram of the recipient celiac axis depicts complete occlusion of the hepatic artery.
A-1: Magnetic resonance angiogram in a transplantation patient with hepatic artery thrombosis. Magnetic resonance angiogram of the recipient celiac axis depicts complete occlusion of the hepatic artery.
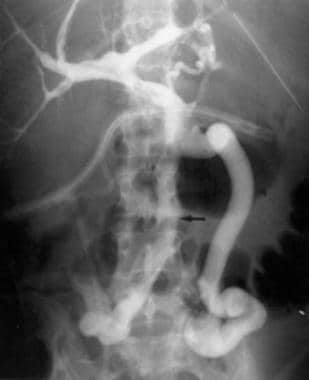
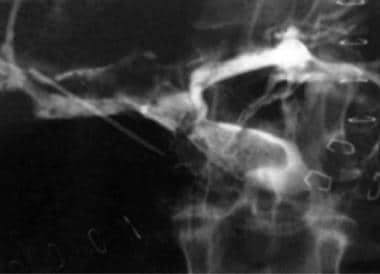 Transjugular portography demonstrates extensive portal vein thrombus in the whole-liver allograft of a 40-year-old woman whose clinical condition rapidly deteriorated on postoperative day 39.
Transjugular portography demonstrates extensive portal vein thrombus in the whole-liver allograft of a 40-year-old woman whose clinical condition rapidly deteriorated on postoperative day 39.
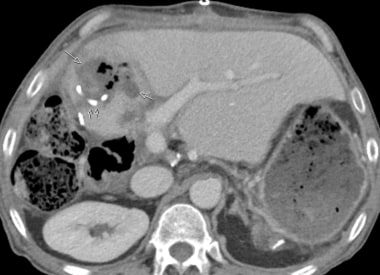
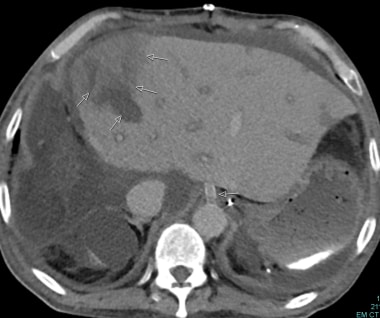 C-3: Infarction of the lateral part of a graft (multiple arrows); thrombosis of the arterial graft (single arrow).
C-3: Infarction of the lateral part of a graft (multiple arrows); thrombosis of the arterial graft (single arrow).
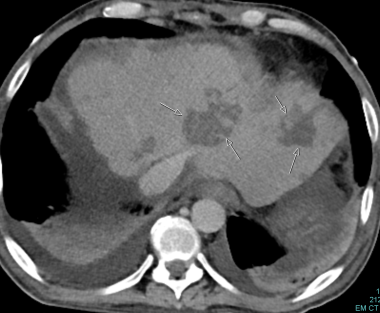 C-5: Multiple infarctions of the left lateral aspect are marked by arrows. Bilateral pleural effusion can be seen.
C-5: Multiple infarctions of the left lateral aspect are marked by arrows. Bilateral pleural effusion can be seen.
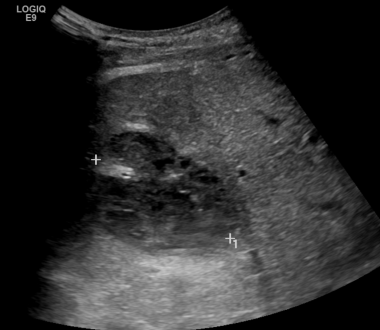 C-6: Ultrasound image of the same patient showing a larger infarction/collection? (between the cursors) of the hepatic graft.
C-6: Ultrasound image of the same patient showing a larger infarction/collection? (between the cursors) of the hepatic graft.
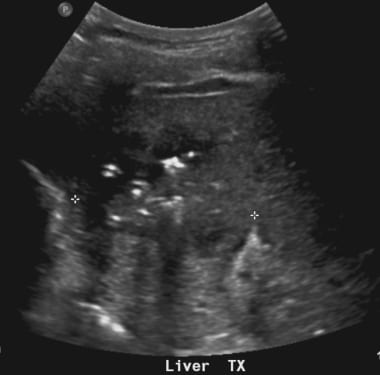 C-7: The patient developed frank abscess after a few days. The hyper-echogenic foci of the air can be easily seen.
C-7: The patient developed frank abscess after a few days. The hyper-echogenic foci of the air can be easily seen.
Lee et al retrospectively enrolled 75 patients who had undergone liver transpalantation and found that although MDCT in the late period should be interpreted with caution in patients with suspected biliary complication, MDCT is a reliable diagnostic technique for the identification of early and late abdominal complications after liver transplantation. [18] In a separate study, Boraschi et al found MDCT to be a reliable diagnostic technique for early abdominal complications of liver recipients. [19]
Indications for OLTX
Orthotopic liver transplantation (OLTX) can provide the definitive treatment for patients with ESLD. Causes of ESLD that can necessitate OLTX in adults include the following [20] :
-
Cirrhosis associated with hepatitis B and hepatitis C
-
Alcoholic cirrhosis
-
Acute liver failure
-
Chronic active autoimmune hepatitis
-
Primary biliary cirrhosis
-
Primary benign and malignant neoplasms [23]
Overview of LDLTX
As OLTX has become more widespread, limited organ availability has become a concern, and recipient waiting lists have lengthened. As a result, much interest has arisen in adult-to-adult, living-donor liver transplantation (LDLTX). This is a technique modified from previously established adult-to-child hepatic transplantation surgery (see the image below). In adult-to-adult LDLTX, a portion of the liver from an immunologically compatible donor, often a relative, is used to replace the entire diseased liver of the recipient. At the author's institution, either the right or left lobe is taken as the graft. The donor is left with enough liver to maintain normal hepatic function, and the recipient is given enough liver to restore function. The donor and the recipient benefit from significant hepatic hyperplasia that begins within 12 hours of surgery.
