Practice Essentials
Pulmonary sequestration is an embryonic mass of lung tissue that has no identifiable bronchial communication and that receives its blood supply from 1 or more anomalous systemic arteries. [1, 2, 3] Multiple feeding vessels may be present. This congenital anomaly can be classified as extralobar sequestration (ELS; 25% of patients with pulmonary sequestration) or intralobar sequestration (ILS; 75% of patients with pulmonary sequestration). Pulmonary sequestration is rare, estimated to be 1-6% of congenital lung malformations. [4, 5, 6]
The position of a lesion and its persistence in a relatively young individual raises the index of suspicion that the underlying pathology may be the result of a sequestered segment. Demonstration of a dominant feeding vessel, usually from the aorta or its major vessels, and venous drainage to the pulmonary veins suggests the diagnosis. Alternative venous drainage patterns in ILS include a route directly into the left atrium via the azygos or hemiazygos systems, into intercostals veins, or into the inferior vena cava (IVC) or superior vena cava (SVC).
Pulmonary sequestration, or bronchopulmonary sequestration, should be considered in patients with a cystic lung mass. CT and MRI findings are variable because of inflammation and infection; as a result, it is often misdiagnosed as pneumonia, lung cancer, mediastinal tumor, or lung cysts. [4, 7, 8, 9]
The finding of alternative venous drainage patterns separates pulmonary sequestration from other diagnoses, such as infection and tumor, round atelectasis, Bochdalek hernia, and pulmonary infarction. Enlargement of the associated abnormal feeding vessels is a constant feature, and the azygos vein is also frequently enlarged.
Multiple supply arteries are found in 15% of sequestrations; 73% of sequestrations develop blood vessels leading off the abdominal aorta, and 18% develop blood vessels leading off the thoracic aorta. Rare documented origins include the ascending aorta and the arch, subclavian, innominate, celiac, right coronary, and circumflex arteries.
(Radiologic characteristics of ILS and ELS lesions are demonstrated in the images below.)
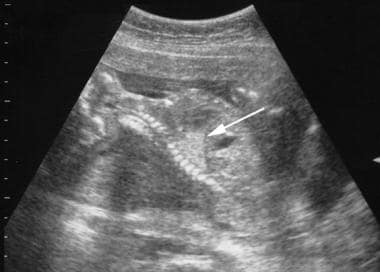 Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a triangular echogenic mass astride the left diaphragm (arrow). A color Doppler sonogram (not shown) revealed an ectopic blood supply to the mass arising from the infradiaphragmatic aorta.
Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a triangular echogenic mass astride the left diaphragm (arrow). A color Doppler sonogram (not shown) revealed an ectopic blood supply to the mass arising from the infradiaphragmatic aorta.
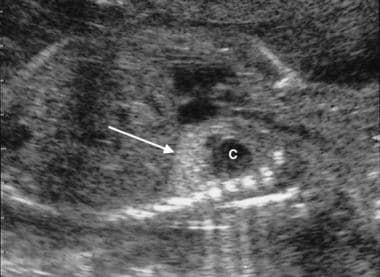 Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a complex mass with a cystic component astride the left diaphragm (arrow) (same patient as in the previous image). A color Doppler sonogram (not shown) failed to demonstrate an ectopic blood supply to the mass. At thoracotomy after birth, an intralobar sequestration was confirmed. Note that identical appearances may occur with congenital cystic adenomatoid malformation.
Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a complex mass with a cystic component astride the left diaphragm (arrow) (same patient as in the previous image). A color Doppler sonogram (not shown) failed to demonstrate an ectopic blood supply to the mass. At thoracotomy after birth, an intralobar sequestration was confirmed. Note that identical appearances may occur with congenital cystic adenomatoid malformation.
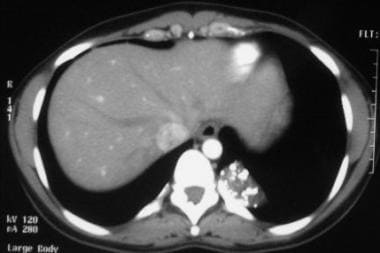 Contrast enhanced computed tomography scan (CT) in a 34-year-old female with an extralobar pulmonary sequestration. This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification.
