Practice Essentials
Barrett esophagus is a metaplastic disorder in which specialized columnar epithelium replaces healthy squamous epithelium. Barrett metaplasia is the most common cause or precursor of esophageal carcinoma. The rate of esophageal adenocarcinoma is increasing in the Western world, and it is associated with a poor prognosis, mainly because individuals present with late-stage disease. [1, 2] Barrett esophagus is considered the only identifiable precursor lesion to esophageal adenocarcinoma. [3, 4]
Early detection of esophageal adenocarcinoma is considered feasible because the majority of cases of esophageal adenocarcinoma develop slowly from Barrett esophagus, a metaplastic condition in which the normal stratified, squamous epithelium of the lower esophagus is replaced with a polarized, columnar-lined epithelium with intestinal-type differentiation. White light endoscopy is considered the gold standard for diagnosing Barrett esophagus. [5, 6]
Chronic gastroesophageal reflux disease (GERD) is a risk factor for Barrett esophagus. The progression of Barrett esophagus to malignancy follows a lengthy course during which it progresses from low-grade dysplasia to high-grade dysplasia and then to adenocarcinoma. The prevalence of Barrett esophagus is estimated to be 1.5% in the general population and up to 15% in patients with GERD. [7, 8]
The risk for Barrett esophagus also increases with age and is often diagnosed in the 6th decade. Although the elderly are at increased risk for development of adenocarcinoma, annual surveillance among elderly patients may be more harmful than beneficial because of more limited life expectancy. [9, 10]
The preferred radiologic examination for Barrett esophagus is a double-contrast esophagography. [11, 12, 13, 14] Imaging modalities that yield less information include nuclear medicine technetium-99m (99mTc) pertechnetate scanning, endoluminal ultrasonography, chromoendoscopy, [15, 16, 17] and computed tomography (CT) scanning. Of the newer technologies, inspection of the mucosa with high-resolution white light endoscopy is the most critical. [18, 19, 20]
Positive findings on a double-contrast esophagogram suggest a diagnosis of Barrett esophagus, in correlation with the clinical history. However, an endoscopic examination with biopsy is required to confirm the diagnosis because columnar metaplasia is diagnosed at microscopy. [21] In addition, the features that suggest columnar metaplasia are not always present on the esophagogram. A Barrett stricture without the other features cannot be distinguished from the other etiologies of a stricture.
(Radiologic characteristics of Barrett esophagus are presented in the images below.)
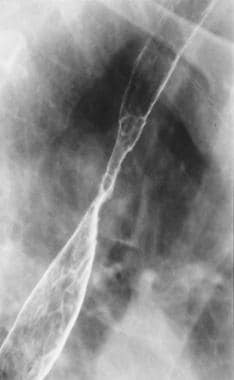 Spot radiograph from double-contrast esophagography shows a smooth stricture in the midesophagus. Multiple ulcerations in the region of the stricture are seen. Note the reticular mucosal appearance extending down from the inferior aspect of the stricture.
Spot radiograph from double-contrast esophagography shows a smooth stricture in the midesophagus. Multiple ulcerations in the region of the stricture are seen. Note the reticular mucosal appearance extending down from the inferior aspect of the stricture.
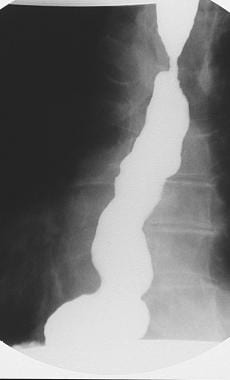 Spot radiograph shows spontaneous severe gastroesophageal reflux extending upward beyond the Barrett stricture.
Spot radiograph shows spontaneous severe gastroesophageal reflux extending upward beyond the Barrett stricture.
