Practice Essentials
Celiac disease is an autoimmune disorder of the small intestine caused by gluten intolerance in genetically predisposed individuals. It is a chronic disease characterized by mucosal lesions of the small bowel that impair nutrient absorption (see the images below). [1, 2, 3] Studies show that a genetic predisposition in certain individuals can cause an immune response, which damages the small intestine mucosa. Celiac disease is strongly associated with HLA class II D-region genes in the major histocompatibility complex on chromosome 6. Approximately 90-95% of patients have either DR3-DQ2 or DR5/7DQ2 haplotypes. [4, 5, 6, 7]
Radiographic findings of celiac sprue are present in virtually all patients in the clinically active phase of the disease. However, these symptoms may also present in patients with a wide variety of other malabsorption disorders. Common findings in celiac sprue include dilatation of the small intestine lumen, intussusception, hypersecretion, segmentation, a mosaic pattern, and jejunoileal fold-pattern reversal. [8]
The definitive diagnosis of celiac sprue is based on a small-bowel biopsy and clinical improvement after the introduction of a gluten-free diet. [9, 10]
Preferred examination
Small-bowel series and enteroclysis are the preferred radiographic examinations for the diagnosis of celiac sprue. CT shows many of the findings well and is often performed when patients have nonspecific findings and when malignancy must be excluded. [11, 12, 13, 14, 15]
When multiple findings of sprue are present on small-bowel follow-though or enteroclysis, the diagnosis is fairly specific. However, up to 25% of patients may not have enough findings for a confident diagnosis, and a small percentage of patients may have multiple findings that are radiographically suggestive of celiac sprue with negative biopsy results. Duodenal biopsy is performed to confirm the diagnosis in many cases and is considered the criterion standard for diagnosing celiac disease.
Serologic testing of celiac patients is a less common means of diagnosis, whereby patients are screened for the presence of immunoglobulin G and immunoglobulin A (IgA) antigliadin antibodies (AGAs) and/or IgA antiendomysial antibodies (EMAs). [16] The sensitivity of AGA tests varies because AGAs are also found in autoimmune disorders other than celiac disease. In addition, AGAs become undetectable after the institution of a gluten-free diet.
IgA EMA screening is considered the most reliable serologic test for the detection of celiac disease, with a specificity of nearly 100%. Nevertheless, EMA testing has its drawbacks, with poor sensitivity in children younger than 2 years and with false-negative results in 2-3% of patients who have selective IgA deficiency.
When all 3 antibodies are screened simultaneously, the positive or negative predictive value is 99%. Therefore, these serologic tests may obviate biopsy, especially if the condition responds to a gluten-free diet. [4]
(See the images below.)
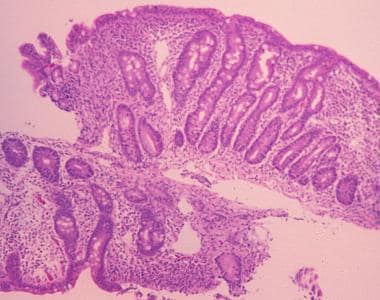 Sprue. High-powered photomicrograph of a duodenal biopsy sample from a patient with celiac disease reveals villous atrophy caused by gluten intolerance in celiac disease.
Sprue. High-powered photomicrograph of a duodenal biopsy sample from a patient with celiac disease reveals villous atrophy caused by gluten intolerance in celiac disease.
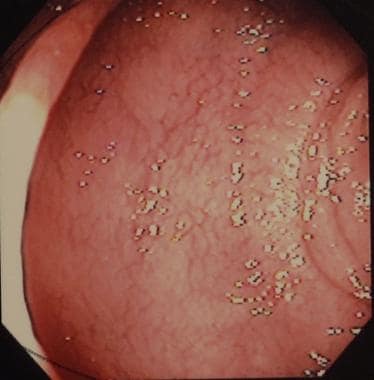 Sprue. Photograph from duodenal endoscopy in a patient with celiac disease provides a macroscopic view of duodenal villous atrophy.
Sprue. Photograph from duodenal endoscopy in a patient with celiac disease provides a macroscopic view of duodenal villous atrophy.
The symptoms of celiac disease are wide ranging, show significant variation among patients, and largely depend on the length and severity of damage to the small bowel. The classic clinical presentation is malabsorption and includes diarrhea, steatorrhea, flatulence, weight loss, and fatigue. Patients with nonclassical celiac disease present without symptoms and signs of malabsorption. Extraintestinal manifestations include neuropathy, aphthous ulcers, and dermatitis herpetiformis. [17] Associated laboratory abnormalities may include iron or folate deficiency anemia, hypocholesterolemia, hypocalcemia, hypoalbuminemia, elevated alkaline phosphatase and liver enzyme levels, and a prolonged prothrombin time. [9]
The American College of Gastroenterology (ACG) recommends that antibody testing, especially immunoglobulin A anti-tissue transglutaminase antibody (IgA TTG), is the best first test for suspected celiac disease, although biopsies are needed for confirmation. In children younger than 2 years, the the ACG recommends that the IgA TTG test should be combined with testing for IgG-deamidated gliadin peptides. [18]
Radiography
Small-bowel series
For a standard barium follow-through examination, the patient ingests approximately 300-450 mL of nonflocculable barium, followed by fluoroscopy. Abdominal images are taken at appropriate intervals until the barium has entered the colon.