Contrast enhanced computed tomography scan (CT) in a 34-year-old female with an extralobar pulmonary sequestration. This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification.
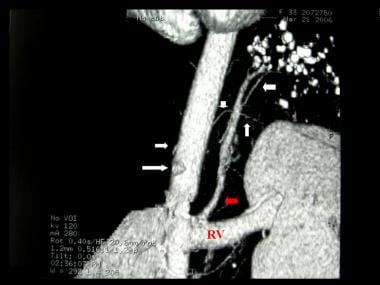 Contrast enhanced computed tomography angiogram in a 34-year-old female with an extralobar pulmonary sequestration (same patient as in the previous image). This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification (confirmed on unenhanced CT scan) with an arterial supply from the celiac axis (white arrows) and venous drainage via the left renal vein (red arrow).
Contrast enhanced computed tomography angiogram in a 34-year-old female with an extralobar pulmonary sequestration (same patient as in the previous image). This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification (confirmed on unenhanced CT scan) with an arterial supply from the celiac axis (white arrows) and venous drainage via the left renal vein (red arrow).
Preferred examination
Chest radiographs can provide a reasonable diagnostic clue to pulmonary sequestration. A mass in the posterobasal segment of the lung in young patients with recurrent, localized pulmonary infections is suggestive of ILS. When such a lesion resolves incompletely with appropriate medical treatment, an underlying sequestration should be considered.
Bronchography (computed tomography [CT] scanning or radiographic studies) may be helpful in excluding other diagnoses. CT scans have 90% accuracy in the diagnosis of pulmonary sequestration. The diagnosis of an intralobar pulmonary sequestration can be confirmed by enhanced contrast helical CT scanning with 3-dimensional (3D) reconstruction, a noninvasive method. [3] Arteriography (conventional or CT angiography [CTA]) is helpful in differentiating the lesion from other abnormalities of the lung, such as pulmonary arteriovenous fistulae, but the CT scans should be correlated with clinical presentation and chest radiographs. [10, 11, 12]
Magnetic resonance imaging (MRI) and MR angiography (MRA) can provide information similar to that on CT scans. [13, 14, 15, 16, 17, 12] Ultrasonography is noninvasive and safe, making its use ideal in prenatal and postnatal settings. [4, 8, 18, 19, 20] The diagnosis can be achieved as early as the second trimester. Color flow and duplex Doppler ultrasonography can elegantly depict the ectopic blood supply and drainage. [21, 22, 23] Radionuclide angiography is another noninvasive technique that may demonstrate the systemic arterial blood supply to the sequestration, thus establishing the diagnosis. [24]
Pulmonary sequestration can be diagnosed by Doppler ultrasound antenatally at 18-19 weeks' gestation. [4, 8]
Limitations of techniques
Use of conventional radiographs must be avoided in women who are pregnant.
Distinguishing ELS from ILS using plain radiographs is difficult, and differentiation is important in selecting the mode of treatment. Infradiaphragmatic ELS is difficult to detect on plain radiographs.
Bronchography is invasive, and its findings are nonspecific.
Diagnosis with arteriography is based on the demonstration of systemic arterial blood supply to the lung sequestration, but arteriography is invasive, and its findings are nonspecific. The same findings have been described in other lung anomalies and in healthy lung segments.
Use of CT scanning requires a high radiation dose; therefore, performing CT scanning in pregnant women and in infants should be avoided if possible. Infants may require sedation or general anesthesia.
MRI has limited availability, it is an expensive tool, and it may require the use of heavy sedation or general anesthesia in young patients.
Ultrasonographic findings are nonspecific in the antenatal and neonatal periods, and the differential diagnosis is wide in these findings. Ultrasonography has limited value in adults with both ILS and ELS.
The experience with nuclear medicine techniques is limited and mostly anecdotal, and the technique requires the use of ionizing radiation. [24, 25]
Extralobar sequestration
In ELS, 80% of sequestrations lie between the lower lobe and the diaphragm. Lesions are usually located in the region of the posterior basal segments of the lower lobes. Left-sided lesions are more common than right-sided lesions. The mass may be closely associated with the esophagus, and fistulae may develop.
Subdiaphragmatic ELS lesions can mimic masses arising in various organs, such as the adrenal gland. In addition, ELS frequently is associated with other congenital extrapulmonary anomalies. Venous drainage occurs via the systemic circulation.
