Practice Essentials
Imaging studies are a major component in the evaluation of patients for the screening, staging, treatment and surveillance of rectal cancer. Rectal cancers are, after colon cancers, the second most common gastrointestinal (GI) carcinoma, and have the best prognosis. The 5-year survival rate is approximately 50%. Almost all rectal cancers are primary adenocarcinomas (see the images below). Adenocarcinoma of the rectum is a major cause of mortality and morbidity in North America and Western Europe. Prognosis is related to the stage of the disease at diagnosis and to initial treatment. Screening for and removing adenomatous polyps may improve survival rates. Systematic preoperative radiochemotherapy and total mesorectal excision are the standard of care for locally advanced rectal carcinoma. [1]
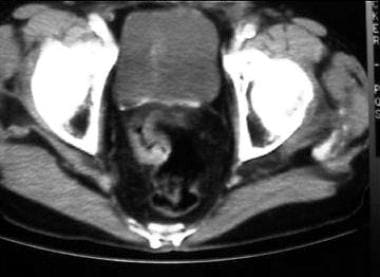
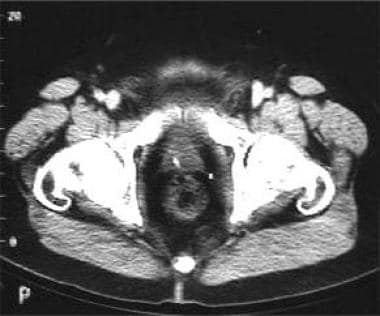 CT scan for low rectal carcinoma preoperative staging. Note circumferential thickening of the rectal wall.
CT scan for low rectal carcinoma preoperative staging. Note circumferential thickening of the rectal wall.
The National Comprehensive Cancer Network (NCCN) guidelines [2] utilizes the The American Joint Committee on Cancer (AJCC) tumor/node/metastasis (TNM) classification and staging system (see Colon Cancer Staging for more information). [2, 3, 4, 5] However, the Dukes classification (or one of its modifications, such as the modified Astler–Coller [MAC]) remains in wide use (see Table 1 for a comparision of anatomic stage/prognostic groups).
Table 1. Anatomic Stage/Prognostic Groups (Open Table in a new window)
| Stage | T | N | M | Dukes | MAC |
|---|---|---|---|---|---|
| 0 | Tis | N0 | M0 | -- | -- |
| I | T1 | N0 | M0 | A | A |
| T2 | N0 | M0 | A | B1 | |
| IIA | T3 | N0 | M0 | B | B2 |
| IIB | T4a | N0 | M0 | B | B2 |
| IIC | T4b | N0 | M0 | B | B3 |
| IIIA | T1-T2 | N1/N1c | M0 | C | C1 |
| T1 | N2a | M0 | C | C1 | |
| IIIB | T3-T4a | N1/N1c | M0 | C | C2 |
| T2-T3 | N2a | M0 | C | C1/C2 | |
| T1-T2 | N2b | M0 | C | C1 | |
| IIIC | T4a | N2a | M0 | C | C2 |
| T3-T4a | N2b | M0 | C | C2 | |
| T4b | N1-N2 | M0 | C | C3 | |
| IVA | Any T | Any N | M1a | D | D |
| IVB | Any T | Any N | M1b | D | D |
| IVC | Any T | Any N | M1c | D | D |
Prognosis is also affected by the histologic grade of the tumor. The complications of rectal cancer include obstruction (common); fistula formation to the small bowel, bladder, or vagina (uncommon); and perforation (rare). (See the table below for the Modified Dukes Classification for 5-year survival rates.) [3]
Table 2. Modified Dukes Classification System and 5-year Survival Rate* (Open Table in a new window)
Stage |
Description |
5-yr Survival Rate, % |
A |
Limited to the bowel wall |
83 |
B |
Extension to pericolic fat; no nodes |
70 |
C |
Regional lymph node metastases |
30 |
D |
Distant metastases (liver, lung, bone) |
10 |
*Modified from Zinkin. [3] |
||
Imaging modalities
Evaluation begins with a history and physical examination, including a digital rectal examination. Inspect the stool and test for occult blood. Order blood tests (ie, complete blood count, liver function tests, and carcinoembryonic antigen levels).
Perform either sigmoidoscopy (rigid or flexible) or a double-contrast barium enema. Perform CT studies to stage the tumor before treatment and to choose the most appropriate treatment. Although magnetic resonance imaging (MRI) is slightly more accurate than CT in staging primary rectal tumors, CT is much more widely available. Most institutions and departments have more extensive experience using CT than MRI and continue to use CT for staging rectal tumors. [6, 7, 8, 9, 10, 11]
Endoscopic ultrasonography (EUS) is an acceptable alternative when MRI is contraindicated.