Small-bowel radiographs are then examined for signs of celiac disease, such as thickening of the primary mucosal folds, lumen dilatation, barium segmentation or flocculation, transient intussusception, prolonged transit time, thickening of duodenal mucosal folds, decreased number of duodenal mucosal folds, asymmetry of duodenal mucosal folds, contrast-agent dilution, "bubbly bulb" duodenal nodules, and reversal of the jejunoileal fold pattern. Most individual radiologic signs are nonspecific but are characteristic of celiac disease when combined.
Both small-bowel series and jejunal biopsy are reliable diagnostic techniques. When combined, these methods detect small-bowel abnormalities in up to 95% of cases, with 2 false-negative and 4 false-positive results reported among 103 patients in one study. A small number of cases are detected by using this technique. Nevertheless, a small-bowel series is noninvasive and inexpensive, and it may be performed on an outpatient basis. For these reasons, this study is often recommended for the initial investigation of possible celiac disease. [19, 13]
Enteroclysis
Enteroclysis, or small-bowel enema study, is a variation of a small-bowel series that has been widely advocated as a noninvasive diagnostic examination in patients with suspected celiac disease. A confident diagnosis can be made in nearly 75% of patients, and this technique also helps to exclude celiac disease in a similar percentage of patients. Enteroclysis helps to screen for celiac disease in patients with an atypical clinical presentation, and it may allow for earlier recognition of a malignant tumor or other complications. [20]
During enteroclysis, approximately 600-800 mL of barium is actively pumped into the small bowel, and imaging is performed at appropriate time intervals. A double-contrast technique is often used with 300-450 mL of thick, air-contrast barium diluted with 450-600 mL of water. The barium is pumped in until it reaches one half to two thirds of the small bowel. This step is followed by the infusion of a dilute methylcellulose solution to propel the barium through the remainder of the small bowel, to distend the lower bowel, and to increase radiolucency.
Small-bowel series and enteroclysis
Both enteroclysis and small-bowel series are often used to investigate celiac disease. By allowing for the visualization of jejunoileal fold pattern reversal or a combination of at least 3 features—fold thickening, jejunal atrophy, jejunization of the ileum, dilatation, or barium flocculation—an accurate diagnosis can be made. In one study, 81% of patients with celiac disease had at least one of these features. [21]
In celiac disease, the small bowel often has a unique appearance consisting of a decreased number of folds in the jejunum and an increased number of folds in the ileum. In particular, there is an increased separation of the jejunal folds, a reduction in the number of folds in the proximal jejunum, an increased thickness of ileal folds, and, in some patients, an increased number of ileal folds. In extreme cases, this results in jejunoileal fold pattern reversal with atrophy of the jejunum and hypertrophy of the ileum. [22]
In one study, 3 or fewer folds per inch in the first loop of jejunum was found to be highly diagnostic of celiac disease, and the presence of 5-7 folds in the jejunum and/or 3 or fewer folds in the ileum was seen as evidence against this diagnosis. [23] Jejunoileal fold pattern reversal is regarded by many as the single most reliable indicator of celiac disease in patients evaluated by small-bowel series or enteroclysis (see the images below).
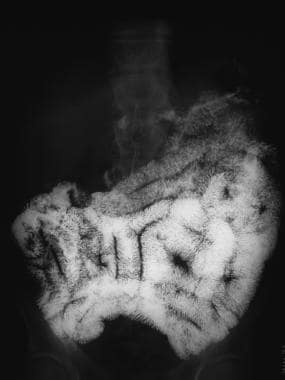 Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows jejunization of the ileal fold pattern; this is characteristic of celiac disease.
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows jejunization of the ileal fold pattern; this is characteristic of celiac disease.
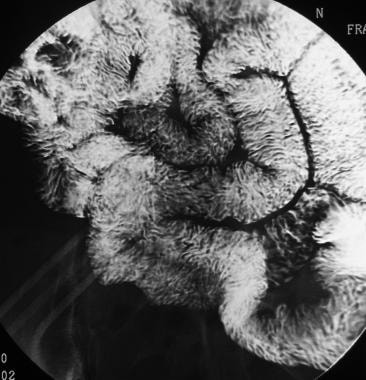 Sprue. Spot radiograph from a small-bowel series in the same patient as in the previous image in Multimedia shows close-up of jejunization of the ileal fold pattern.