Barrett esophagus is named after Numan R. Barrett (1903-1979), a distinguished thoracic surgeon in London who in 1950 wrote an article entitled Chronic Peptic Ulcer of the Oesophagus and "Oesophagitis". [22] He concluded that most of the cases are examples of congenital short esophagus. He suggested that this was a separate entity from reflux esophagitis. [23] In Leeds, England, in 1953, Allison, a thoracic surgeon, and Johnstone, a radiologist, published an article entitled The Oesophagus Lined With Gastric Mucous Membrane. [24] They suggested the term Barrett's ulcers to describe ulcer craters in the columnar cell–lined esophagus. In 1957, Barrett published another article, entitled The Lower Esophagus Lined by Columnar Epithelium, [25] which he presented as a lecture at the Mayo Clinic. He now accepted the view of Allison and Johnstone that this condition involves a columnar cell–lined esophagus and not an extension of the stomach into the mediastinum. His conclusion that a columnar cell–lined esophagus is congenital was later disproved.
Radiography
A specific diagnosis of Barrett esophagus can be suggested if a proximal esophageal stricture, deep penetrating ulcer, or reticular mucosal surface pattern is seen on the esophagogram (as demonstrated in the image below).
 Spot radiograph from double-contrast esophagography shows a smooth stricture in the midesophagus. Multiple ulcerations in the region of the stricture are seen. Note the reticular mucosal appearance extending down from the inferior aspect of the stricture.
Spot radiograph from double-contrast esophagography shows a smooth stricture in the midesophagus. Multiple ulcerations in the region of the stricture are seen. Note the reticular mucosal appearance extending down from the inferior aspect of the stricture.
Although esophageal ulceration in Barrett esophagus can occur anywhere along the columnar epithelium, classically it involves the most proximal portion at or near the squamocolumnar junction, well above the cardia and even as high as the aortic arch. Unlike the shallow ulcerations that usually are caused by reflux esophagitis in the squamous epithelium, a Barrett ulcer tends to be deep, penetrating, and identical to a peptic gastric ulcer. Stricture formation usually accompanies the ulceration. At times, no ulceration is evident, and only a smooth, tapered stricture is present.
The stricture forms at the squamocolumnar junction. The Barrett stricture tends to be short and tight, typically causing eccentric narrowing of the lumen in contrast to the smooth, symmetric, and circumferential luminal narrowing in peptic strictures. A specific sign of Barrett esophagus is the ascending or migrating stricture, in which progressive upward migration of both the squamocolumnar junction and the level of the stricture is depicted on serial esophagograms.
A delicate reticular pattern extending inferiorly for a variable distance from the level of a stricture has been described as a radiologic sign of Barrett metaplasia. However, this appearance is nonspecific, and it has been observed in other conditions such as candidiasis, viral esophagitis, superficial spreading carcinoma, and areae gastricae in a small hiatal hernia.
A sliding hiatal hernia with gastroesophageal reflux (GER) commonly is seen in patients with Barrett esophagus. However, in most patients, a variable length of normal-appearing esophagus separates the Barrett ulcer from the hiatal hernia. This finding is in contrast to that of reflux esophagitis, in which the distal esophagus is abnormal down to the level of the hernia.
Another radiologic sign that raises the possibility of Barrett esophagus is a focal defect in the esophageal contour at least 4 cm proximal to the esophagogastric junction. The contour defect is believed to be an early stage of a midesophageal stricture, a classic feature of Barrett esophagus.
Esophageal contour defects caused by Barrett esophagus simulate normal variations in the caliber of the esophagus. Optimal distention of the esophageal lumen and varying obliquity may be necessary to confirm the presence of restricted distensibility and to identify fixed transverse folds. Subtle contour defects can be observed more readily on double-contrast images because fixation of the esophageal wall may be more conspicuous than on images obtained with a single-contrast technique.
Radiographic findings in short-segment Barrett esophagus are less specific. In one study, 70% of patients with short-segment Barrett esophagus had reflux esophagitis, peptic scarring or strictures, or both on double-contrast esophagograms, and 30% had only hiatal hernias or GER as radiographic findings. [26]
Findings of Barrett esophagus on a double-contrast esophagogram must be confirmed with esophagogastroduodenoscopy (EGD) and biopsy.