Positron emission tomography (PET)/CT colonography is valuable in the evaluation of extracolonic and hepatic disease. PET/CT colonography is useful in patients with obstructing colorectal cancers that cannot be traversed colonoscopically. PET/CT colonography is able to localize synchronous colon cancers proximal to the obstruction precisely. However, there is no definitive evidence to support the routine clinical use of PET/CT colonography. [12]
Limitations of techniques
The 60-cm flexible sigmoidoscope has an increased range over the rigid sigmoidoscope, which at best reaches only to the rectosigmoid junction (20 cm). The sigmoidoscope also is more accurate in the rectum. Sigmoidoscopy detects smaller adenomatous polyps than barium enema; also, polyps may be excised by this method.
Double-contrast barium enema detects most colorectal tumors (80-95%), but it should be preceded by flexible sigmoidoscopy. It has a low perforation rate (1/25,000).
T staging of rectal cancer by MRI is an established modality because MRI can diagnose rectal wall laminar structure. However, on MRI it is difficult to differentiate fibrosis from tumor infiltration, which compromises the ability to distinguish early stage T3 tumors from stage T2 tumors. [12] CT and MRI cannot be used to assess the exact degree of mural invasion of a primary rectal tumor. N staging in patients with rectal cancer is still challenging using any imaging modality. These techniques cannot differentiate between enlarged lymph nodes resulting from tumor and those resulting from inflammation. Normal-sized nodes that contain tumors cannot be detected by CT, MRI, sigmoidoscopy, or barium enema.
EUS cannot fully image high or bulky rectal tumors or regions beyond the immediate area of the primary tumor. [2]
Screening Guidelines
American College of Radiology
The ACR Appropriateness Criteria for rectal cancer includes the following [13] :
-
For average-risk individuals or moderate-risk individuals (eg, first-degree family history of cancer or adenoma), CT colonography is usually appropriate for initial colorectal cancer screening; double-contrast barium enema and MR colonography may be appropropriate.
-
For moderate-risk individuals after positive fecal occult blood test (FOBT) or positive fecal immunochemical test, CT colonography is usually appropriate for colorectal cancer detection; double-contrast barium enema and MR colonography may be appropropriate.
-
For high-risk individuals (eg, hereditary nonpolyposis colorectal cancer, ulcerative colitis, or Crohn colitis), imaging studies are usually not appropriate; colonoscopy is preferred because of its ability to obtain biopsies to look for dysplasia.
-
For colorectal cancer screening after incomplete colonoscopy, CT colonography is usually appropriate for individuals at average, moderate, or high risk for colorectal cancer.
National Comprehensive Cancer Network
The NCCN guidelines for radiographic rectal cancer screening include the following recommendations [5] :
-
Screening should be individualized and include a discussion of the risks and benefits of each modality.
-
CT colonography is an acceptable screening modality for average-risk individuals.
-
CT colonography is not an acceptable screening modality for individuals with an increased or high risk for colorectal cancer.
If the CT colonography is negative and no polyps are identified, rescreening with any modality (colonoscopy, flexible sigmoidoscopy, stool-based testing, or CT colonograpy) is recommended. [5]
When lesions are identified, those that are 5 mm or smaller do not need to be reported or referred for colonscopy. If 1 or 2 lesions that are 6-9 mm are identified, follow-up CT colonography in 3 years or colonoscopy should be performed. A colonoscopy should be performed when there are 3 or more lesions that are 6-9 mm or when there are any lesions that are10 mm or larger. [5]
Staging and Surveillance Guidelines
American College of Radiology
ACR Appropriateness Criteria for rectal cancer pretreatment staging are summarized below. [14]
Locoregional staging of rectal cancer
Transrectal ultrasound (TRUS) and high-resolution MRI are accurate modalities for evaluating local extent of tumor. TRUS may perform better for early-stage tumors (T1-T2) and MRI for more-advanced tumors (T3 and above). High-resolution MRI with phased-array coil has high specificity for determining involvement of the circumferential resection margin (94%), which is an essential factor in presurgical planning. MRI holds advantages over TRUS in lateral pelvic lymph node and superior perirectal lymph node detection. [14]
In patients with advanced-stage rectal carcinoma who cannot undergo MRI and for whom TRUS would be inadequate for evaluating nodes, CT may be appropriate to detect enlarged nodes or local organ invasion. [14]
Evaluation of distant metastases
Liver tumor involvement is best staged with multiphase contrast-enhanced MRI or contrast-enhanced CT (with both modalities, optimization of technique is essential for accuracy). The routine use of PET/CT is likely not indicated; however, it may provide guidance in cases of advanced, bilobar liver disease to exclude extrahepatic metastases prior to surgical intent to cure. [14]
The use of chest CT in preoperative planning is controversial, yet chest CT is still widely performed, along with abdomen-pelvis CT or MRI. [14]