Sprue. Spot radiograph from a small-bowel series in the same patient as in the previous image in Multimedia shows close-up of jejunization of the ileal fold pattern.
The early flocculation of barium is an artifact due to hypersecretion and should alert radiologists to a malabsorptive state. Barium flocculation is associated with celiac sprue in 26% patients, but it is not specific for the disease (see the images below). [21]
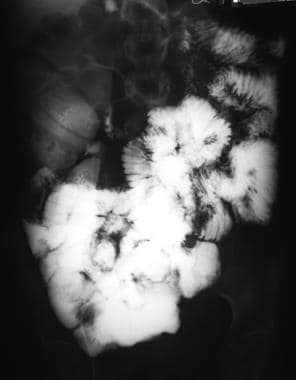 Sprue. Radiograph from a small-bowel series in a patient with celiac disease reveals small-bowel dilatation and contrast-agent dilution.
Sprue. Radiograph from a small-bowel series in a patient with celiac disease reveals small-bowel dilatation and contrast-agent dilution.
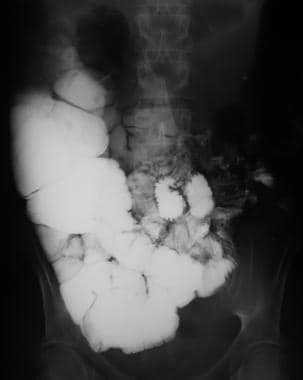 Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation, contrast-agent dilution, and distal small-bowel dilatation.
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation, contrast-agent dilution, and distal small-bowel dilatation.
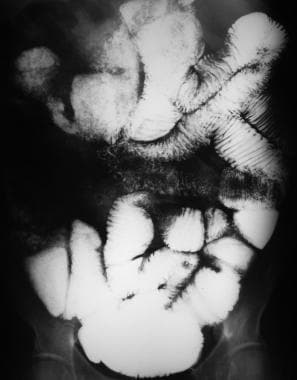 Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows contrast-agent dilution, barium flocculation, increased ileal folds, and intussusception.
Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows contrast-agent dilution, barium flocculation, increased ileal folds, and intussusception.
 Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows dilatation, contrast-agent dilution, intussusception, barium flocculation, and jejunization of the ileum.
Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows dilatation, contrast-agent dilution, intussusception, barium flocculation, and jejunization of the ileum.
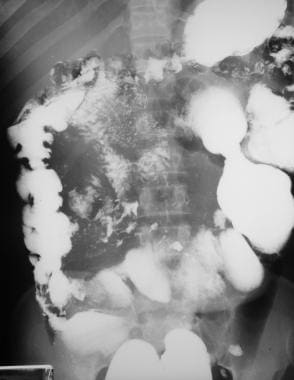 Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows mild bowel dilatation, barium flocculation, and segmentation.
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows mild bowel dilatation, barium flocculation, and segmentation.
 Sprue. Radiograph from a small-bowel series in a patient with celiac disease reveals contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 2 hours after barium ingestion.
Sprue. Radiograph from a small-bowel series in a patient with celiac disease reveals contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 2 hours after barium ingestion.
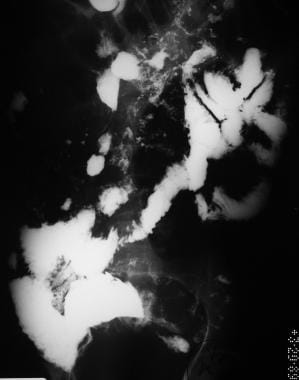 Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation and segmentation, contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 4 hours after barium ingestion.
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation and segmentation, contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 4 hours after barium ingestion.
 Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation and segmentation, contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 4 hours after barium ingestion.
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation and segmentation, contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 4 hours after barium ingestion.
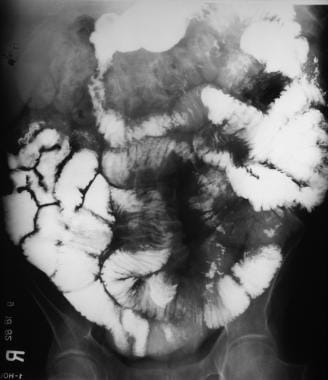 Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows contrast-agent dilution, barium flocculation and segmentation, and an increased number of ileal folds.
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows contrast-agent dilution, barium flocculation and segmentation, and an increased number of ileal folds.