Liver replacement in patients is necessary when the following severe findings are noted:
-
Deterioration of hepatic synthetic function
-
Coagulopathy
-
Variceal bleeding
-
Hypersplenism
For excellent patient education resources, visit eMedicineHealth's Infections Center and Digestive Disorders Center. Also, see eMedicineHealth's patient education articles Liver Transplant, Hepatitis B, Hepatitis C, and Cirrhosis.
Complications
Complications of whole-liver OLTX
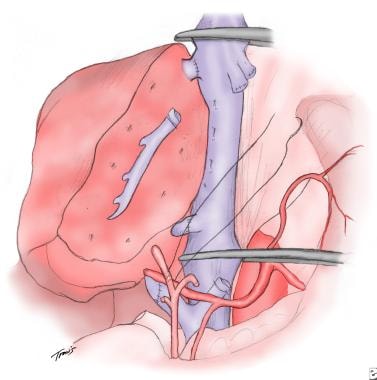
During a standard whole-liver orthotopic liver transplantation (OLTX), the following 4 donor structures must be reconnected sequentially from posterior to anterior to the recipient counterparts:
-
Retrohepatic inferior vena cava (IVC)
-
Main portal vein (MPV)
-
Hepatic artery
-
Extrahepatic bile duct
Complications of OLTX can be mechanical or nonmechanical. Not surprisingly, postoperative mechanical complications are related to problems with the hepatic artery, bile ducts, MPV, and IVC. Nonmechanical complications include allograft nonfunction, rejection, viral reinfection of the transplanted liver by the host, recurrent hepatic malignancy, and posttransplantation lymphoproliferative disease (PTLD). [25] Imaging is important in the detection, evaluation, and (in some instances) nonoperative interventional treatment of mechanical complications. Imaging plays less of a role in the workup of nonmechanical complications of OLTX.
Complications involving the hepatic artery
After OLTX, the biliary tree of the allograft is solely dependent on hepatic artery perfusion to maintain its viability. Disruption of hepatic artery flow results in biliary ischemia or necrosis, with subsequent biliary strictures, bile leaks, bilomas, infected bilomas, and frank intrahepatic abscesses. [25] Hepatic infarction, fulminant hepatic failure, bacteremia, and sepsis can also occur. Complications involving the hepatic artery include hepatic artery thrombosis (HAT), hepatic artery stenosis (HAS), pseudoaneurysm, and arteriovenous fistula. [26]
Hepatic artery thrombosis
HAT is one of the most common and potentially most disastrous arterial complications (see images below). HAT is estimated to occur in 4-12% of adult OLTX patients and in 9-42% of pediatric transplantation patients. [26] Causes include allograft rejection, hepatic artery kinking due to vascular redundancy, underlying HAS, and technical problems at the anastomosis. [27] Doppler ultrasonography (US) has a sensitivity of approximately 90% in detecting HAT. [28]
 Spectral Doppler ultrasonographic waveform of the right hepatic artery in a 60-year-old man, 8 years after orthotopic liver transplantation. The image demonstrates the typical rounded tardus parvus waveform morphology, which is indicative of upstream arterial thrombosis or severe stenosis. Subsequent angiography confirmed occlusion at the hepatic arterial anastomosis.
Spectral Doppler ultrasonographic waveform of the right hepatic artery in a 60-year-old man, 8 years after orthotopic liver transplantation. The image demonstrates the typical rounded tardus parvus waveform morphology, which is indicative of upstream arterial thrombosis or severe stenosis. Subsequent angiography confirmed occlusion at the hepatic arterial anastomosis.
 A-1: Magnetic resonance angiogram in a transplantation patient with hepatic artery thrombosis. Magnetic resonance angiogram of the recipient celiac axis depicts complete occlusion of the hepatic artery.
A-1: Magnetic resonance angiogram in a transplantation patient with hepatic artery thrombosis. Magnetic resonance angiogram of the recipient celiac axis depicts complete occlusion of the hepatic artery.
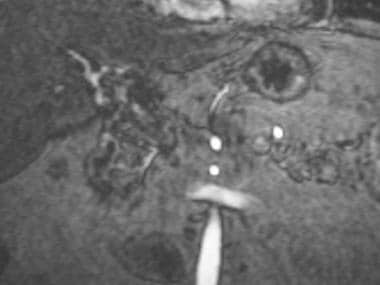 A-2: Magnetic resonance angiogram demonstrates intrahepatic arterial segments reconstituted from mesenteric collaterals. Collateralization explains how, in some patients, intrahepatic flow can be present in the setting of complete extrahepatic arterial occlusion.
A-2: Magnetic resonance angiogram demonstrates intrahepatic arterial segments reconstituted from mesenteric collaterals. Collateralization explains how, in some patients, intrahepatic flow can be present in the setting of complete extrahepatic arterial occlusion.
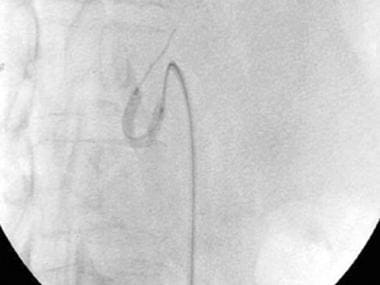
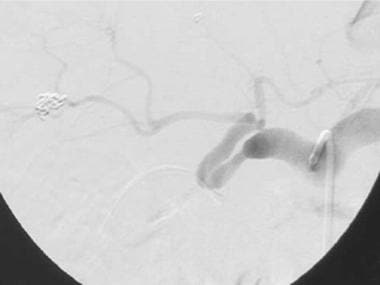
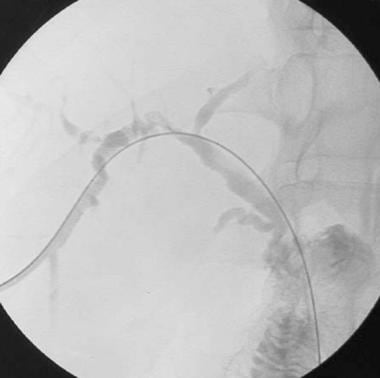
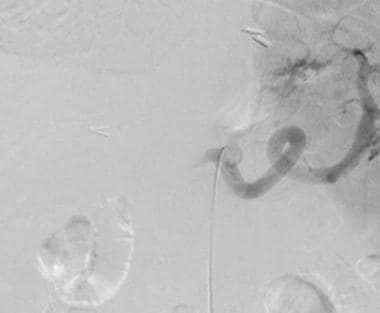 A-3: Digital subtraction angiogram shows complete hepatic artery thrombosis in a liver transplant recipient.
