Practice Essentials
Chest radiographic imaging is an important tool in the examination of patients with an exacerbation of asthma, but patients should not be left waiting in the treatment room for a radiograph before treatment. [1] Chest radiography is the initial imaging evaluation in most individuals with symptoms of asthma. The value of chest radiography is in revealing complications or alternative causes of wheezing in the diagnosis of asthma and its exacerbations. It usually is more useful in the initial diagnosis of bronchial asthma than in the detection of exacerbations, although it is valuable in excluding complications such as pneumonia and asthma mimics, even during exacerbations. [2, 3]
Significant advancements have been made in a number of imaging techniques used for evaluating patients with asthma. CT utilizes specific airway and lung density measurements to identify severity of disease and pathology, hyperpolarized gases are used as MRI contrast media to identify small airway disease, and positron emission tomography (PET) can help identify and target lung inflammation. [4, 5, 6, 7]
(See the images below.)
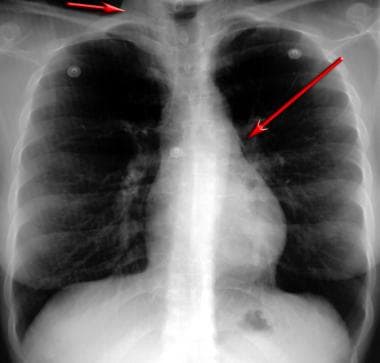 Posteroanterior chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Mediastinal air is noted adjacent to the anteroposterior window and airtrapping extends to the neck, especially on the right side.
Posteroanterior chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Mediastinal air is noted adjacent to the anteroposterior window and airtrapping extends to the neck, especially on the right side.
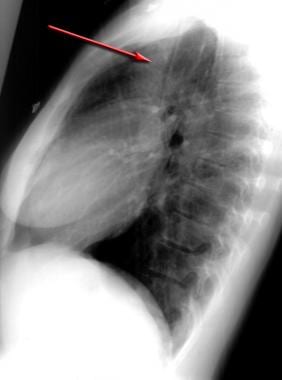 Lateral chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Air is noted anterior to the trachea (same patient as in the previous image).
Lateral chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Air is noted anterior to the trachea (same patient as in the previous image).
Although bronchial thickening, hyperinflation, and focal atelectasis suggest asthma when they are present, chest radiographs obtained during asthma exacerbations can demonstrate normal findings, which reduce its sensitivity as a diagnostic tool. Similarly, identical findings may be observed with chronic bronchitis and viral bronchopneumonia, among other conditions, and these similarities limit the specificity of chest radiography.
HRCT
High-resolution computed tomography (HRCT) is a second-line examination. It is useful in patients with chronic or recurring symptoms and in those with possible complications such as allergic bronchopulmonary aspergillosis and bronchiectasis. [8]
(See the images below.)
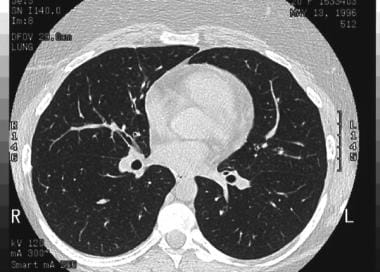 High-resolution CT scan of the thorax obtained during inspiration demonstrates airtrapping in a patient with asthma. Inspiratory findings are normal.
High-resolution CT scan of the thorax obtained during inspiration demonstrates airtrapping in a patient with asthma. Inspiratory findings are normal.
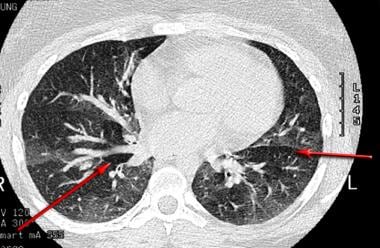 High-resolution CT scan of the thorax obtained during expiration demonstrates a mosaic pattern of lung attenuation in a patient with asthma. Lucent areas (arrows) represent areas of airtrapping (same patient as in the previous image).
High-resolution CT scan of the thorax obtained during expiration demonstrates a mosaic pattern of lung attenuation in a patient with asthma. Lucent areas (arrows) represent areas of airtrapping (same patient as in the previous image).
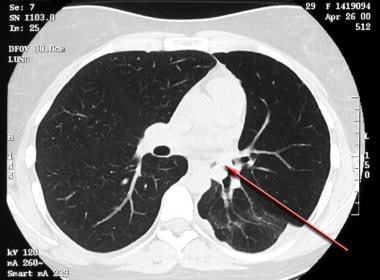 Asthma. High-resolution CT scan of the thorax obtained during inspiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (arrow).
Asthma. High-resolution CT scan of the thorax obtained during inspiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (arrow).
HRCT is more costly than chest radiography and exposes the patient to more radiation. Nevertheless, CT scans can demonstrate a number of findings that support the diagnosis of asthma. HRCT remains the most sensitive study for morphologic changes associated with asthma. HRCT has the potential to aid with the functional assessment of the lungs, such as tests of airtrapping and the bronchodilator response. The specificity of HRCT for bronchial asthma is limited by the similarity of its changes to those of other diseases, such as bronchiectasis, chronic bronchitis, emphysema, and bronchopulmonary aspergillosis.
MRI
Magnetic resonance imaging (MRI) that utilizes hyperpolarized gas can depict the regional distribution of ventilation defects in asthma across the entire lung. [9] In a study of 11 individuals with mild to moderate asthma, hyperpolarized helium 3 MRI ventilation defect volume predicted postbronchodilator forced expiratory volume in 1 second reversibility after 6 years. [9] However, clinical limitations of hyperpolarized MRI include the need for specialized gas mixtures that may only be available to researchers and the need for patients to hold their breath up to 20 seconds, which may not be possible for patients with severe asthma. [10]
Differential diagnosis
The aphorism attributed to Chevallier Jackson states, "All that wheezes is not asthma." This recognition suggests that imaging has an important role in differentiating asthma from its mimics and that further diagnostic evaluation and treatment of nonasthma conditions may be necessary. With knowledge of the imaging findings in alternative disorders, the consulting radiologist may be valuable during the workup in recognizing clinical signs and symptoms that indicate the use of high-resolution chest CT, sinus CT, CT pulmonary angiography, or MRI as the best modality for further imaging in the diagnosis.
Various tracheal tumors, foreign bodies, and other conditions can contribute to wheezing. These may be misdiagnosed for several years before they are recognized.
