Practice Essentials
First described by Hakim and Adams in 1965, normal pressure hydrocephalus (NPH) refers to a clinical entity consisting of the triad of gait disturbance, dementia, and incontinence, coupled with the laboratory findings of normal cerebrospinal fluid (CSF) pressures and radiographic findings of ventriculomegaly. [1] Although NPH is a relatively rare cause of dementia, identifying NPH is important because it is one of the few treatable entities. NPH serves as one of the reasons that all dementia patients should be evaluated with neuroimaging of either CT scanning or MRI as part of their workup. [2, 3, 4, 5, 6, 7, 8]
Normal pressure hydrocephalus can be secondary to a specific cause, such as tumor, head injury, hemorrhage, or meningitis, but more commonly is idiopathic (iNPH). [9] Because the initial symptoms of iNPH are often subtle gait changes, they are easily attributed to normal aging. However, early diagnosis and intervention is crucial, as it is correlated with a more favorable prognosis. [10]
It has been postulated that normal pressure hydrocephalus appears to be a “two-hit” disease: benign external hydrocephalus in infancy, followed by deep white matter ischemia in late adulthood. [11]
Current consensus is that ventriculomegaly resulting from CSF dynamics could be the initiating factor for a vicious cycle of neurologic damage in iNPH. Pathophysiologic factors such as hypoperfusion, glymphatic impairment, disturbance of metabolism, astrogliosis, neuroinflammation, and blood-brain barrier disruption have been shown to cause white matter and gray matter lesions and to ultimately result in iNPH symptoms. [12, 13]
An enlarged ventricular system out of proportion to sulcal atrophy (ventriculosulcal disproportion) is the most important imaging characteristic. Other radiologic markers, such as narrowed temporal horns, have been reported as statistically significant for the diagnosis of NPH. [14]
An ongoing issue in the management of NPH is that clinical features and even some imaging features in patients with NPH can overlap with features of much more common diseases, such as Alzheimer disease with ex vacuo dilatation of the ventricles. Furthermore, the treatment of NPH is quite invasive, requiring intracranial procedures such as ventriculoperitoneal shunting. Thus, much imaging research has been devoted to trying to identify factors that can predict response to shunting.
Fallmer et al showed that several features such as callosal angle, enlarged Sylvian fissures, and focally enlarged sulci provided diagnostic accuracy compared to other differential diagnoses such as vascular dementia. They found that the callosal angle was the most discriminating imaging feature for iNPH. [15]
A study by Rau et al that compared the MRIs of 30 NPH patients with those of 30 healthy controls found that a support vector machine (SVM) algorithm could reliably differentiate between no NPH pattern, possible NPH pattern, and definitive NPH pattern. Accuracy of the SVM algorithm was 0.93, and AUROC (area under the receiver operating characteristic curve) was 0.99. [9]
Imaging modalities
MRI of the brain is the preferred radiologic examination for the diagnosis of NPH. T2-weighted images are especially helpful. CT scanning of the brain is useful if MRI is unavailable. Both radiologic techniques require clinical correlation. The primary role of MR and CT scanning is to assess for hydrocephalus with ventriculosulcal disproportion. This observation is a subjective assessment, and in patients with some sulcal widening or only minimal ventriculomegaly, the studies may not be sensitive or specific. Furthermore, MRI provides pathophysiologic information on CSF flow in patients with NPH. [16] A correlation between clinical symptoms and stiffness values has also been suggested by MRI elastography. [17] One of the most recent advancements with MRI is the assessment of some of the brain metabolic functions in patients with NPH via their glymphatic system. [18]
Radiographic evaluation in the form of pneumoencephalographs has been completely replaced by CT and MRI and is now only of historical interest. Pneumoencephalography was used to demonstrate nonobstructive hydrocephalus. Intrathecally introduced air (via lumbar puncture) was found on radiographs within the enlarged lateral ventricles, not in the subarachnoid convexities. Ultrasonography is not used for the diagnosis of NPH, although some researchers have suggested that reduced cerebral blood flow in NPH can be assessed by using transcranial Doppler ultrasound. [19, 20]
Although neuroimaging is essential to determine the typical findings of NPH, such as ventriculomegaly, disproportionately enlarged subarachnoid space hydrocephalus (DESH), and narrow callosal angle, it is important to evaluate the clinical response to other tests, such as the tap test, before indicating shunting. [2] Other invasive tests such as intracerebral pressure monitoring and CSF dynamics test (CSF space volume load test) may be done depending on available expertise. [3, 21, 22, 23, 24]
(The images below are examples of hydrocephalus ex vacuo and NPH from MRI.)
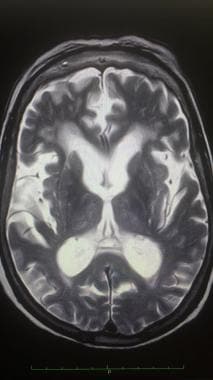 Axial T2-weighted magnetic resonance image of the brain in a patient with hydrocephalus ex vacuo. Note the enlarged ventricular system and noticeable sulcal atrophy.