In patients with renal dysfunction who cannot undergo a contrast-enhanced MRI or CT, either PET/CT or noncontrast MRI may be options to evaluate for metastatic liver disease. Noncontrast CT for liver staging is usually not indicated. However, there is little evidence to support an optimal/standardized imaging algorithm in these patients. Discussion with a radiologist regarding local contrast administration policies and appropriate next steps is recommended. [14]
National Comprehensive Cancer Network
Initial workup and staging
In contrast to ACR, the NCCN guidelines recommend chest CT and abdominal CT or MRI for the initial workup and staging of rectal cancer to assess for distant metastatic disease to the lungs, thoracic and abdominal lymph nodes, liver, peritoneal cavity, and other organs. Abdominal CT should be performed with intravenous (IV) contrast and oral contrast material unless contraindicated. IV contrast is not required for chest CT but is usually given if performed with abdominal CT scan. MRI with gadolinium-based contrast agent (GBCA) can be performed if IV iodinated contrast material is contraindicted. If GBCA is also contraindicated, consider MRI without contrast or PET/CT imaging. [5]
Pelvic MRI is performed with or without contrast, or endorectal ultrasound (EUS) if MRI is contraindicated, to assess T and N stage of the primary rectal tumor. Pelvic MRI may not be required for local staging if the tumor is known to be T1 or if the patient is not a candidate for primary tumor resection. Pelvic MRI or CT can be used for the workup of synchronous metastatic disease. PET/CT is not routinely indicated. [13]
Restaging with chest CT and abdominal CT or MRI and pelvic MRI can be completed before surgery, before adjuvant treatment to assess response to primary therapy or resection, or during reevaluation for conversion to resectable disease. PET/CT is not indicated for restaging. [13]
Surveillance
NCCN offers the following guidance for surveillance imaging by disease stage [13] :
-
Stage I: Routine imaging is not indicated; consider imaging studies based on symptoms and clinical concern for recurrent or metastatic disease.
-
Stage II and III: Chest, abdominal, and pelvic CT every 6-12 months for a total of 5 years; MRI of the rectum or EUS every 3-6 months for 2 years, then every 6 months thereafter for a total of 5 years (for patients with transanal local excision only).
-
Stage IV: Chest, abdominal, and pelvic CT every 3-6 months for the first 2 years, then every 6-12 months thereafter for a total of 5 years; MRI of the rectum or EUS every 3-6 months for 2 years, then every 6 months thereafter for a total of 5 years (for patients with transanal local excision only)
Radiography
Plain abdominal radiographs are useful in patients presenting with large bowel obstruction or perforation. Free gas under the diaphragm is detected best by a plain erect chest radiograph. Rarely, mucin-producing colon cancers demonstrate calcification in the primary tumor and in hepatic and peritoneal secondary deposits.
Most rectal cancers are 3-4 cm in diameter at diagnosis. Polypoid lesions vary from small smooth tumors to larger, lobulated masses with an irregular surface and associated contour deformity along one margin of the bowel wall (see the image below).
Annular lesions result from irregular circumferential masses that severely constrict the bowel lumen.
Margins of the carcinoma show overhanging edges, which are the tumor shelf or shoulder (see the image below).
Mucosal folds in the narrowed segment are destroyed, and ulceration may be present.
Flat lesions are rare and consist of a unilateral broad-based contour defect. Ulceration may be present. Flat lesions may infiltrate the bowel wall and, if extensive, cause areas of nondistensibility.
Early carcinoma/polyps
A small carcinoma usually presents as a polypoid mass with a smooth outline and may be indistinguishable from a benign polyp. Rarely, such carcinomas may present as a small flat lesion.
A polypoid mass is visualized radiologically either as a filling defect in the barium column (single contrast study) or more commonly as a barium-coated soft-tissue mass protruding into the air-filled lumen (double-contrast study).
A sessile polyp may be visualized as a crescent or ringlike shadow on the bowel wall.
Lobulation is common in polypoid lesions larger than 2 cm in diameter.
Pedunculated polyps have stalks that may be identified easily on profile. When the stalk is observed through the polyp itself, this results in a targetlike (or Mexican hat) appearance. Malignant change may occur in the head of a stalked polyp. A long (2 cm or more), thin (5 mm or less) stalk may hinder the spread of carcinoma from the head of the polyp into the wall.
Risk of malignancy
The risk of malignancy in a polyp increases with its size. The risk is less than 1% in polyps less than 1 cm in diameter. This increases to 5% in 1-2 cm adenomas. Polyps larger than 2 cm have a risk of 11-50%. Thus, all 0.5-3 cm polypoid lesions require endoscopic removal and histologic examination.
A large bowel obstruction usually results from an annular carcinoma in the upper rectum or rectosigmoid junction.
A localized perforation, resulting from tumor necrosis, may result in a pararectal abscess that simulates an inflammatory process.
Perforation may also occur proximal to an obstructing tumor, usually in the cecum.
Local invasion of adjacent organs (bladder, uterus, vagina) and fistula formation are late manifestations.
Synchronous lesions
Approximately 5% of colorectal cancers demonstrate multiple lesions at diagnosis. In 35% of patients diagnosed with a primary colorectal carcinoma, an adenomatous polyp is present elsewhere in the colon or rectum. Second tumors are likely to be overlooked ("satisfaction-of-search error").