When asthma does not respond to maintenance treatment, other possible diagnoses, such as cystic fibrosis, primary ciliary dyskinesia, immunodeficiency conditions, or airway and vascular malformations, must be excluded. Risk factors and comorbidities (eg, gastroesophageal reflux, rhinosinusitis, dysfunctional breathing and/or vocal cord dysfunction, obstructive sleep apnea, and obesity) should also be investigated. [11]
Diffuse panbronchiolitis is prevalent in Japan and the Far East, and it may mimic bronchial asthma with wheezing, coughing, dyspnea on exertion, and sinusitis. [12] HRCT findings include centrilobular nodules and linear markings that usually are more profuse than the multifocal bronchiolar impaction sometimes observed with asthma.
Sinus disease, especially in children, is associated with bronchial asthma and wheezing. Although the association is not strong in patients with CT evidence of mild sinus mucosal thickening, a scoring system developed by Newman et al showed that extensive sinus disease was correlated with a substantially higher extent of wheezing than that in patients with only mild thickening. [13] Of 104 adults, 39% had extensive disease, as visualized on CT scans, which was correlated with asthma and peripheral eosinophilia.
In a Finnish study of hospital admissions for acute asthma, admission chest radiographs showed abnormalities in 50% of the patients and resulted in treatment changes in 5%. The numbers were more remarkable when a paranasal sinus series was obtained in unselected patients who presented primarily because of asthma. A sinus abnormality of any kind was found in 85% of patients; maxillary sinus abnormalities occurred alone in 63%. In 29% of patients with a sinus abnormality, treatment was immediately altered. All abnormalities were identified on the Waters view alone, which is 6 times more useful than chest radiography in directing the treatment of acute asthma. [14] Conventional wisdom regarding the sinus radiographic evaluation of chronic coughing and asthma suggests that a workup for chronic coughing should be performed first. [15]
Cough, recurrent bronchitis, pneumonia, wheezing, and asthma are associated with gastroesophageal reflux (GER). [16, 17] The incidence of GER in those with asthma ranges from 38% in patients with only asthma symptoms to 48% in patients with recurrent pneumonia. Scintigraphic studies performed after technetium-99m sulfur-colloid ingestion have shown radionuclide activity in the lungs the next day, but no causal relationship between reflux and asthma has been established. Nevertheless, evidence suggests that increased pulmonary resistance occurs with symptoms of reflux during acid provocation testing; the changes may be sufficiently significant to produce clinically evident bronchospasm. [16]
Pneumothorax may be evident radiographically before it is identified clinically. [18] It often occurs during recurrent episodes of bronchospasm, as well as in other conditions. The presence of an air-fluid level in a hydropneumothorax can be confused with pneumatocele, infected cysts, and cavitary lung disease.
Radiography
In most patients with uncomplicated asthma, radiographic findings are normal. In patients with more advanced asthma, varying stages of hyperinflation are reflected on chest radiographs by a flattening of the hemidiaphragm, increased retrosternal airspace, and relatively minor differences in diaphragmatic positions between inspiration and expiration. Other features of bronchial asthma include a mild prominence of the hilar vasculature that results from transient pulmonary hypertension and mucous plugging with or without atelectasis. [19]
(See the chest radiographic images below.)
 Posteroanterior chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Mediastinal air is noted adjacent to the anteroposterior window and airtrapping extends to the neck, especially on the right side.
Posteroanterior chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Mediastinal air is noted adjacent to the anteroposterior window and airtrapping extends to the neck, especially on the right side.
 Lateral chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Air is noted anterior to the trachea (same patient as in the previous image).
Lateral chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Air is noted anterior to the trachea (same patient as in the previous image).
In early studies, lung opacity on chest radiography was evaluated in 8 regions in patients with asthma; the findings recapitulated the heterogeneous distribution of localized airtrapping seen on radioactive noble gas scintigrams obtained a decade earlier. [20] Airtrapping increases the TLC (total lung capacity) and FRC (functional residual capacity) and reduces the vital capacity (VC) and inspiratory capacity (IC), where IC = TLC – FRC.
FRC, which is the lung volume remaining at the end of expiration, also remains high in the patient with symptomatic asthma; this observation reflects the patient's inability to breathe out in the setting of obstructing secretions, airway narrowing, and edema.
Traditionally, the FRC and TLC have been measured in the pulmonary function laboratory, and planimetry was used in the past to assess the radiographic equivalent of the TLC. A planimeter is a mechanical device used with inspiratory posteroanterior (PA) and lateral chest radiographs. Formulas are used to calculate the lung volume by using a series of virtual sections in which airspace cross-sectional areas are quantified. This procedure was established as a means of diagnosing hyperinflation in bronchial asthma when correlation coefficients of 0.94 were found for helium dilution lung volumes and body plethysmography. A decrease in the TLC after treatment for asthma can be correlated with patient improvement, even when the FEV1 does not improve; this effect likely is related to an improvement in IC. [21]
The reliability of planimetry in the diagnosis of asthma in children also was established, [22] but other findings cast doubt on the usefulness of planimetry in patients with occupational asthma. [23]
Bronchial asthma
The direct measurement of airway wall thickness with chest radiographs was undertaken in patients with mild and severe asthma and in individuals without asthma. The ratio of the internal luminal diameter to the wall thickness was determined by optically measuring the bronchi, as viewed end-on on radiographs, and by reviewing plain radiographic tomograms. The measurements were compared by means of subjective assessment alone. In 11 of 15 patients with severe asthma, subjective assessment results matched the measurements. [24] The authors stated that the finding of more than 2 measurably thickened bronchial walls was rare in individuals without asthma; however, in patients with more severe asthma, the margins of the bronchial walls were delineated better and distinguishable from the findings in individuals without asthma. Ratios varied with bronchiolar luminal diameter, and the authors believed that the ratio was more an index of chronicity than an index of severity.
Nonsegmental, widespread, streaky opacities likely represent focal linear atelectasis resulting from viral superinfection. [25] Segmental opacities may represent localized poor airway mucociliary clearance with atelectasis or early consolidation.
Radiographic correlates of increased TLC that result from airtrapping and small bronchiolar obstruction include hyperinflation; low diaphragms; and, in children, sternal bowing. Sternal bowing reportedly is present in children when the hemidiaphragms are below the 9th or 10th posterior ribs or when the dome of the diaphragm is below the 6th mid anterior rib interspace. [25] However, the value of these findings was disputed. [26] Hemidiaphragms may be flat or inverted, as in tension pneumothorax, and the lateral slips of the diaphragm may be observed, especially on CT scans.
Joorabchi et al, in a study of 65 children hospitalized for asthma, noted the inversion of the pulmonary venous distribution that is typically observed in individuals with left heart failure. [27] The children tended to be younger (6.75 yr vs a group mean of 9.2 yr), and they had tachypnea, retractions, nasal flaring, and tachycardia. The proposed mechanism was increased intrathoracic pressure that led to right ventricular overload, paradoxical septal motion with loss of left ventricular compliance, and elevated left atrial and pulmonary venous pressures.