Many patients with ELS present in infancy with respiratory distress and chronic cough; some lesions are diagnosed coincidentally. [5, 6]
Intralobar sequestration
In ILS, sequestrations occur within pulmonary visceral pleurae and do not communicate with the bronchial tree. ILS is seen in males and females in equal numbers. The lesions of ILS may be solid, fluid, or hemorrhagic or may contain mucus. Cystic or emphysematous elements may be present, and adjacent atelectasis often exists.
Most lesions appear hypervascular, because of abundant systemic vascularization. Super-added infection may lead to some consolidation in adjacent segments, and a chronic inflammatory process may induce localized reactive neovascularization. Mucoid impaction of a bronchus surrounded by a hyperinflated lung is believed to be characteristic of ILS.
ILS is often asymptomatic. Symptoms include recurrent pneumonias, shortness of breath, chronic or recurrent cough and hemoptysis. [5, 6] Intrapulmonary sequestration is usually diagnosed later than ELS, being found in childhood or adulthood when the patient presents with an infection. [26]
Radiography
Conventional chest radiographic findings vary depending on the size of the lesion and whether the lesion is infected. Other factors that cause abnormal radiographic findings are the presence or absence of communication with an airway or contiguous lung tissue and the presence of associated anomalies.
(See the image below.)
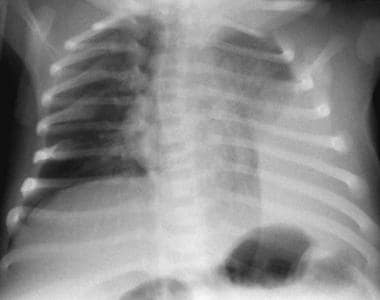 Supine chest radiograph in an infant. This image shows a large opacity at the left costophrenic angle, which can be followed upward to below the diaphragm.
Supine chest radiograph in an infant. This image shows a large opacity at the left costophrenic angle, which can be followed upward to below the diaphragm.
An uninfected sequestration is seen as a well-defined mass or, less commonly, as a cyst in the medial aspect of a posterior lung base. An infected sequestration tends to appear ill defined, may be associated with a parapneumonic effusion, and may contain one or more fluid levels.
Occasionally in ELS, a small bump may be seen on the hemidiaphragm or the inferior paravertebral region.
Rarely, a large sequestration may present with an opaque hemithorax, with or without ipsilateral effusion.
With a barium swallow study, communication between the GI tract and a sequestrated lung segment has been described and may be demonstrated by means of a contrast-enhanced examination of the esophagus.
Mass effect is demonstrated on bronchography as displacement of terminal bronchi by the sequestration. Contrast-agent filling of the sequestered segment in intralobar lesions is uncommon, even when air-fluid levels are present within the cyst. In some patients, a blind intermediate portion of right bronchus may be seen because of hypoplasia of the middle and lower lobes in ELS. CT scans can demonstrate the lack of bronchi entering a sequestration.
Degree of confidence
Chest radiographic findings are usually distinctly abnormal in most patients, and these can provide reasonable diagnostic clues in pulmonary sequestration. An indolent process in the posterobasal segment of the lower lobe in a young person with recurrent, localized pulmonary infections is suggestive of ILS.
Distinguishing ELS from ILS using plain radiographic findings is difficult. Extralobar lesions are more often solid and are associated with elevation of the ipsilateral diaphragm, whereas intralobar lesions appear more cystlike, and air is present if a pulmonary communication exists. The opacity of the sequestration increases with the presence of an infection. When such a lesion resolves incompletely with appropriate medical treatment, an underlying sequestration should be considered.
Lack of filling or lack of demonstration of a communication on bronchography images of the tracheobronchial tree through a normally located bronchus is a characteristic finding that can help make the diagnosis with reasonable certainty in conjunction with other clinical and radiologic findings. Bronchography or CT scanning may be helpful in excluding other diagnoses. The occasional presence of contrast material in the cystic area during bronchography may suggest the confusing diagnosis of cystic bronchiectasis.