A-3: Digital subtraction angiogram shows complete hepatic artery thrombosis in a liver transplant recipient.
A finding on Doppler ultrasound evaluation that would merit close follow-up is the presence of significantly elevated peak systolic velocities in the main hepatic artery, which may be suggestive of hepatic artery stenosis (HAS), an independent risk factor for HAT. [29] Doppler ultrasonographic findings consistent with HAT include absent flow at the porta hepatis (ie, no flow in the donor proper hepatic artery) with absent intrahepatic arterial flow, and absent flow in the donor hepatic artery with abnormal intrahepatic flow. Persistent intrahepatic flow in the setting of complete proper HAT can result from collateralization via nearby superior mesenteric artery branches. Collateralized intrahepatic flow can be differentiated from normal flow by its tardus parvus Doppler waveform morphology. Tardus parvus can be diagnosed objectively when the intrahepatic arterial resistive index (RI) is less than 0.5 and the systolic acceleration time (SAT) is 0.08 second or longer.
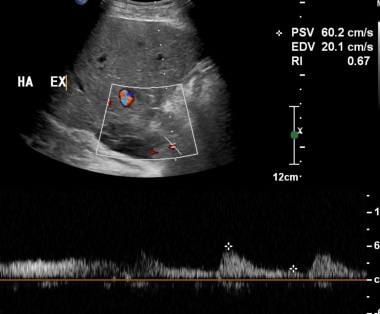 B-1: Extrahepatic artery Doppler ultrasonography shows Doppler spectrum with lower normal limits. Intrahepatic artery spectrum could not be sampled.
B-1: Extrahepatic artery Doppler ultrasonography shows Doppler spectrum with lower normal limits. Intrahepatic artery spectrum could not be sampled.
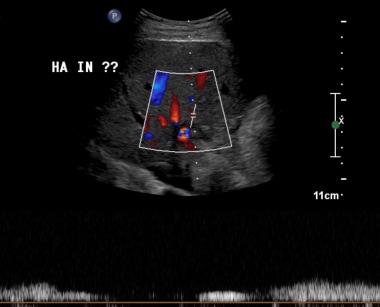 B-2: Extrahepatic artery Doppler ultrasonography shows Doppler spectrum with lower normal limits. Intrahepatic artery spectrum could not be sampled.
B-2: Extrahepatic artery Doppler ultrasonography shows Doppler spectrum with lower normal limits. Intrahepatic artery spectrum could not be sampled.
Without aggressive diagnosis and treatment, HAT is associated with a mortality rate of greater than 80%. Unfortunately, percutaneous thrombolytic therapy of HAT is problematic, because it often occurs soon after OLTX. Thrombolysis and angioplasty of underlying HAS have been successful in a few patients; however, HAT usually requires urgent retransplantation for patient survival.
Hepatic artery stenosis
HAS occurs in approximately 11-13% of OLTXs. [26] HAS can be caused by clamp injury, intimal injury due to perfusion catheters, anastomotic ischemia due to a disrupted vasa vasorum, and rejection. The consequences of hemodynamically significant HAS (>50% stenosis) are essentially identical to those of HAT. Although a few stenoses have been corrected with balloon angioplasty, HAS, similar to HAT, usually requires operative vascular reconstruction or retransplantation (see the images below).
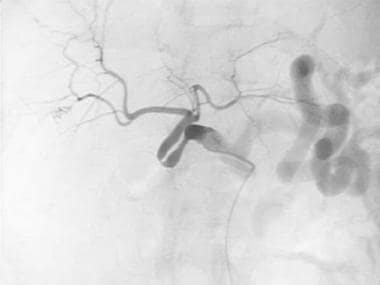 A-4: Digital subtraction angiogram depicts redundant donor-recipient hepatic artery. A stenotic hairpin turn is noted.
A-4: Digital subtraction angiogram depicts redundant donor-recipient hepatic artery. A stenotic hairpin turn is noted.
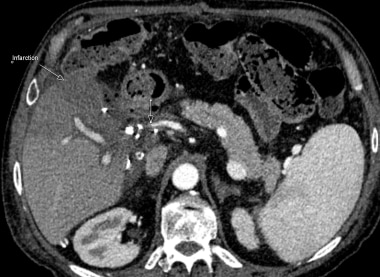 B-3: Hepatic artery graft stenosis: arrows indicate hepatic artery tapering and disappearance. The low attenuation area shows infarction.
B-3: Hepatic artery graft stenosis: arrows indicate hepatic artery tapering and disappearance. The low attenuation area shows infarction.
Doppler US is used to screen for HAS. [5, 6] If the surgical anastomosis can be interrogated directly, stenosis is diagnosed if peak systolic velocity exceeds 200-300 cm/s. Upstream from the stenosis, an abnormal pattern of high resistance and low velocity can be detected. Turbulence is encountered within 1-2 cm downstream of the narrowing.
In most patients, direct Doppler evaluation of hepatic artery anastomosis is not possible, because the donor-recipient arterial anastomosis is tortuous, because it is in an inconsistent position, and because it is usually obscured by overlying bowel gas. However, similar to HAT, hemodynamically significant HAS results in intrahepatic arterial tardus parvus waveform morphology. Using an RI of less than 0.5 in conjunction with an SAT of 0.08 second or longer yields a sensitivity of 45% for the detection of HAS. The sensitivity increases to 70% if 2 of the following 3 criteria are present:
-
RI of less than 0.5
-
SAT of 0.08 second or longer
-
Peak systolic velocity of greater than 200 cm/s at the anastomotic site
Because the specificity of Doppler is only 64% in detecting marked arterial disease (ie, HAT or hemodynamically significant HAS), angiography usually is required to confirm the diagnosis. [30, 31] At the time of diagnostic angiography, a decision can be made regarding the effectiveness of percutaneous correction of documented HAS.