Degree of confidence
Double-contrast barium enemas detect approximately 90% of rectal tumors. The overall detection rate for single-contrast barium enemas is approximately 80%, but it is much lower for small polypoid tumors.
False-positive examinations may result, as residual stool may adhere to the bowel wall and mimic a tumor. A submucosal mass, such as a lipoma, a benign mucosal adenoma, or a hyperplastic polyp, may be indistinguishable from a small polypoid cancer.
False-negative examinations may result from inadequate bowel preparation, in which multiple filling defects resulting from residual stool may obscure carcinoma. In this case, repeat examination or sigmoidoscopy is required.
Small lesions may be missed in a dense pool of barium. Errors of perception account for more than 50% of missed cancers. These can be reduced by having a different observer perform a second reading.
Multiple cancers can produce false negatives, since second lesions are more likely to be overlooked ("satisfaction-of-search error"). Strictures resulting from inflammatory bowel disease, diverticulitis, and radiation colitis may mimic malignant strictures. Extrinsic compression of the rectum by an adjacent mass may mimic a primary rectal tumor.
Computed Tomography
Indications for performing CT in rectal carcinoma include the following:
-
CT is used for staging rectal carcinomas before treatment, for staging of recurrent disease, and for detecting the presence of distant metastases after surgery.
-
In older patients who may be unable to undergo colonoscopy or barium enema, modified CT is performed for primary detection of colorectal tumors.
-
Rectal tumors may be diagnosed on CT as an incidental finding.
CT staging (see Table 2, below) or TNM staging (see Table 3, below) systems may be used to assess colon neoplasms. [15, 16]
Table 2. CT Staging System for Rectal Cancer* [17] (Open Table in a new window)
Stage |
Description |
T1 |
Intraluminal polypoid mass; no thickening of bowel wall |
T2 |
Thickened rectal wall >6 mm; no perirectal extension |
T3a |
Thickened rectal wall plus invasion of adjacent muscle or organs |
T3b |
Thickened rectal wall plus invasion of pelvic side wall or abdominal wall |
T4 |
Distant metastases, usually liver or adrenal |
*Modified from Thoeni. [17] |
|
The rectal tumor is often observed as a focal mass of soft-tissue density adjacent to the gas-filled or Gastrografin-filled bowel lumen; oral water-soluble contrast (1% Gastrografin) is administered at 12 hours and at 2 hours before examination to achieve bowel opacity.
Findings on CT include the following:
-
Malignant strictures are detected by a thickening of the bowel wall (see the first image below); this thickening is concentric if the scanning plane is at right angles to the long axis of the rectum (see the second image below)
-
Extrarectal tumor spread is suggested by a loss of tissue fat planes between the rectum and surrounding tissues, as well as perirectal fat stranding and nodularity
-
Invaded muscle may be enlarged
-
Small strands of tissue may extend from the rectal wall into the perirectal fat
-
CT findings help to determine surgical options; precise information concerning the site and local extent of the tumor is required before the appropriate surgical choice can be made; well-defined tumors (stage T1 or T2) may be amenable to simple resection or low anterior resection, whereas more advanced tumors (T3) may require abdominoperineal resection or anterior resection, depending on their location; perioperative adjuvant radiotherapy or chemotherapy may be used.
Node (N) staging
Nodes greater than 10 mm in diameter are considered abnormal. CT is unable to distinguish enlarged, benign nodes from enlarged, malignant nodes. Furthermore, malignant foci may be present in nodes less than 1 cm in diameter.
Overall, 60% of affected nodes are detected by CT.
Enlarged nodes may be detected in the mesentery and retroperitoneum. Rectal tumors may metastasize to internal iliac nodes.
Metatastasis (M) staging
Hepatic metastases are the most common site of distant tumor spread. CT detects hepatic metastases as well-defined areas of low density (compared to normal liver parenchyma) in the portal venous phase, following injection of intravenous contrast medium (see the image below). In the earlier arterial phase, hepatic metastases may demonstrate rim enhancement or become hyperdense or isodense (in relation to normal liver).
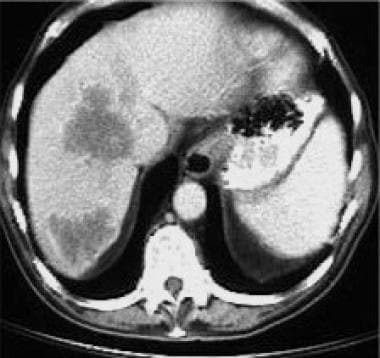 CT scan following intravenous contrast medium, revealing hypodense lesions in the right lobe of the liver from metastases of a rectal adenocarcinoma.
CT scan following intravenous contrast medium, revealing hypodense lesions in the right lobe of the liver from metastases of a rectal adenocarcinoma.