False positives/negatives
Pulmonary sequestration in asymptomatic individuals may be confused with a bronchogenic cyst, congenital cystic adenomatoid malformation (CCAM), Bochdalek hernia, and mediastinal or pulmonary neoplasm. In symptomatic individuals, the differential diagnosis includes pneumatoceles, pneumonia, bronchiectasis, and lung abscess. Lesions considered in the differential diagnosis for infradiaphragmatic lesions include neuroblastoma, teratoma, adrenal hemorrhage, mesoblastic nephroma, and foregut duplication.
The occasional presence of contrast material in the cystic area during bronchography may suggest the confusing diagnosis of cystic bronchiectasis. Bronchographic findings may be misleading, because the failure of the contrast agent to enter the bronchus is not pathognomonic of a sequestrated lung segment and may occur as a result of a foreign body, mucous plug, or bronchial atresia.
Computed Tomography
The role of CT scanning is to define vascular anatomy (as demonstrated in the images below) and to provide supporting evidence that opacities depicted on chest radiographs or antenatal sonograms may be sequestrations. [11]
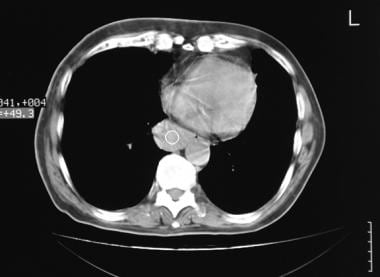 Extralobar pulmonary sequestration. Computed tomography scan in a 55-year-old patient who smokes. This image shows a nonspecific mass in the posterior mediastinum. At thoracotomy, the mass was seen to be attached to the paravertebral region by a feeding artery originating from the descending thoracic aorta. Histologic examination confirmed an extralobar sequestration.
Extralobar pulmonary sequestration. Computed tomography scan in a 55-year-old patient who smokes. This image shows a nonspecific mass in the posterior mediastinum. At thoracotomy, the mass was seen to be attached to the paravertebral region by a feeding artery originating from the descending thoracic aorta. Histologic examination confirmed an extralobar sequestration.
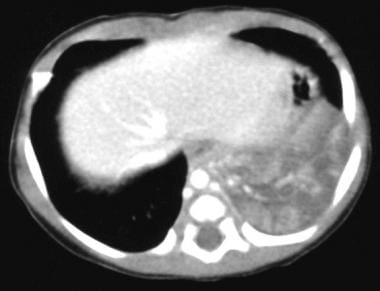 Contrast-enhanced portal venous phase transaxial computed tomography scan through the liver and base of the left lung. This image shows a large mass of mixed attenuation with an arterial supply from the aorta and an enlarged hemiazygos vein from venous return.
Contrast-enhanced portal venous phase transaxial computed tomography scan through the liver and base of the left lung. This image shows a large mass of mixed attenuation with an arterial supply from the aorta and an enlarged hemiazygos vein from venous return.
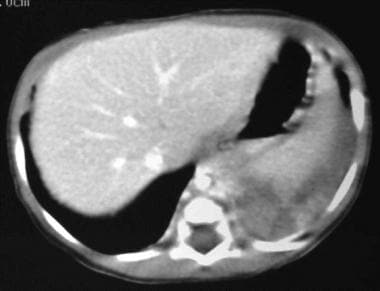 Contrast-enhanced portal venous phase transaxial CT scan through the liver and base of the left lung (same patient as in the previous image). This image shows a large mass of mixed attenuation with an arterial supply from the aorta and an enlarged hemiazygos vein from venous return. At surgery, an extralobar sequestration was confirmed.
Contrast-enhanced portal venous phase transaxial CT scan through the liver and base of the left lung (same patient as in the previous image). This image shows a large mass of mixed attenuation with an arterial supply from the aorta and an enlarged hemiazygos vein from venous return. At surgery, an extralobar sequestration was confirmed.
 Contrast enhanced computed tomography scan (CT) in a 34-year-old female with an extralobar pulmonary sequestration. This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification.
Contrast enhanced computed tomography scan (CT) in a 34-year-old female with an extralobar pulmonary sequestration. This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification.
 Contrast enhanced computed tomography angiogram in a 34-year-old female with an extralobar pulmonary sequestration (same patient as in the previous image). This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification (confirmed on unenhanced CT scan) with an arterial supply from the celiac axis (white arrows) and venous drainage via the left renal vein (red arrow).