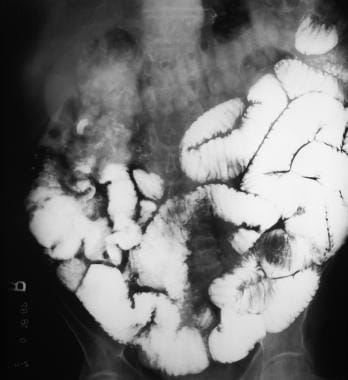 Sprue. Radiograph from a small-bowel series in the same patient as in the previous image reveals intussusception, contrast-agent dilution, increased ileal folds, and small-bowel dilatation.
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image reveals intussusception, contrast-agent dilution, increased ileal folds, and small-bowel dilatation.
In almost all patients with celiac disease, the mucosa is divided into 1- to 2-mm polygonal elevations surrounded by distinct grooves, forming a mosaic pattern in or beyond the duodenal bulb. Despite its prevalence, the mosaic pattern can be identified in only 10% of celiac patients by using enteroclysis, and the jejunal surface usually appears flat and featureless. [23, 20]
Peptic duodenitis occurs in celiac patients with hypersecretion of gastric acid and may cause peptic changes in the duodenal mucosa, including mucosal inflammation, gastric metaplasia, and Brunner gland hyperplasia. Peptic duodenitis may cause the formation of multiple, 1- to 4-mm, round, mucosal nodules in the proximal duodenum, called the bubbly bulb (see the images below). [20]
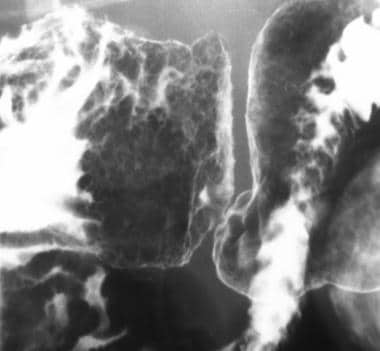 Sprue. Spot radiograph from an air-contrast upper gastrointestinal barium examination in a patient with celiac disease shows the bubbly mucosal pattern in the duodenal bulb, which is caused by peptic duodenitis in patients with celiac disease.
Sprue. Spot radiograph from an air-contrast upper gastrointestinal barium examination in a patient with celiac disease shows the bubbly mucosal pattern in the duodenal bulb, which is caused by peptic duodenitis in patients with celiac disease.
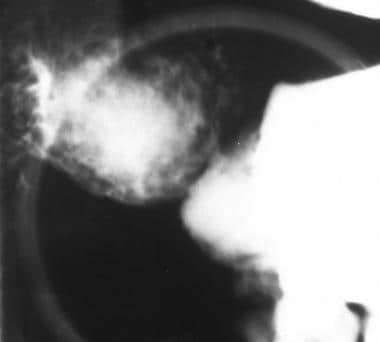 Sprue. Spot radiograph from a single-contrast upper gastrointestinal barium examination in a patient with celiac disease shows the bubbly mucosal pattern of the duodenal bulb, which his caused by peptic duodenitis in patients with celiac disease.
Sprue. Spot radiograph from a single-contrast upper gastrointestinal barium examination in a patient with celiac disease shows the bubbly mucosal pattern of the duodenal bulb, which his caused by peptic duodenitis in patients with celiac disease.
Intussusception is the telescoping or infolding of one segment of intestine within another adjacent segment. Transient, nonobstructive intussusception occurs in about 20% of patients with celiac disease but is not specific for the disease. In barium studies, intussusception has a coiled spring appearance; however, it is rarely observed during small-bowel follow-through or enteroclysis (see the images below). [20, 24]
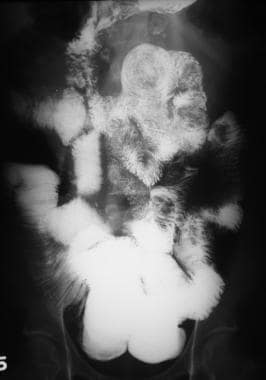 Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows a large intussusception along with numerous smaller intussusceptions, each with a characteristic coiled-spring appearance. Image also demonstrates dilatation, contrast-agent dilution, and jejunization of the ileum.
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows a large intussusception along with numerous smaller intussusceptions, each with a characteristic coiled-spring appearance. Image also demonstrates dilatation, contrast-agent dilution, and jejunization of the ileum.
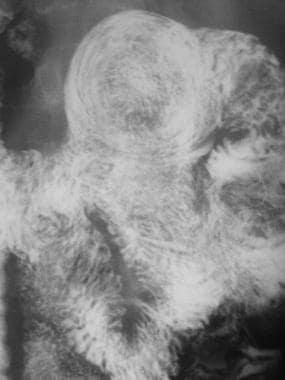 Sprue. Magnified view of the previous image from a small-bowel series in a patient with celiac disease shows coiled-spring appearance of a jejunojejunal intussusception.
Sprue. Magnified view of the previous image from a small-bowel series in a patient with celiac disease shows coiled-spring appearance of a jejunojejunal intussusception.
 Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows contrast-agent dilution, barium flocculation, increased ileal folds, and intussusception.
Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows contrast-agent dilution, barium flocculation, increased ileal folds, and intussusception.
 Sprue. Magnified view of the previous image, a radiograph from a small-bowel series in a patient with celiac disease, shows the characteristic coiled-spring appearance of an intussusception. The central mucosal channel is faintly visible.
Sprue. Magnified view of the previous image, a radiograph from a small-bowel series in a patient with celiac disease, shows the characteristic coiled-spring appearance of an intussusception. The central mucosal channel is faintly visible.
 Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows dilatation, contrast-agent dilution, intussusception, barium flocculation, and jejunization of the ileum.
Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows dilatation, contrast-agent dilution, intussusception, barium flocculation, and jejunization of the ileum.
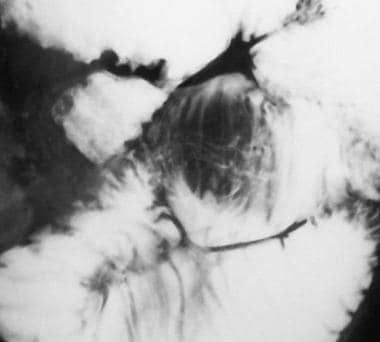 Sprue. Magnification of the intussusception on the radiograph in Image 20, in Multimedia, from a small-bowel series in a patient with celiac disease.
Sprue. Magnification of the intussusception on the radiograph in Image 20, in Multimedia, from a small-bowel series in a patient with celiac disease.
Degree of confidence
When combined, small-bowel follow-through and duodenal biopsy can be used to detect small-bowel abnormalities in up to 95% of cases. [19] Using enteroclysis alone, one can make a confident diagnosis in nearly 75% of patients while also excluding the possibility of celiac sprue in a similar percentage. [20]
Individual radiologic signs are nonspecific, but they are highly characteristic of celiac disease when combined. Small-bowel series and enteroclysis are the preferred radiographic examinations for the diagnosis of celiac disease. Nevertheless, duodenal biopsy is often used to confirm radiographic findings and remains the criterion standard for diagnosing celiac disease.
One study of 103 patients resulted in 4 false-positive results (3.9%) and 2 false-negative results (1.9%) when a small-bowel series was used to evaluate the possibility of celiac disease. [19]
Computed Tomography
CT is the most common cross-sectional study used in the diagnosis of abdominal pathology, and many patients traditionally examined with enteroclysis or a small-bowel series are now initially examined with CT. [25] One advantage of CT over barium studies is that images are not obscured by the presence of intestinal air. [26, 27] As with small-bowel series, most of the CT findings are nonspecific, but when combined, they are highly suggestive of celiac disease.
The nonspecific radiologic features of celiac disease on CT include bowel dilatation predominantly in the mid and distal jejunum, fluid excess, barium flocculation, fold thickening and separation, prolonged small-bowel transit time, abnormalities of the valvulae conniventes, and reversal of the normal small-bowel fold pattern. Additional cross-sectional imaging findings include transient small-bowel intussusceptions, mesenteric and/or retroperitoneal lymphadenopathy, and hyposplenism (see the images below). [25, 28, 29, 30]
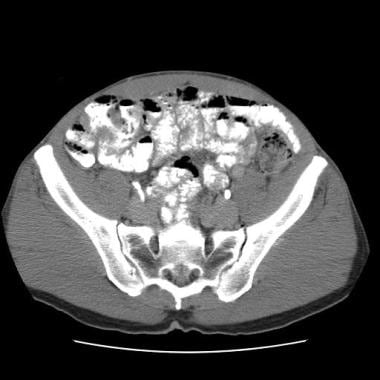 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows an increased number of ileal folds, or jejunization of the ileum.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows an increased number of ileal folds, or jejunization of the ileum.
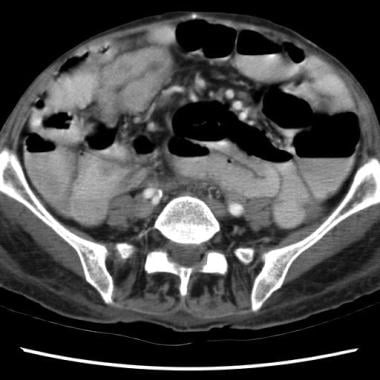 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows small-bowel dilatation and dilution of contrast material.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows small-bowel dilatation and dilution of contrast material.