Hepatic metastases may be suitable for surgical resection if they are small (usually less than 3 cm), number less than 3, and are suitably located. Intra-arterial chemotherapy or radiofrequency (RF) ablation is the approach for metastases that are not suited for resection.
Pulmonary metastases are more frequent from lower rectal carcinomas than upper rectal or colon carcinomas. This is because low rectal tumors drain into the systemic venous system (via the internal iliac veins) rather than into the portal venous system (via the superior and inferior mesenteric veins), as do colon and upper rectal cancers. Thus, lower rectal tumors may have pulmonary metastases and no evidence of hepatic metastases. Although pulmonary metastases may be detected by chest radiography, CT has a higher sensitivity for small pulmonary metastases (< 10 mm).
Other common sites include the adrenals, the peritoneum, and the omentum. Adrenal metastases may occur in as many as 14% of patients with colon carcinoma. They are manifested by enlargement (>2 cm), asymmetry, and heterogeneity.
Bony and cerebral metastases are uncommon.
Complications of the primary tumor
CT scans can demonstrate obstruction, perforation, and fistula formation. A local perforation of a carcinoma may be associated with an extraluminal fluid collection.
Early cancers and polyps
Tumors less than 2 cm in diameter cannot be detected reliably by standard CT techniques.
CT colonography, or virtual colonoscopy, involves a 3-dimensional (3-D) computer reconstruction from a volumetric data set using a workstation, as well as distending a clean colon with air. [18] Images are read as soft-copy from the workstation by paging-through the 2-D axial images, aided by multiplanar and 3-D endoluminal images.
The arrival of multisectional helical scanners reduced the time required to obtain the images (usually 30 seconds for each series, scanning the patient prone and supine using a reduced tube current to minimize the radiation dose). The length of time required for image analysis (currently 5-30 minutes) also has decreased, with the introduction of sophisticated software programs that enable a "mathematically straightened" colon to be viewed, while coreferencing the 3-D images with the cross-sectional images.
Advances in computer-aided diagnosis and display methods are expected to improve the performance of this test and reduce the reading time. The sensitivity of this technique is greater than that of a double-contrast barium enema. For polyps larger than 10 mm, it has a sensitivity of 91% but a specificity of 76%. This sensitivity falls to 81% for 5-10 mm polyps.
CT findings in recurrent rectal cancer
One should obtain a baseline CT study 3 months after resection of a rectal tumor. Recurrent tumor is staged by similar criteria described above for primary cancers. There is a local recurrence rate of 20-40% and a distant metastases rate of approximately 35% after curative resection. Most of these occur within 2 years of surgery.
CT can be used to detect local recurrence, as well as lymphadenopathy and distant metastases. CT criteria for identification of a recurrent tumor include invasion of adjacent structures, increasing size, and associated lymphadenopathy (see the image below).
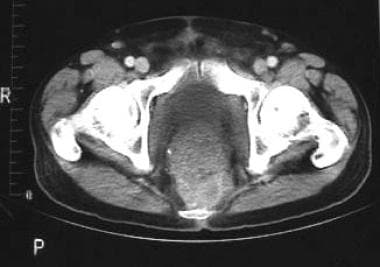 CT scan demonstrating presacral recurrence of a tumor following abdominoperineal resection for carcinoma of the rectum.
CT scan demonstrating presacral recurrence of a tumor following abdominoperineal resection for carcinoma of the rectum.
An inflammatory mass following surgery or radiation therapy may mimic a recurrent tumor and may require biopsy for differentiation (see the image below). Postoperative soft-tissue masses usually are the result of granulation tissue, but they may be caused by a hematoma or abscess. Of these, 60% decrease, but 40% may remain unchanged for up to 2 years.
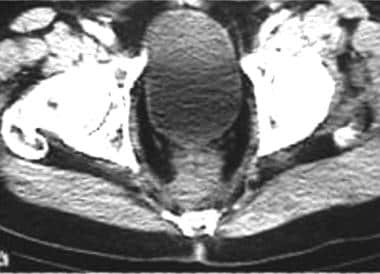 CT scan following abdominoperineal resection. The presacral mass is a result of fibrosis. Distinction from recurrent tumor may be difficult on imaging alone.
CT scan following abdominoperineal resection. The presacral mass is a result of fibrosis. Distinction from recurrent tumor may be difficult on imaging alone.
Both recurrent tumor and inflammatory masses can cause hydronephrosis by ureteric obstruction (see the image below).
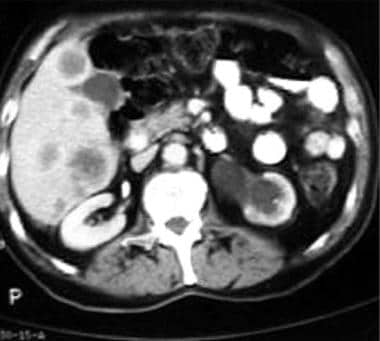 CT scan demonstrating metastases in the right lobe of the liver from a rectal adenocarcinoma. The left kidney is hydronephrotic because of obstruction of the distal left ureter by perirectal spread of the tumor.