Contrast enhanced computed tomography angiogram in a 34-year-old female with an extralobar pulmonary sequestration (same patient as in the previous image). This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification (confirmed on unenhanced CT scan) with an arterial supply from the celiac axis (white arrows) and venous drainage via the left renal vein (red arrow).
Simple sectional CT scan studies can reveal the anatomic position of an abnormality and may contribute to knowledge of the vascular supply in two thirds of patients. With the advent of volumetric slip-ring scanning (either spiral or multisection), the vascular supply and venous drainage of ILS and ELS lesions can be defined with a much higher degree of certainty. Volumetric slip-ring CT scans can provide information regarding the morphologic structure and attenuation values of any focus.
Powerful computing with 3D reconstruction provides excellent spatial resolution and definition of the spatial relationships of structures, which can obviate invasive angiographic procedures.
For optimal CT depiction of lesions with the use of state-of-the-art volumetric scanning, a fast intravenous (IV) contrast injection rate and appropriate volume and delay based on size is required. Multiplanar and 3D minimum ionizing particle (MIP) reconstructions are helpful.
Degree of confidence
Multidetector CTA not only allows simultaneous imaging of the aberrant artery and venous drainage but also has the potential to become the first-line examination in the preoperative assessment of pulmonary sequestration. Accuracy of diagnosis of a pulmonary sequestration is approximately 90% if a lesion is depicted in a typical site, if it has solid and cystic components, and if it is associated with emphysema and an abnormal blood supply and venous drainage into either the pulmonary veins or the systemic veins.
Lung abscess, congenital adenomatoid cystic malformation, lung tumor, round atelectasis, Bochdalek hernia, and pulmonary infarction may mimic pulmonary sequestration. If an aberrant blood supply and drainage cannot be demonstrated, a false-negative examination may occur.
Magnetic Resonance Imaging
Contrast-enhanced MRA or even conventional T1-weighted, spin-echo (SE) images may help in the diagnosis of pulmonary sequestration by demonstrating a systemic blood supply, particularly from the aorta, to a basal lung mass. In addition, MRA may demonstrate venous drainage of the mass and may obviate more invasive investigations.
Degree of confidence
MRI and MRA can provide information similar to that of CT scans without the need for ionizing radiation29; however, MRI is less accessible, takes longer to perform, is subject to motion artifacts, and requires sedation in infants and small children.
Sufficient experience has not been accumulated in the use of MRI in the diagnosis of pulmonary sequestration. Demonstration of aberrant blood supply to the sequestrated segment is pivotal to the diagnosis. Therefore, meticulous technique is necessary, because respiratory and cardiac motion may theoretically degrade the images. Moreover, a systemic artery supplying lung tissue is not pathognomonic of sequestration, because anomalous systemic arterial supply to normal segments of the lung is a rare, but well-recognized, congenital anomaly.
As in any imaging technique, MRI findings must be interpreted in the light of the clinical presentation and the ultrasonographic and chest radiographic findings.
Ultrasonography
ILS lesions (shown below) appear as solid intrathoracic masses that may contain small cystic areas secondary to multiple fluid-filled bronchi. The left lower lobe is the most common site. The appearances are nonspecific and can be complex, solid homogeneous or inhomogeneous lesions and echogenic or cystic, depending on the histologic components in the lesion. These findings are suggestive of a number of possibilities in the pulmonary sequestration spectrum. [22, 23] Pulmonary sequestration can be diagnosed by Doppler ultrasound antenatally at 18-19 weeks' gestation. [4, 8]
 Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a triangular echogenic mass astride the left diaphragm (arrow). A color Doppler sonogram (not shown) revealed an ectopic blood supply to the mass arising from the infradiaphragmatic aorta.
Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a triangular echogenic mass astride the left diaphragm (arrow). A color Doppler sonogram (not shown) revealed an ectopic blood supply to the mass arising from the infradiaphragmatic aorta.
 Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a complex mass with a cystic component astride the left diaphragm (arrow) (same patient as in the previous image). A color Doppler sonogram (not shown) failed to demonstrate an ectopic blood supply to the mass. At thoracotomy after birth, an intralobar sequestration was confirmed. Note that identical appearances may occur with congenital cystic adenomatoid malformation.
Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a complex mass with a cystic component astride the left diaphragm (arrow) (same patient as in the previous image). A color Doppler sonogram (not shown) failed to demonstrate an ectopic blood supply to the mass. At thoracotomy after birth, an intralobar sequestration was confirmed. Note that identical appearances may occur with congenital cystic adenomatoid malformation.
Demonstration of a systemic arterial supply and left atrial venous drainage by color-flow and duplex ultrasonography establishes the diagnosis. Ultrasonographic demonstration of a vascular supply may be difficult, and the failure to depict the supply does not exclude the diagnosis. The arterial supply is most commonly derived from the descending aorta, but it can arise, in descending order of frequency, from the celiac, splenic, intercostal, subclavian, internal thoracic, or pericardiophrenic arteries. In ILS, 16% of lesions can have multiple blood supplies.
Prenatal diagnosis of retroperitoneal ELS is not rare, accounting for 2-5% of all lung sequestrations. [4, 8, 18, 19, 20]
Analysis of ultrasonographically guided fine-needle biopsy specimens of respiratory epithelium confirms the diagnosis of extrapulmonary ELS, but most of the time, surgical resection follows imaging evaluation.
Ultrasonography is useful in the prenatal diagnosis of pulmonary sequestration and its complications, in assessing progression, and in forming a prognosis, which, in turn, is important for appropriate parental counseling and fetal therapy. Serial prenatal sonograms are necessary in patients in whom pulmonary sequestration is suspected, in order to search for poor prognostic factors, such as increasing mediastinal shift and increasing size of the sequestration. In fetuses with chest masses, 8% have additional structural abnormalities and an abnormal karyotype.
In ELS, 65% of patients have associated anomalies, such as an accessory spleen, congenital heart disease, or a diaphragmatic hernia. In patients with ELS, complications can include tension hydrothorax, polyhydramnios, and hydrops fetalis. [21, 27] Ultrasonography can demonstrate absent or reversed diastolic flow in a torsed vascular pedicle, which is believed to cause complications in patients with ELS.
In patients with ILS, prenatal complications are unlikely because the sequestrated segment is well anchored in the thorax and is unable to undergo torsion.
Degree of confidence
Ultrasonography is important in the diagnosis of pulmonary sequestration. This imaging modality is noninvasive and safe, which make its use ideal in the prenatal and postnatal periods. The basal location of most of the lesions provides an excellent acoustic window for ultrasonography. The diagnosis can be made as soon as the early second trimester.
Mimics of ILS include congenital diaphragmatic hernia, CCAM, tracheobronchial atresia, cystic mediastinal teratoma, and bronchogenic and enteric cysts. Absence of peristalsis and presence of an intact diaphragm exclude a diagnosis of diaphragmatic hernia. If the CCAM is microcytic type 3, it can be ultrasonographically indistinguishable from pulmonary sequestration. If bronchial communication occurs after infection in patients with ILS, highly echogenic reverberation artifacts caused by air may be seen.
In the retroperitoneal location, mimics of ELS include neuroblastoma, adrenal hemorrhage, teratoma, and lymphangioma. [28] One ELS is diagnosed for every 2.5 neuroblastomas. A neuroblastoma is characterized by poorly defined margins and low or mixed echogenicity with foci of calcification. A neuroblastoma is more often cystic, right sided, and seen in the third trimester; ELS is more often echogenic and left sided and can possibly be seen as early as the second trimester. Adrenal hemorrhage typically has cystic components and involves the adrenal gland, either wholly or in part. Differentiating adrenal hemorrhage from pulmonary sequestration may be a function of time rather than initial appearances. Teratomas and lymphangiomas occur considerably less frequently.
On antenatal and neonatal sonograms, an ELS may mimic a neuroblastoma. [29] The differential diagnosis of an infradiaphragmatic ELS includes neuroblastoma, teratoma, adrenal hemorrhage, and mesoblastic nephroma and foregut duplication cysts.
Angiography
The blood supply of 75% of pulmonary sequestrations is derived from the thoracic or abdominal aorta. The remaining 25% of sequestrations receive their blood flow from the subclavian, intercostal, pulmonary, pericardiophrenic, innominate, internal mammary, celiac, splenic, or renal arteries. The arterial supply typically enters the lung via the pulmonary ligament if the artery originates above the diaphragm. Arteries originating below the diaphragm reach the sequestration by piercing the diaphragm or via the aortic or esophageal hiatus.