ED management
In a study of 117 patients with asthma who were older than 15 years, hyperinflation and bronchovascular changes were seen on chest radiograph in 31% of patients in whom asthma began before they were 15 years of age. However, these changes were not observed in any patients in whom asthma began after they were 30 years. of age. [28]
In a study of outpatients with acute asthma who presented to an emergency department (ED), a mean of 55% of patients had normal radiographic findings, while 37% had findings of hyperinflation, and 7% had minimal and unchanged interstitial abnormalities. [29] Pneumonia was present in 16% of adults. Despite the large statistical range of patients with only normal findings (30-81%) and despite the discovery of pneumomediastinum in 5% of children, the authors concluded that chest radiography was not helpful unless complications of asthma were suggested clinically.
One of the largest studies of ED visits involving chest radiographs was performed in a large city hospital. In this study, findings in 5,000 patients were reviewed; 2-view radiographs used in two thirds of the patients and only portable radiographs were used in one third. Overall, 35% of the patients with chest symptoms had serious radiographic findings, but only 14% of the patients with symptoms of asthma had serious radiographic abnormalities. However, the applicability of these findings to individual chest radiographic findings of asthma is limited by the small proportion of total radiographs (4.6%) obtained in patients with asthma. [30]
In a British general hospital ED, findings in 695 episodes of acute asthma in adults and children were evaluated. Chest radiographs were obtained in 135 of 695 patients, or 19% of the total instances of asthmatic exacerbation. Of the radiographs, 79% (presumably portable radiographs) demonstrated normal findings. Abnormalities included evidence of infection (13%), hyperinflation (7%), and edema (2%). Increased perihilar markings were observed in only 2 patients. [31]
Hospital admission
Sherman et al examined patients with exacerbations of chronic obstructive airway disease (COPD). More than half of the 242 hospitalized patients had a "predominant clinical pattern of asthma." Wheezing was not specifically listed as a clinical finding for any patient, although cough and dyspnea were included. Only 4.5% of the radiographs resulted in clinically significant findings that changed the treatment planned with clinical and laboratory criteria alone, in the asthma group as well as the whole group. Sherman et al concluded that admission chest radiography is justified only after the following selection criteria are met: WBC more than 15 × 109/L; polymorphonuclear count more than 8 × 109/L; or a history of congestive heart failure, coronary artery disease, chest pain, or edema. [32]
White et al prospectively studied admission chest radiographs in a large-city ED. PA and lateral radiographs were obtained in more than 95% of the patients who eventually were admitted after a 12-hour course of treatment. Major findings, present in 34% of the patients, included focal opacity, increased interstitial markings, cardiomegaly, pulmonary venous congestion, pneumothorax, and new pulmonary nodules. Minor findings, present in 41%, included hyperinflation, pleural thickening, and calcified granulomas. Focal opacities or increased interstitial markings were correlated with subsequent antibiotic use, independent of an elevated WBC or body temperature. [33]
Pediatric asthma
In children, the natural overlap of nonbacterial bronchiolitis with bronchial asthma accounts for their similar findings on radiographs. Findings of an increased retrosternal airspace and flattened hemidiaphragms are sometimes accompanied by peripheral arterial attenuation. These findings are components of the hyperinflation observed with both entities. [34]
Gershel et al, in a study of 371 children with first-time wheezing, identified criteria for obtaining chest radiographs. The criteria included a heart rate higher than 160 bpm or a respiratory rate higher than 60 per minute, localized rales or localized decreased breath sounds before treatment, and/or persistent localized rales and localized wheezing after treatment. [35] Roback et al al evaluated the use of chest radiography in children with first-time wheezing by using the practice parameters of Gershel et al as a yardstick with which to compare actual clinical practice. The retrospective study revealed that of the 41% of the patients who underwent chest radiography, 24% had a clinically significant abnormality such as local consolidation, pneumothorax, pneumomediastinum, asymmetric opacity, hyperinflation, segmental atelectasis, edema, cardiomegaly, or airway compression. [36]
Rubenstein et al compared the usefulness of routine spirometry with that of chest radiography in patients with mild ambulatory asthma in a university student population. Although 36% of the patients had spirometric results consistent with airway obstruction (predicted FEV1 < 80%, predicted peak expiratory flow rate [PEFR] < 85%, or 20% improvement with bronchodilators), 59% had abnormal radiographic findings consisting of hyperinflation, increased perihilar markings, and peribronchial or peribronchiolar cuffing. Bronchitis and/or bronchiolitis and bronchial asthma caused the radiographic findings. [37]
Computed Tomography
The role of computed tomography (CT) in the imaging of airway disease increased after the development of lung high-resolution CT (HRCT). The technical progress of thin-section acquisition, high-spatial-frequency data reconstruction (ie, bone algorithm technique), and targeted reconstruction has allowed the visualization of finer details on HRCT scans; these details include airtrapping, measurable bronchial wall thickening, atelectasis, centrilobular nodules due to mucous plugging, and acinar nodules due to low-grade inflammatory changes. [38, 39, 40, 41, 42, 43]
King et al discuss details of HRCT methods for evaluating the airways in obstructive pulmonary disease. [44] They discuss the technical features of HRCT and review its use in the assessment of obstructive airway disease.
(See the asthma-related HRCT images below.)
 High-resolution CT scan of the thorax obtained during inspiration demonstrates airtrapping in a patient with asthma. Inspiratory findings are normal.
High-resolution CT scan of the thorax obtained during inspiration demonstrates airtrapping in a patient with asthma. Inspiratory findings are normal.
 High-resolution CT scan of the thorax obtained during expiration demonstrates a mosaic pattern of lung attenuation in a patient with asthma. Lucent areas (arrows) represent areas of airtrapping (same patient as in the previous image).
High-resolution CT scan of the thorax obtained during expiration demonstrates a mosaic pattern of lung attenuation in a patient with asthma. Lucent areas (arrows) represent areas of airtrapping (same patient as in the previous image).
 Asthma. High-resolution CT scan of the thorax obtained during inspiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (arrow).
Asthma. High-resolution CT scan of the thorax obtained during inspiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (arrow).
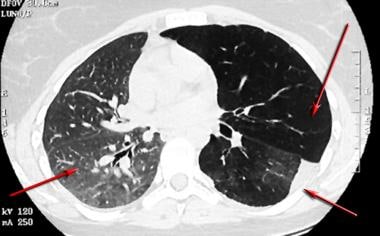 Asthma. High-resolution CT scan of the thorax obtained during expiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (same patient as in the previous image). Note the normal increase in right lung attenuation during expiration (right arrow). The left lung remains lucent, especially the upper lobe, secondary to bronchial obstruction with airtrapping (left upper arrow). The vasculature on the left is diminutive, secondary to reflex vasoconstriction. Left pleural thickening and abnormal linear opacities are noted in the left lower lobe; these are the result of prior episodes of postobstructive pneumonia (left lower arrow).