Axial T2-weighted magnetic resonance image of the brain in a patient with hydrocephalus ex vacuo. Note the enlarged ventricular system and noticeable sulcal atrophy.
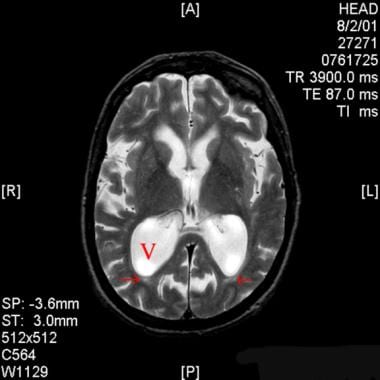 Axial T2-weighted magnetic resonance image of the brain in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system, especially the atria of the lateral ventricles (V), which is out of proportion with sulcal atrophy.
Axial T2-weighted magnetic resonance image of the brain in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system, especially the atria of the lateral ventricles (V), which is out of proportion with sulcal atrophy.
Computed Tomography
In patients with NPH, CT scans demonstrate hydrocephalus with ventriculomegaly that is out of proportion to sulcal atrophy. This so-called ventriculosulcal disproportion differentiates NPH from ex vacuo ventriculomegaly, in which sulcal atrophy should also be present. CT scans depicting NPH are presented below.
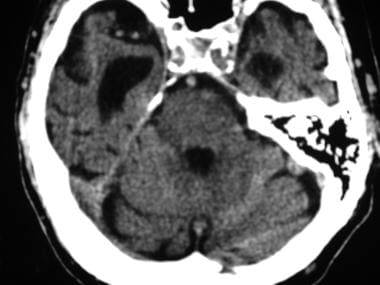 Axial nonenhanced computed tomography (CT) scan of the head of a patient with normal pressure hydrocephalus at the level of the middle cranial fossa. Note the disproportionately enlarged temporal horns of the lateral ventricles compared with the relatively normal sulcal size.
Axial nonenhanced computed tomography (CT) scan of the head of a patient with normal pressure hydrocephalus at the level of the middle cranial fossa. Note the disproportionately enlarged temporal horns of the lateral ventricles compared with the relatively normal sulcal size.
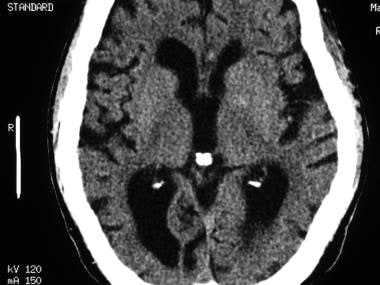 Axial nonenhanced computed tomography (CT) scan at the level of the basal ganglia in a patient with normal pressure hydrocephalus. Note the prominent lateral ventricles, which are disproportionately dilated in comparison with the mild sulcal widening.
Axial nonenhanced computed tomography (CT) scan at the level of the basal ganglia in a patient with normal pressure hydrocephalus. Note the prominent lateral ventricles, which are disproportionately dilated in comparison with the mild sulcal widening.
In NPH, ventriculomegaly is prominent in all 3 horns of the lateral ventricles and in the third ventricle, but there is relative sparing of the fourth ventricle. Frontal and occipital periventricular hypoattenuating areas, which may represent transependymal CSF flow, may be noted in NPH. This sign is infrequent, and it may also represent periventricular leukoencephalopathy of microangiopathic disease. Another finding possibly associated with NPH is corpus callosal thinning, but this finding is nonspecific and can be associated with many other conditions.
CT scanning alone cannot be used to make a diagnosis of NPH, since the clinical picture and CSF pressures also are necessary in diagnosis. With an appropriate clinical picture and ventriculosulcal disproportion demonstrated on either CT or MRI scans, 50-70% of patients are likely to respond favorably to a CSF-shunting procedure.
After studying 12 patients using spectral domain-optical coherence tomography (SD-OCT), Afonso et al found significant changes in choroidal thickness that support the hypothesis of choroidal susceptibility to hemodynamic alterations in idiopathic NPH. [25]
In the diagnosis of NPH, the exact percentage of false-positive and false-negative CT scan findings is unknown. This is partially because NPH remains an incompletely understood entity, and no criterion standard test exists with which to make an unequivocal diagnosis. Assessing for the ability to predict response to surgery seems more appropriate. Unfortunately, individual patient response to CSF shunting in NPH is variable, and the exact percentage of false-positive and false-negative findings of suggestive CT scans is unclear. Disease entities that may mimic the CT scan findings of NPH include obstructive hydrocephalus, ex vacuo dilatation secondary to cerebral atrophy, and idiopathic arrested hydrocephalus.