In the rare instance of sequestration in an upper lobe, arterial supply from the internal thoracic artery has been reported. If aortography (seen in the images below) is unrevealing, a coronary source should be included in the preoperative search.
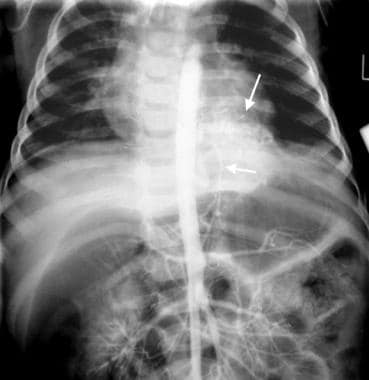 Intralobar pulmonary sequestration. Aortogram in an 8-year-old patient who presented with signs of an acute chest infection. A chest radiograph showed a left lower lobe consolidation (not shown). After appropriate medical treatment, the child improved clinically, but an opacity in the left lower lobe persisted. A sequestrated lung segment was suspected because of a history of several previous respiratory infections from age 3 years and older. This aortogram shows contrast material injected within the upper abdominal aorta. An anomalous artery is arising from the infradiaphragmatic portion of the aorta (bottom, shorter arrow) and is supplying a supradiaphragmatic mass in the left lower lobe (top, longer arrow).
Intralobar pulmonary sequestration. Aortogram in an 8-year-old patient who presented with signs of an acute chest infection. A chest radiograph showed a left lower lobe consolidation (not shown). After appropriate medical treatment, the child improved clinically, but an opacity in the left lower lobe persisted. A sequestrated lung segment was suspected because of a history of several previous respiratory infections from age 3 years and older. This aortogram shows contrast material injected within the upper abdominal aorta. An anomalous artery is arising from the infradiaphragmatic portion of the aorta (bottom, shorter arrow) and is supplying a supradiaphragmatic mass in the left lower lobe (top, longer arrow).
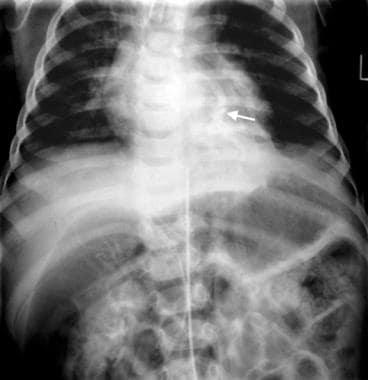 Intralobar pulmonary sequestration. An 8-year-old patient presented with signs of an acute lung infection (same patient as in the previous image). The venous phase of aortogram shows pulmonary venous drainage into the left atrium (arrow).
Intralobar pulmonary sequestration. An 8-year-old patient presented with signs of an acute lung infection (same patient as in the previous image). The venous phase of aortogram shows pulmonary venous drainage into the left atrium (arrow).
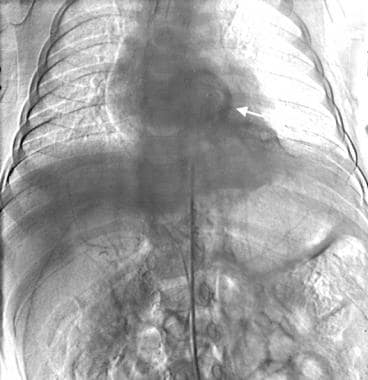 Intralobar pulmonary sequestration (same patient as in the previous 2 images). Radiographic subtraction of the image directly previous to this one shows pulmonary venous drainage (arrow).
Intralobar pulmonary sequestration (same patient as in the previous 2 images). Radiographic subtraction of the image directly previous to this one shows pulmonary venous drainage (arrow).
The arterial supply is usually composed of a single vessel that is disproportionately large. This vessel is typically 0.5-2.0 cm in diameter, and multiple arteries are present in 15-20% of cases in which the arteries are 3 mm or smaller in diameter. Venous drainage occurs most often via the pulmonary vein in ILS, establishing a left-to-right shunt; in ELS, the drainage occurs via bronchial or other systemic veins. Occasionally, drainage is solely to the azygos or hemiazygos system. In rare cases, drainage is to the intercostal, innominate, or portal veins. Dual venous drainage to both pulmonary and systemic veins is the most uncommon situation.