Asthma. High-resolution CT scan of the thorax obtained during expiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (same patient as in the previous image). Note the normal increase in right lung attenuation during expiration (right arrow). The left lung remains lucent, especially the upper lobe, secondary to bronchial obstruction with airtrapping (left upper arrow). The vasculature on the left is diminutive, secondary to reflex vasoconstriction. Left pleural thickening and abnormal linear opacities are noted in the left lower lobe; these are the result of prior episodes of postobstructive pneumonia (left lower arrow).
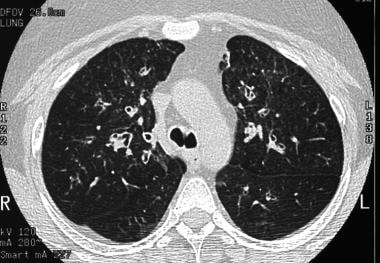 Asthma. High-resolution CT scan of the thorax demonstrates mild bronchial thickening and dilatation in a patient with bilateral lung transplants and bronchial asthma.
Asthma. High-resolution CT scan of the thorax demonstrates mild bronchial thickening and dilatation in a patient with bilateral lung transplants and bronchial asthma.
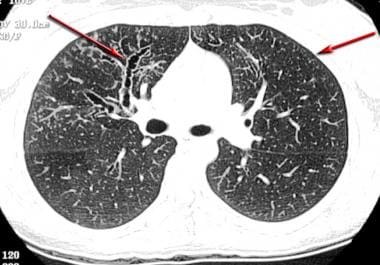 Asthma. High-resolution CT scan of the thorax demonstrates central bronchiectasis, a hallmark of allergic bronchopulmonary aspergillosis (right arrow), and the peripheral tree-in-bud appearance of centrilobular opacities (left arrow), which represent mucoid impaction of the small bronchioles.
Asthma. High-resolution CT scan of the thorax demonstrates central bronchiectasis, a hallmark of allergic bronchopulmonary aspergillosis (right arrow), and the peripheral tree-in-bud appearance of centrilobular opacities (left arrow), which represent mucoid impaction of the small bronchioles.
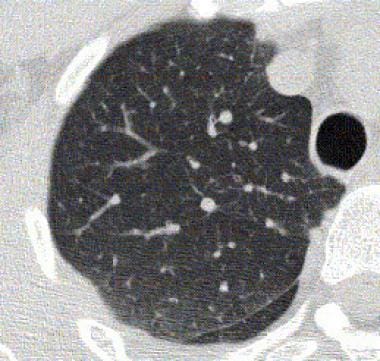 Baseline high-resolution CT scan of the thorax obtained during expiration in a patient with bronchial asthma.
Baseline high-resolution CT scan of the thorax obtained during expiration in a patient with bronchial asthma.
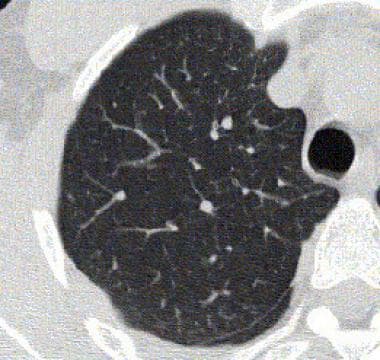 Asthma. High-resolution CT scan of the thorax obtained during expiration and after a methacholine challenge in the same patient as in the previous image. Note the greater degree of airtrapping in the posterior subpleural aspects of the right upper lobe after methacholine is administered.
Asthma. High-resolution CT scan of the thorax obtained during expiration and after a methacholine challenge in the same patient as in the previous image. Note the greater degree of airtrapping in the posterior subpleural aspects of the right upper lobe after methacholine is administered.
Bronchial asthma
HRCT findings in bronchial asthma include the following:
-
Bronchial wall thickening
-
Bronchial dilatation
-
Cylindrical and varicose bronchiectasis
-
Reduced airway luminal area
-
Mucoid impaction of the bronchi
-
Centrilobular opacities, or bronchiolar impaction
-
Linear opacities
-
Airtrapping, as demonstrated or exacerbated with expiration
-
Mosaic lung attenuation, or focal and regional areas of decreased perfusions
Emphysema and airtrapping
Kinsella et al evaluated the coexistence of emphysema and asthma findings using HRCT. In comparing 10 nonsmoking patients with asthma with 10 matched cigarette smokers with severe airflow obstruction, an emphysema grade of 0% was observed in the nonsmokers and 100% in smokers; the emphysema score reflected vascular disruption, bullae, and low-attenuating areas. Although all smokers with a TLC greater than 120% had at least some emphysema, no nonsmoking patients with asthma had emphysema. [45]
Paganin et al studied airway remodeling in nonsmokers with allergic asthma and in those with nonallergic asthma. On HRCT scans, the authors observed emphysema, cylindrical and varicose bronchiectasis, bronchial wall thickening (ie, bronchial recruitment), and linear opacities ("sequellar line shadows"). The findings were significantly more prevalent in individuals with nonallergic asthma than in individuals with allergic asthma. Scores of the findings were significantly greater in both groups and were associated with the severity and duration of asthma. [46]
Whether true emphysema exists in patients with asthma or whether only terminal airspace enlargement is involved in bronchial asthma, [47] the severity of the findings appears to be correlated with the clinical measures of severe asthma. Paganin et al suggested that some form of airway remodeling accounted for the findings and that the process likely differed in allergic asthma versus nonallergic asthma. An interesting speculation is that interstitial emphysema and peribronchial fibrosis may be the result of rupture of the dilated bronchial glands that are present in bronchial asthma. [48]
Confirming earlier findings, authors from Japan also showed that smokers with moderately severe asthma have a significantly higher emphysema score (13.7% vs 2.3%) than that of nonsmokers. As expected, the diffusion capacity was correlated with the emphysema score and the pack-years of cigarette smoking. [49]
Mochizuki et al studied individuals with reversible asthma, and they were stratified in terms of absent, mild, or severe emphysema. Neither the duration nor the severity of asthma was correlated with the presence of emphysema, whereas smoking history, sex, and age were strongly correlated. Patients with long-standing and partially reversible bronchial asthma did not have emphysema if they were nonsmokers. [50]
The correlation of airtrapping with pulmonary function was studied by using HRCT in 74 patients with chronic airway disease, including asthma, [51] and it was found that on expiratory HRCT scans, the airtrapping and expired volume scores were inversely correlated with FEV1, FEV1/FVC, and FEF25. The TLC was not correlated with any of the imaging, age, sex, cigarette smoking history, or visual HRCT scores. Airtrapping was found, even when PFT results were normal; this finding suggests a complementary role for HRCT in the functional evaluation of asthma. [48]
Gevenois et al demonstrated that the distribution of lung attenuation, as visualized on CT scans, depends on the TLC and, to a lesser degree, age. [52] However, Biernacki et al showed a considerable overlap in lung attenuation, as measured in Hounsfield units, in the evaluation of patients with chronic asthma, patients with chronic bronchitis and emphysema, and control subjects without asthma. The authors confirmed a correlation (r = 0.63) between TLC and the index of lung attenuation. [53]
Ng et al investigated airtrapping as an expression of small airway narrowing. The authors examined 106 patients with small airway disease and 19 healthy individuals. They found that decreased attenuation was more prominent on expiratory HRCT scans than on inspiratory HRCT scans. [54]