CT scan demonstrating metastases in the right lobe of the liver from a rectal adenocarcinoma. The left kidney is hydronephrotic because of obstruction of the distal left ureter by perirectal spread of the tumor.
Consensus in the literature indicates that local excision of early rectal carcinoma results in low morbidity and better functional results but achieves inferior oncologic outcomes, such as postoperative complications and recurrence, when compared with radical resection. Khalid et al found that higher local recurrence with local excision was attributed to occult lymph node disease and inadequate adjunctive therapy due to suboptimal staging. They found no difference in 5-year survival between these treatment approaches and concluded that longer follow-up is needed to confirm whether survival rates diverge after 5 years. [19]
Degree of confidence
CT is more accurate in assessing T4 cancers; however, the spatial resolution of CT is too low to distinguish T2 from T3 lesions. CT has a 50% sensitivity for local invasion, but it does not distinguish between direct tumor infiltration and an inflammatory reaction induced by the tumor. CT accuracy rates vary from 53 to 94% for depth of penetration and from 54 to 70% for lymph node metastases, but CT is unable to detect tumors in normal-sized nodes (< 1 cm in diameter). In most lymph nodes, metastases are less than 1 cm in diameter. Nodes may be enlarged for other reasons, such as infection. Rectal lesions smaller than 2 cm may not be detected. The accuracy and quality of CT scans can be increased by using an intravenous (IV) contrast medium, rectal contrast (air or Gastrografin), smooth muscle relaxants, and laxatives.
The sensitivity of CT colonography is greater than that of double-contrast barium enema. For polyps larger than 10 mm, the technique has a sensitivity of 91% (81% for 5- to 10-mm polyps) but a specificity of 76%. Its future role in colorectal polyp screening is assured. [20]
False positives/negatives
CT signs for rectal cancer are not specific and may be caused by any disease associated with focal thickening of the rectal wall, including Crohn disease. Besides being caused by a rectal carcinoma, a polypoid mass may result from an adenoma, carcinoid tumor, or lymphoma.
In cachectic patients, absence of fat planes is a result of nutritional status, not tumor invasion.
Enlarged lymph nodes may result from inflammation rather than tumor. Additionally, lymph nodes of normal size may contain tumor.
Hypodense hepatic lesions may be simple cysts rather than hepatic metastases. Hepatic metastases do not enhance following injection of an IV-contrast medium and appear as hypodense lesions (see the following images).
 CT scan following intravenous contrast medium, revealing hypodense lesions in the right lobe of the liver from metastases of a rectal adenocarcinoma.
CT scan following intravenous contrast medium, revealing hypodense lesions in the right lobe of the liver from metastases of a rectal adenocarcinoma.
 CT scan demonstrating metastases in the right lobe of the liver from a rectal adenocarcinoma. The left kidney is hydronephrotic because of obstruction of the distal left ureter by perirectal spread of the tumor.
CT scan demonstrating metastases in the right lobe of the liver from a rectal adenocarcinoma. The left kidney is hydronephrotic because of obstruction of the distal left ureter by perirectal spread of the tumor.
Recurrent tumor (see the first image below) may be difficult to differentiate from postoperative fibrosis on imaging grounds alone (see the second image below) and may require biopsy.
 CT scan demonstrating presacral recurrence of a tumor following abdominoperineal resection for carcinoma of the rectum.
CT scan demonstrating presacral recurrence of a tumor following abdominoperineal resection for carcinoma of the rectum.
 CT scan following abdominoperineal resection. The presacral mass is a result of fibrosis. Distinction from recurrent tumor may be difficult on imaging alone.
CT scan following abdominoperineal resection. The presacral mass is a result of fibrosis. Distinction from recurrent tumor may be difficult on imaging alone.
Magnetic Resonance Imaging
Rectal tumors have low signal intensity (similar to adjacent skeletal muscle) on T1-weighted sequences, which facilitates their differentiation from high-signal perirectal fat (see the image below). [6, 7, 8, 9, 10]
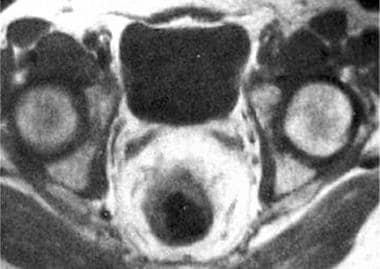 Axial MRI scan of a T3a rectal carcinoma, revealing mural thickening from the tumor and extension of the tumor into the perirectal fat.
Axial MRI scan of a T3a rectal carcinoma, revealing mural thickening from the tumor and extension of the tumor into the perirectal fat.
T2-weighted images are used to detect pelvic sidewall invasion.