CT is useful in diagnosing intussusception of the small bowel. CT in patients with intussusception shows soft-tissue masses with well-defined enhancing rims, fat-density centrally, and an irregular central attenuation. No oral contrast material or air is usually seen within the masses. Depending on their orientation to the scanning axis, the masses may appear round, oval, or pseudo-kidney shaped. The internal appearance is the same regardless of mass orientation. [26]
Jejunoileal fold pattern reversal can be demonstrated on CT. In one study, CT proved useful for measuring the number of jejunal and ileal folds in 19 of 22 patients with celiac disease. The number of folds in healthy subjects was 4-6 and 2-7 per 2.5 cm of jejunum and of ileum. In patients with celiac disease, the number of folds was 0-6 and 4-7 per 2.5 cm of jejunum and ileum. Jejunization of the ileum was observed in 17 of 22 patients, and jejunoileal fold pattern reversal was observed in 15 of 22 celiac patients and in no control subjects. [25]
Previously described CT appearances have focused on the GI tract; however, diagnosis of celiac disease may be suggested by recognition of signs of increased splanchnic circulation using cross-sectional imaging. Increased splanchnic circulation can be observed radiographically as dilatation of the superior mesenteric artery (SMA), superior mesenteric vein, or portal vein. CT findings are similar to those reported with ultrasonography and angiography (see the images below).
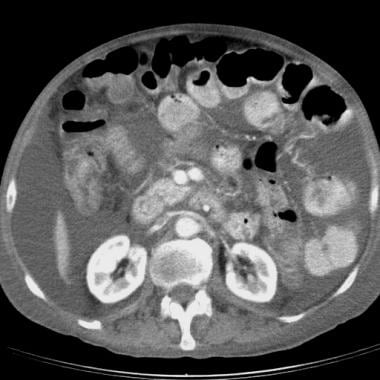 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast material in a patient with celiac disease reveals ascites, small-bowel dilatation, and increased diameter of the superior mesenteric artery and superior mesenteric vein.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast material in a patient with celiac disease reveals ascites, small-bowel dilatation, and increased diameter of the superior mesenteric artery and superior mesenteric vein.
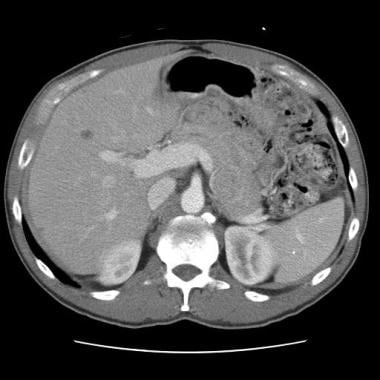 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast material in a patient with celiac disease. Image shows a dilated portal vein, which is characteristic of increased splanchnic circulation.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast material in a patient with celiac disease. Image shows a dilated portal vein, which is characteristic of increased splanchnic circulation.
 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows an increased number of ileal folds, or jejunization of the ileum.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows an increased number of ileal folds, or jejunization of the ileum.
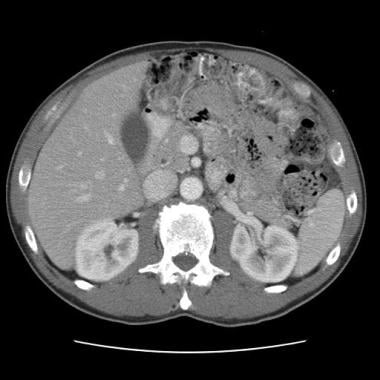 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows dilation of the superior mesenteric artery and superior mesenteric vein. This finding is characteristic of increased splanchnic circulation.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows dilation of the superior mesenteric artery and superior mesenteric vein. This finding is characteristic of increased splanchnic circulation.
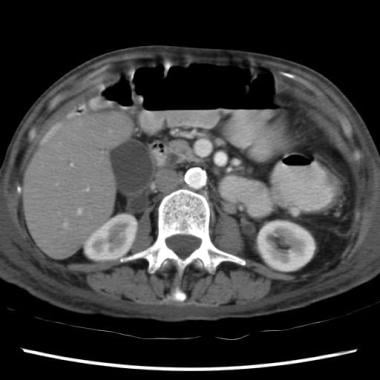 Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows a marked increase in the diameter superior mesenteric artery.
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows a marked increase in the diameter superior mesenteric artery.