Pseudoaneurysm
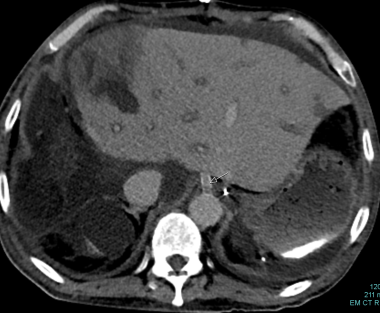
Hepatic artery pseudoaneurysms can be extrahepatic/paraportal or intrahepatic. Both types appear as spherical, hypoechoic fluid collections on gray-scale US. Pseudoaneurysms can be filled with a variable amount of mural thrombus. Spectral and color Doppler US can be used to document monophasic flow within the patent lumen of a pseudoaneurysm and the typical to-and-fro flow pattern at its neck. On computed tomography (CT) scans, a pseudoaneurysm is depicted as a focal lesion with central enhancement following arterial blood-pool attenuation.
Extrahepatic pseudoaneurysms occur after OLTX in approximately 2% of patients with a mortality following rupture of nearly 69%. [32] Early detection is a key determinant of survival. [33] The most common location of pseudoaneurysm formation is at the donor-recipient arterial anastomosis. A less common site is the ligation of the donor gastroduodenal artery. Pseudoaneurysms can be caused by infection or technical failure. Because of the potential for rupture and life-threatening hemorrhage, pseudoaneurysms need to be treated promptly. To ensure maximum arterial flow, most extrahepatic lesions are repaired surgically.
Intrahepatic pseudoaneurysms usually result from percutaneous biopsy, biliary procedures, or infection. Pseudoaneurysms, particularly if mycotic, can result in fistulas to the PV or biliary tree. Intrahepatic pseudoaneurysms or arterial fistulas can cause hemobilia, gastrointestinal bleeding, and hemoperitoneum. Most intrahepatic lesions can be treated by transcatheter embolization or stent placement. Direct percutaneous puncture with a 22-gauge needle followed by the injection of coils or particulate thrombotic agents is sometimes necessary.
Biliary complications
Biliary complications occur in approximately 13-19% of adults following OLTX. With adult OLTXs, a primary, end-to-end choledochocholedochostomy is performed after donor cholecystectomy. In most cases of LDLTX, a biliary-enteric hepatojejunostomy is used to reconstitute bile drainage. T tubes no longer are used for either type of biliary anastomosis; therefore, T-tube complications are not considered in this article.
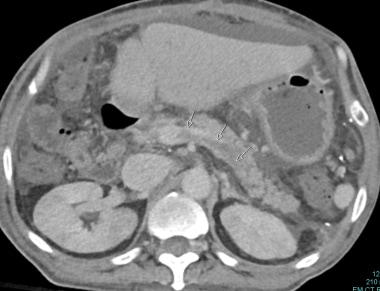
Bile duct strictures can be anastomotic or nonanastomotic. Some anastomotic strictures result from technical difficulties. Other anastomotic strictures are caused by fibrosis and scarring that can be associated with bile leaks. Anastomotic stenosis can be related to marginal blood supply of the cut ends of the donor and recipient ducts. Nonanastomotic strictures can be caused by HAS, HAT, prolonged cold ischemia time, rejection, cytomegalovirus infection, intraductal sludge and stone formation, and recurrent primary sclerosing cholangitis in the allograft (see image below). [21, 22, 34]
 Multiple ischemic biliary strictures documented during interventional external-internal drainage procedure.
Multiple ischemic biliary strictures documented during interventional external-internal drainage procedure.
Nonischemic strictures can develop over time and present with deterioration of liver function tests and bouts of cholangitis. Dominant biliary strictures can be detected using US, CT scanning, MRI, magnetic resonance cholangiography (MRC), percutaneous transhepatic cholangiography (PTC), and endoscopic retrograde cholangiopancreatography (ERCP). [35]
In a study by Boraschi et al to assess the diagnostic value of MRCP and MR imaging at 3 T for biliary complications, sensitivity was found to be 99%; specificity 96%; PPV 95%; and NPV 99%. [36]
The choices for mechanical revision of a bile duct stricture include PTC, ERCP, and repeat operation. Ischemic bile duct strictures can present with rapid onset of allograft dysfunction and sepsis. Unlike the native biliary tree, which is supplied by collaterals from the gastroduodenal artery, the harvested donor bile duct and the anastomotic end of the recipient duct are solely dependent on the hepatic artery. Compromise of the hepatic artery by HAS or HAT results in abrupt ischemia and necrosis of the biliary epithelium. This causes strictures, disruption of the ducts, bile leaks, bilomas, infected bilomas, and abscesses.
Timely angiographic correction of HAS or HAT occasionally can result in graft salvage. [30, 31] Percutaneous drainage of associated fluid collections is often only a temporizing measure, because severe underlying biliary tree damage has usually occurred. Patients with sepsis who have severe biliary and hepatic damage from HAS or HAT require retransplantation for ultimate survival.
Complications involving the portal vein
Complications involving the PV occur in approximately 1-13% of OLTX recipients. Portal vein stenosis (PVS) usually occurs at the donor-recipient anastomosis (see the images below). Portal vein thrombosis (PVT) often involves the main extrahepatic segment (see the images below). Causes of PVS and PVT include surgical difficulties, thrombus formation from the portal venous bypass cannula, excessive vessel redundancy, and hypercoagulability. PVS can lead to PVT. PVT and hemodynamically significant PVS cause graft dysfunction and portal hypertension.
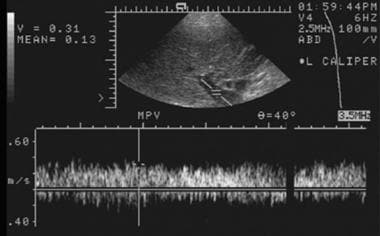 Early posttransplantation evaluation of the portal vein by using spectral Doppler ultrasonography depicts a normal waveform with peak flow velocity of approximately 31 cm/s.