Tumor enhancement can be achieved by paramagnetic agents such as gadolinium.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Decisions about neoadjuvant therapy, radical resection, or local excision depend on accurate preoperative staging. High-resolution MRI plays an important role in preoperative staging of rectal cancer. MRI provides greater contrast in soft tissues than CT. MRI is more accurate than CT at preoperative staging of rectal and rectosigmoid tumors and detecting of direct tumor spread into the perirectal fat and adjacent pelvic organs. [21, 22, 23, 24, 25]
Synthetic MRI
Zhu et al assessed the preoperative diagnostic performance of multiparametric quantitative assessment in rectal carcinoma using synthetic MRI (SyMRI) and found that SyMRI can provide multiparameter quantitative image maps allowing improved measurement and slightly shorter acquisition time than conventional MRI. [26]
Degree of confidence
MRI accuracy varies from 66 to 92% for depth of penetration and from 60 to 90% for lymph node metastases. (CT accuracy varies from 53 to 94% for depth of penetration and from 54 to 70% for lymph node metastases.)
MRI has a higher sensitivity (91%) than CT (82%) in detecting local recurrence, as well as a higher specificity (100%) than CT (69%); however, most centers continue to use CT rather than MRI for staging and follow-up imaging of rectal neoplasms. This is because of the wider availability of CT and because centers have much longer experience with CT. This is likely to change in the future.
MR colonography can detect colon polyps and may compete with CT colonography in screening programs.
Ultrasonography
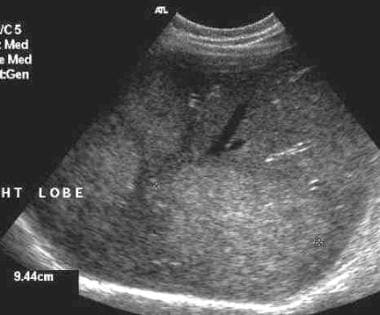
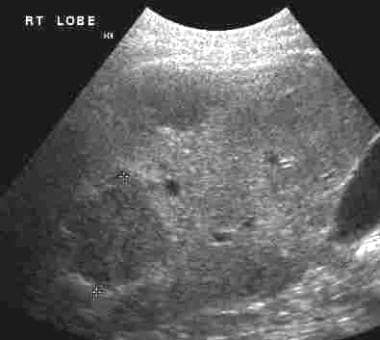
The primary role of ultrasound is in detecting liver metastases. Ultrasonographic sensitivity is as high as 85%. Hepatic metastases resulting from rectal carcinoma usually are hyperechoic (see the first image below) but may be hypoechoic (see the second image below). [27, 28]
Unlike CT and MRI, transrectal ultrasound can depict individual rectal wall layers. The extent of spread through the rectal wall may be assessed by means of a rotating high-frequency probe placed in the rectum (see the image below).
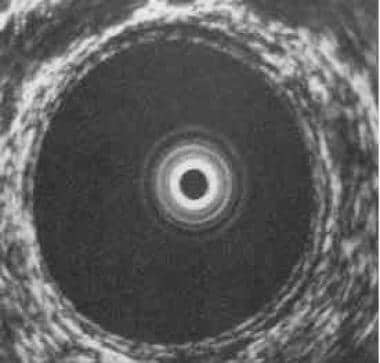 Transrectal ultrasound demonstrating the 5 concentric layers of the normal rectal wall. The mucosa (innermost ring), the submucosa (middle ring), and the serosa (outermost ring) are echogenic (white rings). They are separated by 2 hypoechoic (black) rings, the muscularis mucosa (adjacent to the mucosa) and the muscularis propria (adjacent to the serosa); the rings are best seen in the 5-o'clock position in the full-size view.
Transrectal ultrasound demonstrating the 5 concentric layers of the normal rectal wall. The mucosa (innermost ring), the submucosa (middle ring), and the serosa (outermost ring) are echogenic (white rings). They are separated by 2 hypoechoic (black) rings, the muscularis mucosa (adjacent to the mucosa) and the muscularis propria (adjacent to the serosa); the rings are best seen in the 5-o'clock position in the full-size view.
The rectal wall is visualized as 5 concentric bands as follows:
-
Mucosa (echogenic)
-
Muscularis mucosa (hypoechoic)
-
Submucosa (echogenic)
-
Muscularis propria (hypoechoic)
-
Serosa (echogenic)
The rectal tumor is demonstrated as a hypoechoic mass with varying mural invasion (see the image below). Invasion of the bladder, prostate, and adjacent lymph nodes may be demonstrated. Lymph nodes involved by tumor become spherical and hypodense rather than oval and hyperdense, as is seen in normal lymph nodes.
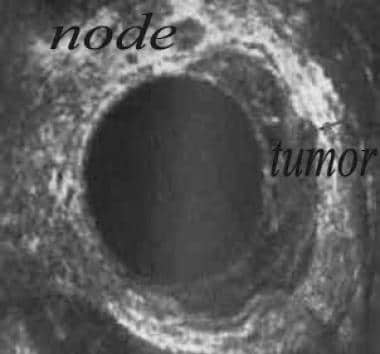 Large tumor in left lateral rectal wall with invasion of perirectal fat. A large node is present at the 12-o'clock position.