Degree of confidence
The definitive diagnosis is made by using angiography (conventional, CTA, or MRA), which delineates the feeding vessel to the sequestration along with its venous system. Aortograms and pulmonary angiograms may be needed in some patients in whom pulmonary sequestration is suspected.
Arteriography is helpful in differentiating pulmonary sequestration from other abnormalities of the lung, such as pulmonary arteriovenous fistulae. However, demonstration of a systemic artery supplying lung tissue is not pathognomonic of sequestration, because a congenital, anomalous, systemic arterial supply to normal segments of the lung is rare but well known.
Arteriography must be interpreted along with clinical and chest radiographic findings.
-
Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a triangular echogenic mass astride the left diaphragm (arrow). A color Doppler sonogram (not shown) revealed an ectopic blood supply to the mass arising from the infradiaphragmatic aorta.
-
Intralobar pulmonary sequestration. Mid trimester fetal sonogram shows a complex mass with a cystic component astride the left diaphragm (arrow) (same patient as in the previous image). A color Doppler sonogram (not shown) failed to demonstrate an ectopic blood supply to the mass. At thoracotomy after birth, an intralobar sequestration was confirmed. Note that identical appearances may occur with congenital cystic adenomatoid malformation.
-
Intralobar pulmonary sequestration. Aortogram in an 8-year-old patient who presented with signs of an acute chest infection. A chest radiograph showed a left lower lobe consolidation (not shown). After appropriate medical treatment, the child improved clinically, but an opacity in the left lower lobe persisted. A sequestrated lung segment was suspected because of a history of several previous respiratory infections from age 3 years and older. This aortogram shows contrast material injected within the upper abdominal aorta. An anomalous artery is arising from the infradiaphragmatic portion of the aorta (bottom, shorter arrow) and is supplying a supradiaphragmatic mass in the left lower lobe (top, longer arrow).
-
Intralobar pulmonary sequestration. An 8-year-old patient presented with signs of an acute lung infection (same patient as in the previous image). The venous phase of aortogram shows pulmonary venous drainage into the left atrium (arrow).
-
Intralobar pulmonary sequestration (same patient as in the previous 2 images). Radiographic subtraction of the image directly previous to this one shows pulmonary venous drainage (arrow).
-
Extralobar pulmonary sequestration. A solid mass in the posterior mediastinum (arrow) in a 55-year-old patient who smokes. A bronchogenic neoplasm was suspected.
-
Extralobar pulmonary sequestration. Computed tomography scan in a 55-year-old patient who smokes. This image shows a nonspecific mass in the posterior mediastinum. At thoracotomy, the mass was seen to be attached to the paravertebral region by a feeding artery originating from the descending thoracic aorta. Histologic examination confirmed an extralobar sequestration.
-
Supine chest radiograph in an infant. This image shows a large opacity at the left costophrenic angle, which can be followed upward to below the diaphragm.
-
Color Doppler sonogram. This image shows an infradiaphragmatic artery arising from the aorta and a vein draining back into the hemiazygos vein.
-
Contrast-enhanced portal venous phase transaxial computed tomography scan through the liver and base of the left lung. This image shows a large mass of mixed attenuation with an arterial supply from the aorta and an enlarged hemiazygos vein from venous return.
-
Contrast-enhanced portal venous phase transaxial CT scan through the liver and base of the left lung (same patient as in the previous image). This image shows a large mass of mixed attenuation with an arterial supply from the aorta and an enlarged hemiazygos vein from venous return. At surgery, an extralobar sequestration was confirmed.
-
Contrast enhanced computed tomography scan (CT) in a 34-year-old female with an extralobar pulmonary sequestration. This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification.
-
Contrast enhanced computed tomography angiogram in a 34-year-old female with an extralobar pulmonary sequestration (same patient as in the previous image). This image shows a 5- x 2-cm subpulmonic mass with punctuate calcification (confirmed on unenhanced CT scan) with an arterial supply from the celiac axis (white arrows) and venous drainage via the left renal vein (red arrow).