Early posttransplantation evaluation of the portal vein by using spectral Doppler ultrasonography depicts a normal waveform with peak flow velocity of approximately 31 cm/s.
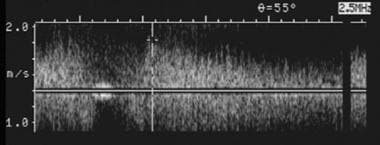 Spectral Doppler ultrasonogram of the portal vein was obtained at the onset of graft dysfunction. Compared with the baseline examination, markedly accelerated flow to 150 cm/s was documented. Subsequent catheter venography was performed that documented a high-grade venous stenosis. The stenosis was treated successfully by using balloon angioplasty and stent placement.
Spectral Doppler ultrasonogram of the portal vein was obtained at the onset of graft dysfunction. Compared with the baseline examination, markedly accelerated flow to 150 cm/s was documented. Subsequent catheter venography was performed that documented a high-grade venous stenosis. The stenosis was treated successfully by using balloon angioplasty and stent placement.
 Transjugular portography demonstrates extensive portal vein thrombus in the whole-liver allograft of a 40-year-old woman whose clinical condition rapidly deteriorated on postoperative day 39.
Transjugular portography demonstrates extensive portal vein thrombus in the whole-liver allograft of a 40-year-old woman whose clinical condition rapidly deteriorated on postoperative day 39.
 After aggressive percutaneous thrombolysis, balloon dilatation, mechanical thrombectomy, and systemic anticoagulation, patency of the portal vein has been restored. Follow-up portography shows that the portal vein is open. Competing collaterals communicating with the superior mesenteric vein (arrow) and the inferior mesenteric vein (arrowhead) were noted. Subsequently, the collateral gonadal vein draining the superior mesenteric vein to the inferior vena cava was embolized with coils to improve hepatopetal flow.
After aggressive percutaneous thrombolysis, balloon dilatation, mechanical thrombectomy, and systemic anticoagulation, patency of the portal vein has been restored. Follow-up portography shows that the portal vein is open. Competing collaterals communicating with the superior mesenteric vein (arrow) and the inferior mesenteric vein (arrowhead) were noted. Subsequently, the collateral gonadal vein draining the superior mesenteric vein to the inferior vena cava was embolized with coils to improve hepatopetal flow.
US, CT scanning, MRI, and MRV can be used to detect PVS and PVT, as follows:
-
Short-segment PV narrowing can be depicted by using gray-scale US.
-
An abrupt 3- to 4-fold increase in velocity occurs within the PV, as documented with spectral Doppler US.
-
Aliasing on color Doppler US reflects turbulent flow associated with PVS.
-
PVS can be demonstrated by using conventional CT scanning or MRI; however, this finding is uncommon. Unlike US, conventional CT scanning and MRI are not routinely used to screen for postoperative vascular complications. CT scanning and MRI are more expensive modalities, and the findings can be suboptimal because the PV is oriented obliquely to axial sections, making stenoses and partial thromboses more difficult to appreciate. In addition, PV flow artifacts can be difficult to assess on routine MRI scans.
-
With state-of-the-art magnets and pulse sequences, PVS can be displayed by using a variety of coronal MRV techniques. MRV is usually reserved to confirm PVS suggested by findings on screening US scans.
-
Digital subtraction angiography (DSA) can ultimately be used to diagnose and treat PVS. Splenic, mesenteric, transjugular, and transhepatic portography can be employed. PVS can be successfully corrected in many patients by using percutaneous transluminal balloon angioplasty.
With noninvasive imaging, PVS must be diagnosed with caution. Hemodynamically significant PVS must be distinguished from PV pseudostenosis. Pseudostenosis results when the recipient PV is somewhat larger than the donor PV. The difference in caliber causes increased velocity and turbulence at the anastomosis that are not physiologically significant. If PV narrowing is associated with a velocity increase of less than 3- to 4-fold on spectral Doppler ultrasonographic analysis, the narrowing likely is pseudostenosis. Unlike PVS, pseudostenosis is not associated with impaired graft function or clinical signs of portal hypertension. In equivocal cases, DSA may be necessary to measure the actual pressure gradient across the anastomosis.
PVT also can be diagnosed noninvasively, as described below:
-
On gray-scale sonograms, a hypoechoic–to–mildly hyperechoic filling defect can be seen within the PV. Because a fresh clot can be anechoic and not perceptible on gray-scale US scans, all US examinations should include spectral and color Doppler ultrasonographic analysis of the PV to confirm vessel patency. In patients with complete PVT, no detectable flow is visualized by spectral or color interrogation. Partial PVT may appear as a nonocclusive filling defect on US. Resultant luminal narrowing can be mistaken for PVS with gray-scale, spectral, and color Doppler US. As previously noted, PVS can underlie PVT, which can be difficult to determine by using noninvasive imaging.
-
PVT can be diagnosed with the help of CT scans. The presence of a thrombus is diagnosed most accurately with the use of intravenous (IV) contrast material during the portal venous phase of dynamic scanning. Caution must be exercised not to mistake laminar flow artifact for PVT.
-
Conventional MRI can demonstrate PVT. The age of the clot can affect its signal intensity on T1- and T2-weighted pulse sequences. Because flow artifacts commonly occur in the PV on MRI images, an accurate diagnosis of PVT can be difficult using conventional sequences. A confident diagnosis of PVT often requires the use of robust, nonenhanced or IV contrast-enhanced MRV pulse sequences, usually obtained in the coronal plane.
-
DSA can be used to diagnose and treat PVT. Portography can confirm the presence and extent of a thrombus in the PV. Percutaneous intervention can be successful in restoring venous patency. Techniques include infusion of a thrombolytic (eg, recombinant tissue thromboplastin activator), angioplasty of associated PVS (if present), mechanical thrombectomy, and catheter embolization of competing collaterals.
-
Surgical options in PVT include thrombectomy, segmental PV resection, placement of a jump graft, or creation of a portosystemic shunt. PVT can necessitate retransplantation.