Large tumor in left lateral rectal wall with invasion of perirectal fat. A large node is present at the 12-o'clock position.
Degree of confidence
Transrectal ultrasonography is limited to lesions located less than 14 cm from the anus and may not be used for the upper rectum. It may overestimate tumor size and extent as a result of tumor inflammatory response. Spread beyond the rectal wall to the pelvic cavity cannot be detected. Transrectal ultrasonography only detects adjacent lymph nodes.
The sensitivity of transrectal ultrasonography for detection and local staging of rectal tumors (within 14 cm of the anus) is 90-100% (CT is 50-80%), and its specificity is 75% (CT is 33-80%). Transrectal ultrasonography cannot assess the extent of any distant spread beyond its narrow range. Although MRI scanning with the use of an endorectal coil may have a slightly higher accuracy for detecting lymph nodes (up to 90%), transrectal ultrasonography has been shown to be the most accurate method for the determination of the depth of wall penetration (62-92%) and is comparably accurate for lymph node metastases (67-78%). [2]
Nuclear Imaging
Nuclear medicine studies have an increasing role in colorectal cancer. [29, 30]
Radioimmunoglobulin scintigraphy uses a monoclonal antibody that recognizes carcinoembryonic antigen or tumor-associated glycoprotein 72 and may be used in the detection of disease recurrence in the pelvis or extrahepatic abdomen. This technique is being replaced by positron emission tomography (PET).
PET may detect recurrent or metastatic disease using fluorodeoxyglucose (FDG).
In patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy, FDG-PET findings are reliable prognostic predictors of both overall survival and disease-free survival. The 5-year overall survival rate was 91% in patients with a negative PET after radiochemotherapy, versus 72% in those with a positive PET (P=0.024) after radiochemotherapy, whereas disease-free survival was 81% and 62% (P=0.003) for those with negative and positive PET findings, respectively. [31]
In a study evaluating the impact of FDG-PET on the management of patients with colorectal carcinoma, [32] a change was noted in the clinical stage and major management decisions in approximately 40% of patients. Of changes to clinical tumor staging in 25 patients, the disease was upstaged in 20 patients (80%) and downstaged in 5 patients (20%). As a result of FDG-PET findings, physicians avoided major surgery in 41% of patients for whom surgery was the intended treatment.
False-positive results may occur with FDG-PET in patients with abscesses from nonspecific inflammatory reactions following radiotherapy or tracer uptake in the bowel, bladder, or ureters.
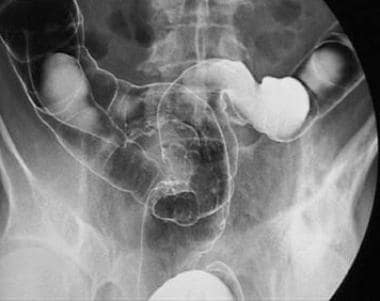
-
Polypoid carcinoma of the upper rectum.
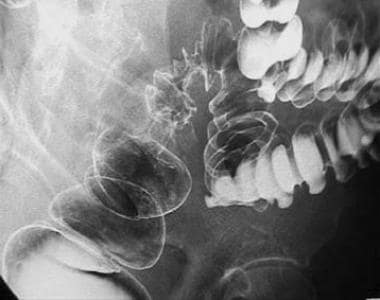
-
Annular carcinoma in the upper rectum.
-
CT scan of wall thickening in a rectal carcinoma.
-
CT scan for low rectal carcinoma preoperative staging. Note circumferential thickening of the rectal wall.
-
CT scan following intravenous contrast medium, revealing hypodense lesions in the right lobe of the liver from metastases of a rectal adenocarcinoma.
-
CT scan demonstrating presacral recurrence of a tumor following abdominoperineal resection for carcinoma of the rectum.
-
CT scan following abdominoperineal resection. The presacral mass is a result of fibrosis. Distinction from recurrent tumor may be difficult on imaging alone.
-
CT scan demonstrating metastases in the right lobe of the liver from a rectal adenocarcinoma. The left kidney is hydronephrotic because of obstruction of the distal left ureter by perirectal spread of the tumor.
-
Axial MRI scan of a T3a rectal carcinoma, revealing mural thickening from the tumor and extension of the tumor into the perirectal fat.
-
Hyperechoic hepatic metastases from rectal adenocarcinoma.
-
Ultrasound of liver revealing hypoechoic metastasis from a rectal carcinoma.
-
Transrectal ultrasound demonstrating the 5 concentric layers of the normal rectal wall. The mucosa (innermost ring), the submucosa (middle ring), and the serosa (outermost ring) are echogenic (white rings). They are separated by 2 hypoechoic (black) rings, the muscularis mucosa (adjacent to the mucosa) and the muscularis propria (adjacent to the serosa); the rings are best seen in the 5-o'clock position in the full-size view.
-
Large tumor in left lateral rectal wall with invasion of perirectal fat. A large node is present at the 12-o'clock position.