Bronchiectasis and bronchial dilatation
Studies of HRCT images in asthma consistently reveal the presence of bronchiectasis in patients with asthma but not allergic bronchopulmonary aspergillosis (ABPA). In ABPA, bronchiectasis often is considered part of the definition of the disease. Dilated airways may take the form of cylindrical, varicose, or cystic bronchiectasis. Park et al observed bronchial dilatation in 31% of patients with asthma versus 7% of control subjects. The authors measured bronchoarterial ratios but did not find a statistically significant difference between the groups. [55]
Lynch et al showed that dilated bronchi, defined as bronchi that are larger than accompanying arteries in which the tapering pattern is not lost, were observed in 59% of the control subjects, as compared to 77% of the patients with asthma. Other researchers found no or few such features in control subjects. A decreased arterial diameter with hypoventilation and hypoxic vasoconstriction, a sectioning artifact near the branching arteries and bronchi, a bronchodilator effect on medium-sized airways, and subclinical ABPA are potential explanations for the unexpectedly high percentage of findings in control subjects. [56]
In a study by Grenier et al, subsegmental and distal bronchiectasis was more common in patients with asthma (29%) than in healthy volunteers (7%). The changes were considered permanent, especially if they were varicose or cystic; the prevalence of these changes and the number of involved lobes increased with disease severity. The authors studied interobserver variability and found that interobserver and intraobserver agreement (k = 0.40) were clinically acceptable for bronchial wall thickening, bronchial dilatation, small centrilobular opacities, and decreased lung attenuation. Interobserver and intraobserver agreement was not clinically acceptable with subtypes of bronchiectasis, such as the cylindrical and varicose subtypes. [57]
Compared with the value of the traditional modality of bronchography, the value of thoracic HRCT in demonstrating central bronchiectasis in ABPA was proven in all 21 patients with the disease and in most of the segments. Central and peripheral bronchiectasis, but not peripheral bronchiectasis alone, have been evaluated by using both chest radiography and HRCT images as a diagnostic criteria for ABPA. Angus et al observed bronchial dilatation in 82% of their 17 patients and in 41% of the affected lobes in patients with ABPA versus 18% and 5%, respectively, in patients with asthma and in those without ABPA. However, peripheral bronchiectasis alone was not found in any of the patients with ABPA. [58]
Mucoid impaction is a well-defined finding in patients with ABPA. It may appear as centrilobular bronchiolar plugging or have a tree-in-bud appearance on HRCT scans. Mucoid impaction is believed to be one of the physiologic origins of mosaic lung attenuation. [19] Paganin et al attributed the development of varying degrees of cylindrical bronchiectasis to sequela of multifocal mucoid impactions and bronchial hypersecretion in asthma [46]
Grenier et al found a 21% incidence of centrilobular opacities on HRCT scans obtained in patients with asthma, compared with 5% in individuals without asthma. The authors believed that these opacities and the decreased lung attenuation can be related to the severity of asthma. The authors studied intraobserver and interobserver variability and found that, with bronchial wall thickening, bronchial dilatation, small centrilobular opacities, and decreased lung attenuation, intraobserver (k = 0.60-0.79) and interobserver (k = 0.40-0.64) agreement was clinically acceptable. [57]
In a study by Lo et al of 30 children (median age, 12 yr; range, 5-16 yr) with difficult-to-treat asthma, abnormal CT findings were highly prevalent in a cohort of children with severe asthma, with bronchiectasis identified in approximately 27%. Bronchial wall thickening was observed in 80%, and air trapping in 60%. [59]
Bronchial wall thickening
Carroll et al found that, in cartilaginous airways, the total areas of the inner wall and outer wall, smooth muscle, mucous gland, and cartilage were greater in fatal cases of asthma than in control and nonfatal cases. [60] The internal size of segmental to sixth-generation bronchi was studied in healthy control subjects by using HRCT. Measurements ranged from 0.8 to 8 mm in diameter, with the use of 2-HU windows, 5X optical magnification, and automated luminal area calculation. The authors used a 2-HU window to clarify the edges of the bronchial walls to enhance the reproducibility of the measurement. [61]
Hudon et al used HRCT to show that bronchial thickening in patients with asthma and irreversible airflow obstruction was significantly greater (2.4 mm) than that of patients with completely reversible asthma (2 mm) despite the similar internal diameters of their airways. [62]
Lynch et al observed bronchial wall thickening on chest radiographs and HRCT scans in 71% and 92% of individuals with asthma, respectively (vs HRCT in 19% of control subjects). The authors' patient selection was somewhat biased toward those with asthma complications and smokers (44%). [63]
Park et al found bronchial wall thickening proportional to severity in 44% of stable nonsmokers with asthma versus 4% of control subjects. Bronchial wall thickening occurred in 83% of patients with severe airflow obstruction versus 35% in patients with mild obstruction and 38% in control subjects. [55]
Grenier et al found bronchial wall thickening in 82% of patients with asthma versus 7% of control subjects; this finding established one of the largest differentials between these groups, although the measurements were solely subjective. Nevertheless, the method of measurement appeared to be reliable in terms of intraobserver and interobserver variability. [57]
Awadh et al studied airway wall thickening and found no significant difference in the ratio of wall thickness to outer diameter or the percentage of wall area to the total outside cross-section in patients with near-lethal asthmatic attacks versus patients with moderate asthma. [64] Both groups differed from patients with mild asthma and from individuals without asthma. Nevertheless, even the group with mild asthma differed from individuals without asthma; this finding confirmed those of others and demonstrated that individuals with mild asthma can have airway thickening if the condition is chronic. The findings were present in both the small airways (< 2 mm) and the larger airways (>2 mm).