-
Occasionally, PVT is detected in patients with normal allograft function and without portal hypertension. In these patients, sufficient hepatopetal collateralization has developed to maintain adequate venous inflow.
In a study by Torres et al of off-label use of contrast-enhanced ultrasonography to identify circulatory complications after liver transplantation in children, positive predictive value for diagnosing arterial circulatory complications was 80%, and for diagnosing portal vein circulatory complications, 66.7%. [10]
Complications involving the IVC and associated hepatic vein
The development of posttransplantation IVC stenosis or thrombosis is an uncommon complication that occurs in approximately 1-4% of cases. IVC stenosis and thrombosis tend to occur at the superior and inferior caval anastomoses. Causes include technical problems, IVC compression due to fluid collection or a hematoma, and mass effect from hepatic regeneration. Patients with suprahepatic lesions tend to present with signs and symptoms of Budd-Chiari syndrome; examples of these include pleural effusion, hepatic enlargement, and ascites. IVC obstruction can cause lower-extremity edema, which can be a prominent feature of infrahepatic stenosis. Clinically, leg swelling helps distinguish a suprahepatic caval complication from a PV problem; both of these complications are associated with features of portal hypertension. [24]
US is the imaging study of choice for screening for IVC complications. A stenosis is detected as a gray-scale narrowing with a 3- to 4-fold increase in velocity on spectral Doppler analysis and associated color Doppler aliasing. Indirect findings of suprahepatic IVC stenosis or thrombosis include distention of the hepatic veins (HVs) with dampening of the hepatic venous spectral Doppler waveform and loss of its usual phasicity. Hemodynamically significant IVC stenosis can be differentiated from pseudostenosis by the presence of features of Budd-Chiari syndrome and elevated Doppler velocity measurements.
Stenosis and thrombosis of the IVC can be detected by using CT scans. Findings include narrowing of the retrohepatic segment, demonstration of an intraluminal thrombus, distention of the IVC and HVs, and imaging features of Budd-Chiari syndrome and/or portal hypertension. Analogous changes can be depicted with MRI and MRV.
Inferior venacavographic results can confirm stenosis and thrombosis. Pressure gradient measurements can distinguish physiologically significant lesions from pseudostenoses. Balloon angioplasty and stent placement can be used to correct an IVC stenosis, but restenosis requiring repeat angioplasty with high-pressure balloon catheters is not uncommon.
Isolated complications of the HV are rare. Strictures at the suprahepatic caval anastomosis can involve the confluence of the HV. Like caval strictures, lesions of this vein are amenable to percutaneous transjugular angioplasty and stent placement.
Postoperative Fluid Collections
Postoperative fluid collections are common and can be supradiaphragmatic and infradiaphragmatic. As with other major hepatic surgeries, small, right-sided pleural effusions almost always develop after liver transplantation. These rarely complicate the postoperative course and usually resolve quickly.
In most patients, small parahepatic hematomas are present after surgery. Small hematomas are frequently noted on postoperative ultrasonograms obtained between 24 hours and 1-2 weeks after transplantation. Hematomas tend to be located in the gallbladder fossa and the hepatorenal space. They are depicted ultrasonographically as 2- to 3-cm lesions of variable echogenicity. Analogous findings are seen on CT and MRI scans. Virtually all small hematomas resolve spontaneously and without complications.
Occasionally, imaging shows small intraparenchymal fluid collections and defects in the transplanted liver. These include small contusions, hematomas, and innocuous bilomas. Fluid can be seen to accumulate in the fissure of the ligamentum teres. In patients who are doing well, these also are of little consequence.
A large volume of ascites can form after liver transplantation; they can result from cardiac decompensation with passive hepatic congestion, from allograft dysfunction and rejection, or from vascular complication. Because tense ascites can impair hepatic perfusion, damaging the allograft further, drainage of the ascites becomes necessary. This is easily performed by using imaging guidance and routine 8F catheter placement.
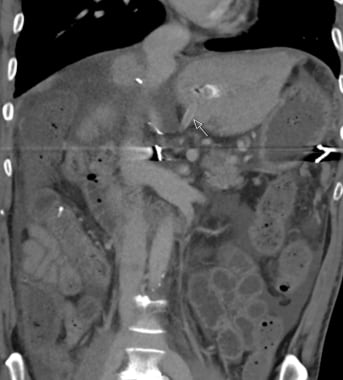
Bilomas are often associated with serious postoperative complications. They can reflect dehiscence of a choledochocholedochostomy or of a biliary-enteric anastomosis. Such dehiscence can result from technical problems or from ischemia at the anastomosis. As previously noted, ischemia resulting in biliary necrosis and biloma formation often is caused by hepatic artery stenosis (HAS) or thrombosis (HAT). In living-donor liver transplantation (LDLTX), bile can leak from the cut surface of the allograft if accessory or aberrant ducts are not noted and ligated or coagulated during surgery.
Bilomas can demonstrate a spectrum of imaging findings. Extrahepatic bile leaks can appear as free fluid or large, loculated collections adjacent to the liver or in other peritoneal spaces. During US and CT scanning, bilomas also can be seen as discrete, rounded, hypoechoic or hypoattenuated intraparenchymal lesions.
Bilomas can manifest as periportal cuffing, mimicking periportal edema. Although often amorphous, these peculiar-appearing bilomas usually reflect severe and extensive bile duct necrosis secondary to HAS or HAT and suggest a poor outcome for the graft.
Bile leaks can be confirmed by using nuclear medicine hepatobiliary scans. Small leaks can seal spontaneously. Other leaks require interventional or surgical correction.
Image-guided aspiration of a fluid collection can be necessary to determine its composition. Small, sterile parahepatic or intrahepatic bilomas can be treated expectantly or can be aspirated dry during sampling. Infected bilomas require percutaneous 8F catheter drainage, which is often performed in conjunction with other biliary and arterial interventions if underlying bile duct or vascular complications are present. [37] In patients with irreversible graft damage, percutaneous control of bilomas, particularly infected ones, can be a temporizing measure while retransplantation is pending.