The fine reticular pattern inferior to the stricture in some patients with Barrett esophagus also may be observed when the areae gastricae, which is the normal appearance of the gastric mucosa on a double-contrast image, is visualized within a small hiatal hernia.
Computed Tomography
CT scanning is not the modality of choice for the diagnosis of Barrett esophagus. However, CT scans obtained for reasons other than the evaluation of Barrett esophagus may incidentally reveal a deep Barrett ulcer in the mid-to-distal esophagus. [27] In addition, in the event of transformation in an area of Barrett metaplasia to esophageal adenocarcinoma, CT may reveal a focal esophageal soft tissue mass. [28] In patients with these findings, CT is useful in staging the cancer and in predicting its response to treatment.
An esophageal ulcer or a mass lesion found incidentally on CT scans must be further evaluated with endoscopy and, probably, biopsy because these findings are nonspecific and may occur in other conditions. [29] A deep ulcer is a nonspecific finding and may be present in other conditions such as esophagitis related to human immunodeficiency virus (HIV) or cytomegalovirus (CMV) infection. Unless contiguous involvement of surrounding tissues exists, distinguishing between a malignant neoplasm and a benign lesion, such as a leiomyoma, may be difficult.
Because CT scanning has a poor yield in the detection of mucosal lesions, it is not the appropriate test, by itself, for the diagnosis of Barrett esophagus.
Ultrasonography
Ultrasonography (US) also is not the modality of choice in the diagnosis of Barrett esophagus. However, endoscopic US is used to evaluate early submucosal or mucosal cancer in the surveillance of patients with Barrett esophagus. [30, 31] Intraluminal US may reveal an esophageal neoplasm, which is depicted as a solid mass lesion that disrupts the normal layers of the esophagus. In addition, extension of the neoplasm beyond the confines of the esophageal wall also may be determined with US.
Findings at intraluminal esophageal US performed for reasons other than the investigation of Barrett esophagus warrant further evaluation with endoscopy and biopsy.
Nuclear Imaging
Radionuclide examination with intravenously administered technetium-99m (99mTc) pertechnetate may show uptake by the gastric type of mucosa; this uptake may be observed in Barrett metaplasia. However, because intestinal metaplasia of any length is the currently accepted modality for the diagnosis of Barrett esophagus, the significance of a positive finding on a pertechnetate scan is uncertain.
In addition, nuclear scanning is not the investigation of choice in the diagnosis of Barrett esophagus. Positive uptake in an area of Barrett metaplasia may be incidentally observed on scans obtained for reasons other than the assessment of Barrett esophagus.
Positive pertechnetate uptake in the region of the esophagus suggests the presence of gastric mucosa. This finding should be evaluated further with endoscopy and, probably, biopsy.
Because intestinal metaplasia of any length is the currently accepted modality for the diagnosis of Barrett esophagus, the significance of a positive finding on a pertechnetate scan is uncertain; it signifies ectopic gastric mucosa in the esophagus. In addition, uptake may be observed in remnants of the heterotopic gastric epithelium in the subcricoid area, termed inlet patch, which has no malignant potential.
Sufficient care must be taken to ensure that positive uptake does not represent the passage of swallowed saliva through the esophagus. This finding can be confirmed by asking the patient to drink a glass of water and then by imaging the lower chest again.
In addition, because Barrett metaplasia may consist of only intestinal metaplasia without the presence of gastric mucosa, no uptake of 99mTc pertechnetate may occur in the region of Barrett esophagus.
-
Spot radiograph from double-contrast esophagography shows a smooth stricture in the midesophagus. Multiple ulcerations in the region of the stricture are seen. Note the reticular mucosal appearance extending down from the inferior aspect of the stricture.
-
Spot radiograph shows spontaneous severe gastroesophageal reflux extending upward beyond the Barrett stricture.