Bronchial responsiveness
Okazawa et al evaluated the exaggerated airway response to bronchoconstricting stimuli. Patients with mild to moderate asthma and control subjects received a methacholine challenge, and airway lumen narrowing was normalized for FRC. In both groups, the site (small, < 2 mm; medium, >2 mm) and extent of airway luminal narrowing on HRCT scans were similar, as were the reductions in FEV1 values. Only patients with asthma had extensive small airway wall thickening without an increased airway wall area; this finding did not change much after a bronchoconstrictor was administered. Control subjects did not have wall thickening, and their airway wall area decreased. [65]
In a study by Kee et al of bronchoeffector agents, the appearance of the airways on HRCT scans showed that airway internal luminal diameter slightly decreased in individuals with mild asthma and that specific airway resistance increased after methacholine administration; this effect completely reversed after the bronchodilator agent albuterol was administered, and an improvement compared with baseline values was even observed. Airway wall thickness did not change in terms of the diameter, and pulmonary functions did not change with treatment. The investigators were able to quantify the changes in patients with asthma and control subjects by using HRCT scans. [66]
In attempting to differentiate COPD from asthma with HRCT scans, Park et al showed that bronchial walls were thicker in bronchial asthma (2.3 mm thicker than normal) than in COPD (0.9 mm thicker than normal). However, the ratio of wall thickness to luminal diameter was not correlated with clinical features such as smoking history, duration of symptoms, physiologic measures (eg, FEV1), specific airway conductance, and a provocative concentration of the bronchoconstrictor methacholine. HRCT findings of tubular bronchiectasis, emphysema, and mosaic lung attenuation were correlated with a long history of asthma symptoms, compromised lung function, and decreased bronchial hyperresponsiveness. [55]
Carr et al studied the role of the small airways in severe asthma by using HRCT. Inspiratory and expiratory scans were obtained with an electron-beam scanner. The mean decrease in the expiratory-to-inspiratory cross-sectional area was measured: Findings were 76% in asthma patients versus 45% in control subjects. The results showed marked initial inspiratory airway narrowing, and further narrowing with expiration in patients with asthma was limited. The authors also found that FEV1 was correlated with this narrowing and with CT features of airtrapping, but not with features of airway wall thickening or airway dilatation. Airtrapping was observed with and without overt bronchiectasis in some lung regions. [67]
Guckel et al evaluated the source of mosaic attenuation on HRCT scans and observed the influence of oxygen administration on this appearance. In 22 patients with asthma who received a methacholine challenge, high-flow oxygen administered by face mask at a rate of 12 L/min produced the greatest increase in volume-corrected attenuation in regions of mosaic attenuation, compared with the nasal administration of oxygen at a rate of 5 L/min or the use of room air. The proposed and plausible explanation is that hypoxic vasoconstriction, another known cause of mosaic attenuation (airtrapping) besides bronchial narrowing, may account for foci of decreased attenuation in patients with asthma. [68]
Effect of treatment
Paganin et al found both reversible and irreversible findings on HRCT scans of individuals with asthma. Mucoid impaction, acinar opacities, and lobar collapse resolved within 2 weeks of treatment with oral steroids. Bronchiectasis, bronchial wall thickening, linear opacities, and emphysema were unchanged during that interval and were considered permanent. While chest radiographs alone showed abnormal findings in 38% of patients, CT demonstrated abnormal findings in 72% of patients. [69]
Grenier et al studied the effect of treatment in patients with asthma without ABPA who had more mucoid impaction or lobar collapse on HRCT scans than on chest radiographs alone. The features tended to resolve with use of corticosteroids. [57]
Another study of bronchoeffector agents and the appearance of airways on HRCT scans revealed that airway internal luminal diameter slightly decreased and specific airway resistance increased after the administration of methacholine in patients with mild asthma. These effects completely reversed after the bronchodilator agent albuterol was administered, and an improvement compared with baseline values was observed. Airway wall thickness did not change with treatment in these patients or in the control subjects. In the control subjects, neither airway luminal diameter nor pulmonary function changed. [66]
Goldin et al examined 15 patients with asthma and 8 control subjects by using spirometry and HRCT and by using a methacholine challenge and albuterol inhalant reversal (see the images below). The authors showed a shift in the frequency distribution curve of lung attenuation and small airway cross-sectional area after bronchoprovocation; the findings reversed after bronchodilators were administered. The findings were correlated with changes in FEV1 in individuals with asthma and with a lack of changes in control subjects. [70]
 Baseline high-resolution CT scan of the thorax obtained during expiration in a patient with bronchial asthma.
Baseline high-resolution CT scan of the thorax obtained during expiration in a patient with bronchial asthma.
 Asthma. High-resolution CT scan of the thorax obtained during expiration and after a methacholine challenge in the same patient as in the previous image. Note the greater degree of airtrapping in the posterior subpleural aspects of the right upper lobe after methacholine is administered.
Asthma. High-resolution CT scan of the thorax obtained during expiration and after a methacholine challenge in the same patient as in the previous image. Note the greater degree of airtrapping in the posterior subpleural aspects of the right upper lobe after methacholine is administered.
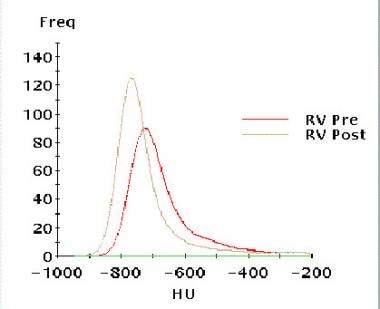 Asthma. Graph demonstrates results in right upper lobe matched pairs before and after a methacholine challenge. The resulting frequency distribution of regional lung density in the midright upper lobe demonstrates a leftward shift to lower attenuation after methacholine administration. Courtesy of Jonathan Goldin, MD, University of California, Los Angeles.
Asthma. Graph demonstrates results in right upper lobe matched pairs before and after a methacholine challenge. The resulting frequency distribution of regional lung density in the midright upper lobe demonstrates a leftward shift to lower attenuation after methacholine administration. Courtesy of Jonathan Goldin, MD, University of California, Los Angeles.
Magnetic Resonance Imaging
Aside from cardiovascular applications, MRI of the thorax is used primarily as a problem-solving modality in the workup of patients with lung, mediastinal, or pleural lesions. MRI is a useful alternative to CT pulmonary angiography in evaluating possible pulmonary embolic disease in patients in whom iodinated contrast agent cannot be administered and when the avoidance of ionizing radiation is preferred. In bronchial asthma, the most promising work appears to involve the use of special paramagnetic gases, which amplify the low signal-to-noise ratio of conventional spin-echo and gradient-echo techniques by several thousand times. The use of such gases offsets the disadvantages of the large magnetic susceptibility states with consequent shortened T2* signals induced by the air-alveolar interfaces.