In the setting of postoperative fever and sepsis, some hematomas must be aspirated to determine if superinfection is present. As determined by stat Gram staining and subsequent culturing, sterile hematomas are left to resolve spontaneously. Catheter placement in an uninfected hematoma is avoided, because semisolid subacute blood is not amenable to catheter drainage and because superinfection (by leaving an indwelling catheter) of an otherwise sterile collection is to be avoided.
Hepatic lymphatics are completely disrupted in the recipient. In the early period after transplantation, periportal fluid can originate in an accumulation of lymph; however, unlike renal transplantation, lymphoceles do not complicate liver transplantation. This is probably because of the intraperitoneal placement of the hepatic allograft, which allows lymph to be reabsorbed by the peritoneum.
Postoperative pyogenic abscesses in the abdomen or retroperitoneum can be treated percutaneously. Abscesses that have evolved from infected bilomas and vascular compromise require correction of the underlying problem. The occasional routine postoperative abscess unassociated with intrinsic graft complications can be treated successfully by using standard, image-guided, percutaneous catheter placement. In patients with uncomplicated abscess, 95% of isolated abscesses can be cured by means of catheter drainage and appropriate antibiotic coverage.
Postoperative Malignancy
Immunosuppressive therapy to prevent rejection is requisite after liver transplantation. However, the treatment is associated with an increased risk of malignancy in the recipient. [38] Of the neoplasms that also occur in immunocompetent individuals, skin and cervical carcinomas are more likely to occur in transplantation patients.
Immunocompromised hepatic allograft recipients are at risk for posttransplantation lymphoproliferative disease (PTLD). Approximately 1-2% of patients receiving livers develop PTLD. Low-dose and steroid-free regimens probably reduce the frequency of PTLD.
Some orthotopic liver transplantations (OLTXs) are performed in patients with known hepatobiliary malignancy or in persons at high risk for it. Patients with hepatocellular carcinoma (HCC) are candidates for OLTX if the disease is unilobar and if no lesion exceeds 5 cm in diameter. [23]
In patients who develop recurrent or metastatic HCC after OLTX, 46% have metastases in the allograft. Other sites of metastatic disease include the lungs, regional lymph nodes, adrenal glands, and bones. Patients with primary sclerosing cholangitis (PSC) and cirrhosis are commonly considered candidates for liver transplantation. [22]
US, CT scanning, and MRI can be used to detect posttransplantation neoplasms. In patients with suspected hepatic involvement, US is a reasonable screening tool, depicting approximately 85% of focal lesions larger than approximately 2 cm. However, US is less effective in demonstrating extrahepatic malignancy, because other abdominal organs are partially or completely obscured by bowel gas, fat, and bone.
CT scanning is excellent in the detection and diagnosis of most posttransplantation malignancies. Dynamic, contrast-enhanced scans of the entire chest, abdomen, and pelvis can be obtained quickly and can provide a complete overview of the disease's extent. Approximately 90% of focal lesions larger than 2 cm can be demonstrated. CT scan–guided fine-needle aspirations and core-needle biopsies can usually provide adequate material to establish a tissue diagnosis.
State-of-the-art MRI is competitive with CT scanning in the evaluation of the abdomen and pelvis for posttransplantation malignancy. Similar to CT scanning, MRI shows 90% of focal liver lesions. As a result of inherently better contrast resolution and the availability of unique contrast agents, some lesions (especially those in the liver) can be imaged and characterized only by using MRI and not by using CT. Unfortunately, MRI adds little to the evaluation of the thorax. Although MRI scans can depict mediastinal pathology, only the largest of parenchymal lung nodules can be demonstrated.
No noninvasive cross-sectional imaging modality is effective in detecting posttransplantation cholangiocarcinoma in patients with PSC. Most tumors are not conspicuous during US, CT scanning, or MRI. Occasionally, an isolated dilated bile duct, hilar or intrahepatic mass, or enlarged regional node suggests the diagnosis. However, in most patients, percutaneous transhepatic cholangiopancreatography (PTC), endoscopic retrograde cholangiopancreatography (ERCP), or cholangioscopy with brushings and biopsy of suggested malignant bile duct strictures are repeated until the diagnosis is established.
Postoperative Recurrence of Hepatobiliary Disease
US, CT scanning, and MRI are insensitive in detecting recurrent, diffuse hepatocellular diseases. Other than excluding other causes of graft dysfunction, the modalities are not helpful in diagnosing recurrent hepatitis B virus (HBV), HCV, primary biliary cirrhosis, or alcohol-related liver disease. Although imaging occasionally can demonstrate severe allograft damage, in most patients the diagnosis of recurrent hepatocellular disease is made clinically based on serum chemistry, immunologic tests, viral serology, and liver biopsy results.
Routine cross-sectional imaging is of little value in detecting primary sclerosing cholangitis (PSC) recurrence. Because of inadequate resolution of peripheral intrahepatic bile ducts, even magnetic resonance cholangiopancreatography (MRCP) is insensitive. Percutaneous transhepatic cholangiopancreatography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP) is required to delineate the early ductal changes of PSC in a transplanted liver.
Living-Donor Liver Transplantation
Complications following living-donor liver transplantation (LDLTX) are essentially identical to those of whole-liver orthotopic liver transplantation (OLTX). The major problems encountered include biliary stenosis, bile leaks, HAS, and HAT. [39] Like OLTX, recurrent hepatocellular disease can affect LDLTXs. Imaging LDLTX complications is analogous to imaging in patients with OLTX.
With LDLTXs, anastomosed bile ducts are necessarily smaller, representing a greater technical challenge. End-to-end bile duct reconstruction is often possible, and in other cases, biliary-enteric anastomosis is performed. As with OLTXs, biliary stricture can occur. Additionally, bile leaks in the donor and in the recipient can result if anomalous ducts crossing the plane of section in the donor liver are not recognized by preoperative imaging or at surgery. Bilomas can form from weeping of the cut liver surfaces, probably because small radicles are coagulated incompletely. As with other fluid collections, LDLTX bilomas can become superinfected.