Magnetic Resonance Imaging
Compared with other studies, MRI is less frequently used for evaluation of the small bowel; however, it has potential based on its excellent soft-tissue contrast and multiplanar imaging capabilities. [31]
HASTE
MRI with a half-Fourier acquired single-shot turbo spin echo (HASTE) sequence has been studied and promoted as a noninvasive, feasible technique for the diagnosis of celiac disease based on its increased sensitivity to fluid detection in the small bowel. HASTE sequences allow for the acquisition of breathing-independent T2-weighted images at a rate of approximately 1 section per second, and imaging can be performed with or without the use of an oral contrast agent. [10, 32, 33, 34]
Parameters used to evaluate celiac disease with MRI include the small-bowel diameter, wall thickness, thickness of valvulae conniventes, and percentage of small-bowel loops containing intraluminal fluid. In one study, HASTE sequencing in a celiac patient revealed fluid-filled jejunal and ileal loops and widening of the small intestine measuring 3 cm in the jejunum and 3.5 cm in the ileum. Jejunal and ileal wall thickness averaged 3 mm, and jejunization of the ileum was clearly present. [33]
The advantages of HASTE sequence MRI include elimination of breathing-related and bowel motion artifact and decreased magnetic susceptibility artifact when compared with conventional gradient-echo sequences. [35, 36] Because contrast agent is not required, HASTE allows for small-bowel imaging in a more natural and physiologic state without ionizing radiation. HASTE sequencing does have disadvantages, including an inability to show superficial small-bowel lesions and a current lack of optimal positive or negative contrasting agent, though polyethylene glycol has been studied. [10, 32, 33, 37]
It is important to note that in 29% of adults with biopsy-proven celiac disease, no small-bowel abnormalities were observed by using the HASTE technique. This finding is not surprising and mirrors data from conventional barium studies in which as many as 25% of celiac patients had no radiographic manifestations of the disease. [10]
Ultrasonography
Ultrasonography can be used as a means of initial investigation or to establish a diagnosis in celiac patients by evaluating small-bowel abnormalities and/or hemodynamic disturbances of the superior mesenteric artery (SMA).
Common sonographic signs in adult celiac patients include increased fluid in the small intestine, moderately dilated small intestine, thickening of the small-bowel wall, increased peristalsis, enlarged mesenteric lymph nodes, dilated SMA (SMA of 8-11 mm, as measured 2-3 cm distal to the artery origin) or portal vein, free fluid in the abdominal cavity, and liver steatosis. [9] Intussusception has a characteristic appearance described as concentric hypoechoic rings separated by a hyperechoic ring; these represent the muscular layers of the intussusceptum and the intussuscipiens with the trapped mesenteric fat, respectively. [38]
In infants, sonographic signs associated with celiac disease include abnormal appearance of the small-bowel wall, hyperperistalsis, slight ascites, pericardial fluid, and changes in the text of the liver tissue. [39, 40]
Ultrasonography with polyethylene glycol electrolyte solution (PEG-ELS) has been suggested as a less-expensive but more time-consuming alternative to traditional barium studies. The patient ingests 200-800 mL of PEG-ELS prior to sonography to facilitate visualization of the small bowel and improve measurement of wall thickness and lumen diameter. PEG-ELS eliminates 2 drawbacks of traditional abdominal sonography: luminal collapse and abdominal gas obscuring the small bowel.
Ultrasonography can also be used to evaluate intestinal blood flow in patients with celiac disease by measuring the hemodynamic parameters of the SMA.
Mean basal SMA blood flow is approximately 50% higher, and postprandial mesenteric blood flow is increased and delayed in time in celiac patients compared with healthy control subjects. Successful treatment of celiac patients with a strict gluten-free diet reduces the hemodynamic abnormalities of active celiac disease. Similar splanchnic circulation changes may be seen in patients with inflammatory bowel disease. [41, 42]
Each sonographic sign in children or adults is nonspecific and can occur in various other small-bowel diseases. However, a combination of signs is highly indicative of celiac disease and warrants further investigation (see the image below). [9, 43]
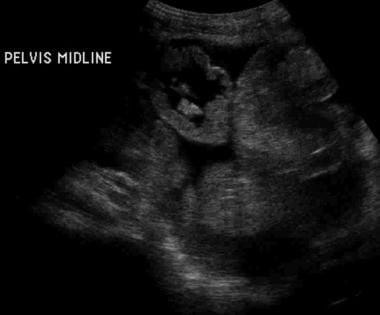 Sprue. Sonogram of the abdomen in a patient with celiac disease shows ascites, bowel-wall thickening, and increased intraluminal fluid.
Sprue. Sonogram of the abdomen in a patient with celiac disease shows ascites, bowel-wall thickening, and increased intraluminal fluid.