Using hyperpolarized helium (3He) produced as needed in a local laser laboratory, de Lange and colleagues performed 32 MRI examinations with a 2-dimensional fast low-angle shot (FLASH) sequence and an interleaved echo-planar sequence immediately after the patient inhaled 1-2 L of freshly prepared gas. The imaging required short-to-intermediate breath holds (approximately 5-22 sec), a set of Helmholtz coils centered over the anterior and posterior thorax, and a special radiofrequency receiver tuned to the 48-MHz Larmor frequency of 3He gas. The gas is prepared with an optical pumping technique by which energy is transferred by laser to a small quantity of a rubidium agent, which, in turn, conveys it to low-energy-state dipoles of the resident 3He. In healthy individuals, 3He gas is transferred immediately and completely to the most peripheral airways and airspaces because of its high intrinsic diffusibility. [71, 72]
When ventilation defects are observed, healthy areas continue to have a homogeneous distribution. One patient in the de Lange study had a history of asthma and normal findings with initial testing. One week later, when the patient had mild seasonal allergies, repeat examination revealed 2 new, discrete, peripheral ventilation defects when the patient had a new onset of allergic symptoms. The findings subsequently resolved on MRIs obtained 1 week later and after treatment. [73]
A later study demonstrated similar reversibility in patients receiving the bronchodilator albuterol (see the images below). [74] The proposed mechanism of action is mucous plugging or bronchospasm, although peripheral defects alone are not believed to be unique to asthma, and they also reflect small airway processes such as emphysema, bronchiolitis, and cystic fibrosis.
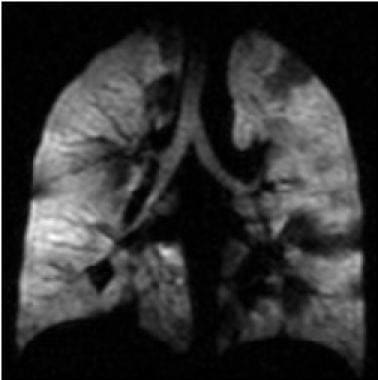 Asthma. Coronal hyperpolarized helium (He-3) MRI in a patient with moderately persistent asthma who underwent imaging twice: This first image was obtained before treatment with an inhaled bronchodilator (ie, albuterol). Multiple dark areas of wedge-shaped ventilation defects improve or resolve after albuterol treatment. Courtesy of T. Altes, MD, and E. de Lange, MD, University of Virginia.
Asthma. Coronal hyperpolarized helium (He-3) MRI in a patient with moderately persistent asthma who underwent imaging twice: This first image was obtained before treatment with an inhaled bronchodilator (ie, albuterol). Multiple dark areas of wedge-shaped ventilation defects improve or resolve after albuterol treatment. Courtesy of T. Altes, MD, and E. de Lange, MD, University of Virginia.
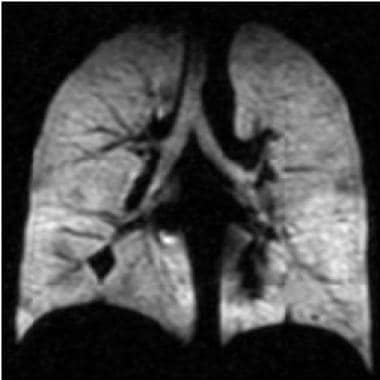 Asthma. Coronal hyperpolarized helium (He-3) MRI in a patient with moderately persistent asthma who underwent imaging twice: This second image was obtained 40 minutes after treatment with an inhaled bronchodilator (ie, albuterol). Multiple dark areas of wedge-shaped ventilation defects improve or resolve after albuterol treatment. The forced expiratory volume in 1 second improved from 83% of the predicted value to 93% after treatment (same patient as in the previous image). Courtesy of T. Altes, MD, and E. de Lange, MD, University of Virginia.
Asthma. Coronal hyperpolarized helium (He-3) MRI in a patient with moderately persistent asthma who underwent imaging twice: This second image was obtained 40 minutes after treatment with an inhaled bronchodilator (ie, albuterol). Multiple dark areas of wedge-shaped ventilation defects improve or resolve after albuterol treatment. The forced expiratory volume in 1 second improved from 83% of the predicted value to 93% after treatment (same patient as in the previous image). Courtesy of T. Altes, MD, and E. de Lange, MD, University of Virginia.
In comparison to the results of nuclear medicine ventilation lung scanning with xenon-133 gas, the resolution of ventilation defects on MRIs has been found to be substantially superior. [73, 74] Additional studies have been performed by using hyperpolarized xenon-129 gas. Oxygen has significant paramagnetic properties and, when used in a 100% concentration, it obviates the use of specialized materials and equipment that is required in 3He hyperpolarized gas. The use of oxygen requires specialization of the pulse sequences, but it is highly diffusible, cheap, and available, and oxygen can be used readily without modifications to the basic MRI unit.
A study by Tahir et al compared lobar lung ventilation computed from expiratory and inspiratory CT data with direct measurements of ventilation at 3He MR imaging by using same-breath hydrogen 1 (1H) MR imaging examinations. According to the authors, percentage of regional ventilation per lobe calculated at CT was comparable to a direct measurement of lung ventilation at 3He MR imaging, providing evidence for the validity of the CT model, and same-breath 1H MR imaging enabled regional interpretation of 3He ventilation MR imaging of the underlying lung anatomy on thin-section CT. [75]
A study by Hahn et al quantified the redistribution of ventilation-weighted signal in the lungs of asthmatics during a breath-hold using high temporal-spatial resolution 3He MRI. The 39 study subjects were classified as healthy/nondiseased (N=14), mild-to-moderate asthma (N=17), or severe asthma (N=8). Mild to moderate asthmatics showed the greatest rate of signal change, even though severe asthmatics had the greatest end-inspiration ventilation heterogeneity. [76]
Svenningsen et al used temporal and spatial information available via 3He MRI to generate pulmonary ventilation temporal-spatial maps to measure, optimize, and guide asthma therapy. The maps identified temporally persistent and intermittent ventilation defects. Intermittent ventilation defect percent was significantly greater in the posterior and inferior lung, as compared to the anterior and superior lung. Persistent and intermittent ventilation defect percent were strongly correlated with forced expiratory volume in 1 second/forced vital capacity. [77]
In animal and human studies, Chen et al have shown the effectiveness of centrically reordered single-shot rapid acquisition with relaxation enhancement, a short effective echo time, and short interecho spacing. [78, 79] Oxygen-enhanced MRI techniques also show great promise in functional imaging of the airways. [80, 81]
Nuclear Imaging
Nuclear medicine technology has been used in the study of aerosol and particulate distribution in the airways. Technetium-99m DTPA radioaerosol lung scintigraphy is a classic technique that shows the extent of major airway distribution, peripheral distribution (depending on particle size), and absorption in the oronasal air passages. Time-activity curves of the radioaerosol have been generated as an index of bronchoalveolar epithelial permeability in asthmatic and nonasthmatic house painters occupationally exposed to isocyanates and have shown a positive correlation between the rate of clearance and work duration. [82]
Technetium-99m radioaerosol has been used to show improved peripheral lung distribution of corticosteroid both in normal subjects and in persons treating their asthma using dry-powder inhalers as opposed to pressurized metered-dose inhalers (pMDIs) with a spacer device. One study has shown improved peripheral deposition of inhaled corticosteroid and several measures of lung function after 1 week of pretreatment with a bronchodilator. However, another study showed no significant change in peripheral radioaerosol distribution after 2 months of pMDI administration of corticosteroid with a spacer, despite improvements in FVC and a serum marker of asthmatic inflammation. [83, 84, 85, 86]