Hepatic artery stenosis (HAS) and thrombosis (HAT) also can occur after LDLTX. The small caliber of the donor right hepatic artery increases the possibility of subsequent arterial complication. The necessity for multiple anastomoses in patients with anomalous branching of the right hepatic artery compounds the potential.
Aberrant hepatic venous and portal venous anatomy can increase the difficulty of performing LDLTXs compared with that of performing OLTXs. However, thorough preoperative imaging and careful surgical dissection allow aberrations in the HV and PV to be identified and dealt with satisfactorily. Significant venous problems are unusual after LDLTX.
Questions & Answers
Overview
What is the prevalence of liver transplantation?
What is the role of imaging in detecting post-liver transplantation complications?
What is adult-to-adult, living-donor liver transplantation (LDLTX)?
What are the indications for liver transplantation?
What are the possible complications of whole-liver orthotopic liver transplantation (OLTX)?
How are the biliary complications of whole-liver orthotopic liver transplantation (OLTX) treated?
Which malignancies are associated with whole-liver orthotopic liver transplantation (OLTX)?
What are the possible complications of living-donor liver transplantation (LDLTX)?
-
Drawing of the basic anastomoses of an adult-to-adult living-donor liver transplantation. At the author's institution, the right lobe is usually harvested from adult donors and transplanted in orthotopic position after the recipient's native liver is extirpated. Although large vessels and bile ducts must be ligated, control of hemorrhage and bile leakage from the cut surface is possible by performing tissue coagulation with a harmonic scalpel.
-
Drawing of an orthotopic liver transplant and the basic surgical anastomoses.
-
Spectral Doppler ultrasonographic waveform of the right hepatic artery in a 60-year-old man, 8 years after orthotopic liver transplantation. The image demonstrates the typical rounded tardus parvus waveform morphology, which is indicative of upstream arterial thrombosis or severe stenosis. Subsequent angiography confirmed occlusion at the hepatic arterial anastomosis.
-
A-1: Magnetic resonance angiogram in a transplantation patient with hepatic artery thrombosis. Magnetic resonance angiogram of the recipient celiac axis depicts complete occlusion of the hepatic artery.
-
A-2: Magnetic resonance angiogram demonstrates intrahepatic arterial segments reconstituted from mesenteric collaterals. Collateralization explains how, in some patients, intrahepatic flow can be present in the setting of complete extrahepatic arterial occlusion.
-
A-3: Digital subtraction angiogram shows complete hepatic artery thrombosis in a liver transplant recipient.
-
A-4: Digital subtraction angiogram depicts redundant donor-recipient hepatic artery. A stenotic hairpin turn is noted.
-
A-5: Digital subtraction angiogram obtained during balloon angioplasty of a stenotic artery.
-
A-6: Digital subtraction angiogram shows a widely patent hepatic artery after angioplasty.
-
Multiple ischemic biliary strictures documented during interventional external-internal drainage procedure.
-
Early posttransplantation evaluation of the portal vein by using spectral Doppler ultrasonography depicts a normal waveform with peak flow velocity of approximately 31 cm/s.
-
Spectral Doppler ultrasonogram of the portal vein was obtained at the onset of graft dysfunction. Compared with the baseline examination, markedly accelerated flow to 150 cm/s was documented. Subsequent catheter venography was performed that documented a high-grade venous stenosis. The stenosis was treated successfully by using balloon angioplasty and stent placement.
-
Transjugular portography demonstrates extensive portal vein thrombus in the whole-liver allograft of a 40-year-old woman whose clinical condition rapidly deteriorated on postoperative day 39.
-
After aggressive percutaneous thrombolysis, balloon dilatation, mechanical thrombectomy, and systemic anticoagulation, patency of the portal vein has been restored. Follow-up portography shows that the portal vein is open. Competing collaterals communicating with the superior mesenteric vein (arrow) and the inferior mesenteric vein (arrowhead) were noted. Subsequently, the collateral gonadal vein draining the superior mesenteric vein to the inferior vena cava was embolized with coils to improve hepatopetal flow.
-
The portal-venous phase computed tomography (CT) scan shows somewhat amorphous and irregular fluid-density material surrounding the portal vein and tracking along the portal triads. This is the typical appearance of a branching biloma that results from the diffuse, extensive extravasation of bile from ischemic ducts. This type of bile duct injury is caused by either severe hepatic artery stenosis or hepatic artery thrombosis.
-
Contrast-enhanced computed tomography (CT) scan shows a 4 x 5-cm hepatic artery pseudoaneurysm in the porta hepatis. Its contrast enhancement is equivalent to that of the arterial blood pool. The peripheral hypoattenuation is consistent with mural thrombus.
-
B-1: Extrahepatic artery Doppler ultrasonography shows Doppler spectrum with lower normal limits. Intrahepatic artery spectrum could not be sampled.
-
B-2: Extrahepatic artery Doppler ultrasonography shows Doppler spectrum with lower normal limits. Intrahepatic artery spectrum could not be sampled.
-
B-3: Hepatic artery graft stenosis: arrows indicate hepatic artery tapering and disappearance. The low attenuation area shows infarction.
-
C-1: Gortex hepatic artery graft thrombosis in a 59-year-old man (shown by arrow).
-
C-2: Gortex hepatic artery graft thrombosis in a 59-year-old man (shown by arrow).
-
C-3: Infarction of the lateral part of a graft (multiple arrows); thrombosis of the arterial graft (single arrow).
-
C-4: Splenic vein thrombosis of the same patient is marked by arrows.
-
C-5: Multiple infarctions of the left lateral aspect are marked by arrows. Bilateral pleural effusion can be seen.
-
C-6: Ultrasound image of the same patient showing a larger infarction/collection? (between the cursors) of the hepatic graft.
-
C-7: The patient developed frank abscess after a few days. The hyper-echogenic foci of the air can be easily seen.
-
C-8: The CT done on the following day confirmed the abscess. A drain was inserted (double arrows)
-
C-7: The patient developed frank abscess after a few days. The hyper-echogenic foci of the air can be easily seen.