Ultrasonography does not replace biopsy and clinical improvement on a gluten-free diet as the essential methods of diagnosing celiac disease. However, sonography is often obtained in patients with nonspecific abdominal complaints, and the recognition of findings of celiac disease may speed the diagnostic process, especially in patients with atypical clinical presentations. [9]
For PEG-ELS sonography, one study revealed an overall sensitivity of 72% and specificity of 100%, though a substantial number of false-negative outcomes occur by using this technique. [37]
Angiography
Celiac disease is associated with disturbances in intestinal circulation reflected in superior mesenteric artery (SMA) dilatation and shortened intestinal circulation time. Angiography is not a radiographic technique frequently used to evaluate celiac patients. However, it is a feasible method for observing circulatory changes and may be performed to exclude other causes of small-bowel disease, such as vasculitis or intestinal ischemia or angina. [44]
Although stenosis or obstruction of the SMA can cause malabsorption, celiac disease tends to feature dilatation of the arteries supplying the affected intestine. Angiographic evaluation of patients with celiac disease has demonstrated a significant increase in the diameter of the SMA and branch vessels with an increase in capillary blush and a shortened intestinal circulation time reflecting increased small-bowel blood flow. [44, 45]
Intestinal blood circulation can be estimated by using a variety of parameters, such as SMA diameter and circulation time. In one study, SMA diameter averaged 8.5 mm ± 0.4 in healthy subjects, while SMA diameter significantly increased in patients with celiac disease, averaging 11.1 mm ± 0.4. A shortened circulation time in celiac patients may result from increased blood flow or decreased blood volume, which is attributed to increased activity of vasodilatory agents, destruction of small-intestine mucosal capillary beds, and arteriovenous shunting. [9, 44]
-
Sprue. High-powered photomicrograph of a duodenal biopsy sample from a patient with celiac disease reveals villous atrophy caused by gluten intolerance in celiac disease.
-
Sprue. Photograph from duodenal endoscopy in a patient with celiac disease provides a macroscopic view of duodenal villous atrophy.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows jejunization of the ileal fold pattern; this is characteristic of celiac disease.
-
Sprue. Spot radiograph from a small-bowel series in the same patient as in the previous image in Multimedia shows close-up of jejunization of the ileal fold pattern.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows small-bowel dilatation and contrast-agent dilution, as well as a slight increase in the number of ileal folds. Individual findings are nonspecific, but when combined, they are increasingly characteristic of celiac disease.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease reveals small-bowel dilatation and contrast-agent dilution.
-
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation, contrast-agent dilution, and distal small-bowel dilatation.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows a large intussusception along with numerous smaller intussusceptions, each with a characteristic coiled-spring appearance. Image also demonstrates dilatation, contrast-agent dilution, and jejunization of the ileum.
-
Sprue. Magnified view of the previous image from a small-bowel series in a patient with celiac disease shows coiled-spring appearance of a jejunojejunal intussusception.
-
Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows contrast-agent dilution, barium flocculation, increased ileal folds, and intussusception.
-
Sprue. Magnified view of the previous image, a radiograph from a small-bowel series in a patient with celiac disease, shows the characteristic coiled-spring appearance of an intussusception. The central mucosal channel is faintly visible.
-
Sprue. Radiograph from a small-bowel series on a patient with celiac disease shows dilatation, contrast-agent dilution, intussusception, barium flocculation, and jejunization of the ileum.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows mild bowel dilatation, barium flocculation, and segmentation.
-
Sprue. Radiograph from a small-bowel series on a patient with celiac disease reveals contrast-agent dilution and bowel dilatation.
-
Sprue. Spot radiograph from an air-contrast upper gastrointestinal barium examination in a patient with celiac disease shows the bubbly mucosal pattern in the duodenal bulb, which is caused by peptic duodenitis in patients with celiac disease.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease reveals contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 2 hours after barium ingestion.
-
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image with celiac disease reveals barium flocculation and segmentation, contrast-agent dilution, moderate small-bowel dilatation, and delayed small-bowel transit. This image obtained more than 4 hours after barium ingestion.
-
Sprue. Spot radiograph from a single-contrast upper gastrointestinal barium examination in a patient with celiac disease shows the bubbly mucosal pattern of the duodenal bulb, which his caused by peptic duodenitis in patients with celiac disease.
-
Sprue. Radiograph from a small-bowel series in a patient with celiac disease shows contrast-agent dilution, barium flocculation and segmentation, and an increased number of ileal folds.
-
Sprue. Radiograph from a small-bowel series in the same patient as in the previous image reveals intussusception, contrast-agent dilution, increased ileal folds, and small-bowel dilatation.
-
Sprue. Magnification of the intussusception on the radiograph in Image 20, in Multimedia, from a small-bowel series in a patient with celiac disease.
-
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast material in a patient with celiac disease reveals ascites, small-bowel dilatation, and increased diameter of the superior mesenteric artery and superior mesenteric vein.
-
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast material in a patient with celiac disease. Image shows a dilated portal vein, which is characteristic of increased splanchnic circulation.
-
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows an increased number of ileal folds, or jejunization of the ileum.
-
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows dilation of the superior mesenteric artery and superior mesenteric vein. This finding is characteristic of increased splanchnic circulation.
-
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows a marked increase in the diameter superior mesenteric artery.
-
Sprue. Helical CT scan of the abdomen obtained with oral and intravenous contrast enhancement in a patient with celiac disease shows small-bowel dilatation and dilution of contrast material.
-
Sprue. Sonogram of the abdomen in a patient with celiac disease shows ascites, bowel-wall thickening, and increased intraluminal fluid.