Ventilation scanning with 99mTc DTPA has also been used as an indicator of ventilation defects in asthmatic children, demonstrating an improvement in homogeneity in distribution of radioaerosol after inhaled steroid therapy. Decreased oral deposition has also been shown with spacer devices and has been linked to a lower prevalence of oral candidiasis and systemic absorption. [87, 88]
The formulation of nonchlorofluorocarbon propellants—namely hydrofluoroalkane (HFA)—has allowed the production of substantially smaller particle size (mass median aerodynamic diameter of 1.2 micrometers rather than the 3.8-micrometer size of the chlorofluorocarbon formulation). This has allowed better drug deposition to the small airways, less oropharyngeal deposition, a low risk of systemic absorption, and small improvements in secondary efficacy measures (eg, as-needed albuterol use, asthma symptoms). Because studies have shown that the inflammatory response in the distal lung in asthma can exceed that in the large airways, HFA-based corticosteroids have the potential to treat asthma more effectively and at reduced steroid doses. [89]
In children given a beclomethasone dipropionate/HFA formulation, lung deposition increased with age among groups aged 5-7 years, 8-10 years, and 11-14 years and positively correlated with FEV1 and FVC. The gastrointestinal dose correlated negatively with age, height, and extent of obstructive disease in these individuals. An argument has been put forward that given the difficulty in conducting direct measurements of the clinical responses to inhaled asthma drugs, lung deposition data could be used as a surrogate for the clinical response to new agents. Such data could help save significant time in the drug development process. [90, 91]
While conventional lung scintigraphy has involved the process of physically associating pharmaceuticals in a nebulizer, pMDI, or dry powder form, the physical dissociation of the drug from radioaerosol has limited investigations of drug kinetics. Positron emitters such as carbon-11 and fluorine-18 can be directly incorporated into the drug formulations and then evaluated using positron emission tomography (PET) technology. Not only are 3-dimensional and higher-resolution images possible, but now evaluations of drug uptake and metabolism are possible. [92]
Berridge has demonstrated the following: (1) that central airway (ie, tracheal and major bronchial) deposition of triamcinolone aerosol is demonstrated much better by PET than would have been expected with standard 99mTc planar imaging; (2) that a rapid fall-off occurs in the drug formulation due to mucociliary clearance; and (3) that despite the fall-off of radiotracer in the peripheral lung, the therapeutic effects likely relate to the presumably steroid-receptor–rich target. [93]
Another use of PET has been in the differentiation of COPD from asthma. Jones et al used 18-fluorodeoxyglucose and carbon-11 PK11195 to show that in situ neutrophil uptake of 18-fluorodeoxyglucose was greater in COPD patients than in normal individuals or those with asthma. Mean uptake of carbon-11 PK11195 into macrophages was mostly greater in both the COPD and asthmatic patients than in control subjects in this pilot study. [94]
-
Posteroanterior chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Mediastinal air is noted adjacent to the anteroposterior window and airtrapping extends to the neck, especially on the right side.
-
Lateral chest radiograph demonstrates a pneumomediastinum in bronchial asthma. Air is noted anterior to the trachea (same patient as in the previous image).
-
High-resolution CT scan of the thorax obtained during inspiration demonstrates airtrapping in a patient with asthma. Inspiratory findings are normal.
-
High-resolution CT scan of the thorax obtained during expiration demonstrates a mosaic pattern of lung attenuation in a patient with asthma. Lucent areas (arrows) represent areas of airtrapping (same patient as in the previous image).
-
Asthma. High-resolution CT scan of the thorax obtained during inspiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (arrow).
-
Asthma. High-resolution CT scan of the thorax obtained during expiration in a patient with recurrent left lower lobe pneumonia shows a bronchial mucoepidermoid carcinoma (same patient as in the previous image). Note the normal increase in right lung attenuation during expiration (right arrow). The left lung remains lucent, especially the upper lobe, secondary to bronchial obstruction with airtrapping (left upper arrow). The vasculature on the left is diminutive, secondary to reflex vasoconstriction. Left pleural thickening and abnormal linear opacities are noted in the left lower lobe; these are the result of prior episodes of postobstructive pneumonia (left lower arrow).
-
Asthma. High-resolution CT scan of the thorax demonstrates mild bronchial thickening and dilatation in a patient with bilateral lung transplants and bronchial asthma.
-
Asthma. High-resolution CT scan of the thorax demonstrates central bronchiectasis, a hallmark of allergic bronchopulmonary aspergillosis (right arrow), and the peripheral tree-in-bud appearance of centrilobular opacities (left arrow), which represent mucoid impaction of the small bronchioles.
-
Baseline high-resolution CT scan of the thorax obtained during expiration in a patient with bronchial asthma.
-
Asthma. High-resolution CT scan of the thorax obtained during expiration and after a methacholine challenge in the same patient as in the previous image. Note the greater degree of airtrapping in the posterior subpleural aspects of the right upper lobe after methacholine is administered.
-
Asthma. Graph demonstrates results in right upper lobe matched pairs before and after a methacholine challenge. The resulting frequency distribution of regional lung density in the midright upper lobe demonstrates a leftward shift to lower attenuation after methacholine administration. Courtesy of Jonathan Goldin, MD, University of California, Los Angeles.
-
Asthma. Coronal hyperpolarized helium (He-3) MRI in a patient with moderately persistent asthma who underwent imaging twice: This first image was obtained before treatment with an inhaled bronchodilator (ie, albuterol). Multiple dark areas of wedge-shaped ventilation defects improve or resolve after albuterol treatment. Courtesy of T. Altes, MD, and E. de Lange, MD, University of Virginia.
-
Asthma. Coronal hyperpolarized helium (He-3) MRI in a patient with moderately persistent asthma who underwent imaging twice: This second image was obtained 40 minutes after treatment with an inhaled bronchodilator (ie, albuterol). Multiple dark areas of wedge-shaped ventilation defects improve or resolve after albuterol treatment. The forced expiratory volume in 1 second improved from 83% of the predicted value to 93% after treatment (same patient as in the previous image). Courtesy of T. Altes, MD, and E. de Lange, MD, University of Virginia.