Magnetic Resonance Imaging
As in CT scanning, the first abnormality that should be noted on MRI views is ventriculomegaly out of proportion with sulcal atrophy. More specifically, the temporal horns of the lateral ventricles may show dilatation out of proportion with hippocampal atrophy. MRI scans depicting NPH are presented below.
 Axial T2-weighted magnetic resonance image of the brain in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system, especially the atria of the lateral ventricles (V), which is out of proportion with sulcal atrophy.
Axial T2-weighted magnetic resonance image of the brain in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system, especially the atria of the lateral ventricles (V), which is out of proportion with sulcal atrophy.
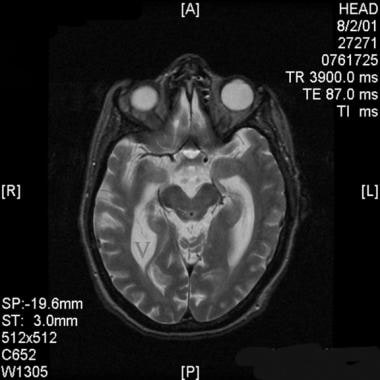 Axial T2-weighted magnetic resonance image through the level of the superior colliculi in a patient with normal pressure hydrocephalus. Note the enlarged temporal horns of the lateral ventricles (V). Also, note the cerebrospinal fluid (CSF) flow void in the cerebral aqueduct (arrow). This flow void lacks signal and appears black, while nonturbulent CSF, as imaged in the ventricles, is hyperintense on T2-weighted images.
Axial T2-weighted magnetic resonance image through the level of the superior colliculi in a patient with normal pressure hydrocephalus. Note the enlarged temporal horns of the lateral ventricles (V). Also, note the cerebrospinal fluid (CSF) flow void in the cerebral aqueduct (arrow). This flow void lacks signal and appears black, while nonturbulent CSF, as imaged in the ventricles, is hyperintense on T2-weighted images.
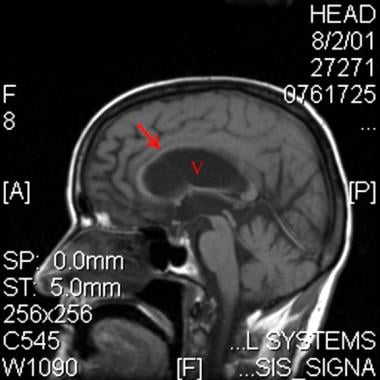 Midline sagittal T1-weighted magnetic resonance image in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system (V), which is out of proportion with sulcal atrophy. Also note the thinned corpus callosum (arrow).
Midline sagittal T1-weighted magnetic resonance image in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system (V), which is out of proportion with sulcal atrophy. Also note the thinned corpus callosum (arrow).
The degree of confidence of MRI in helping diagnose NPH or, more importantly, in helping to predict a positive result with neurosurgical CSF shunting is unknown. Positive surgical results have been demonstrated in 50-70% of patients with a strong clinical history of NPH and classic NPH findings on magnetic resonance images or CT scans. [26]
On T2-weighted images, regions of hyperdynamic CSF demonstrate no signal instead of the increased signal observed in slow-moving CSF, similar to the flow effects seen with vascular flow voids. This “flow void” finding was correlated with shunt success in historic literature. [27]
With the advent of faster MR machines and more efficient acquisition sequences, such as fast/turbo spin echo, the study of CSF and the accurate prediction of treatment success has become more complex.
Nowadays, the stroke volume through the acqueduct can be calculated; however, the value of the finding “flow void” and, further, the stroke volume in patients with NPH has come into question. [28]
A jet of turbulent CSF flow may be observed distal to the aqueduct in the fourth ventricle. This finding appears as a hypointense or absent signal in the proximal fourth ventricle on proton density– and T2-weighted images, with surrounding CSF appearing isointense on proton density–weighted images or hyperintense on T2-weighted images.
MRI may show transependymal CSF flow in the form of a periventricular high signal on T2-weighted images, primarily anterior to the frontal horns or posterior to the occipital horns of the lateral ventricles. However, as with CT imaging, these periventricular abnormalities may be confused with leukoencephalopathy resulting from microvascular ischemia.
Tsunoda and colleagues used 3-dimensional MRI volume-acquisition techniques to objectively assess ventriculosulcal disproportion. [29] They measured ventricular volume (VV) and intracranial CSF space volume (ICV), and then calculated the VV/ICV ratio. They found that patients with NPH (N=16) had significantly higher VV/ICV ratios than did the young control patients (N=14), elderly control patientts (N=13), and patients with cerebrovascular disease (N=16). The authors found that 13 of the 16 patients with NPH had a VV/ICV ratio greater than 30%, while no patients in the other groups had ratios higher than 30%. [30]
Tawfik et al conducted a study of 32 patients using phase contrast MRI pulse sequences with cine acquisition to assess intraobserver and interobserver variability on the manual segmentation of the cerebral aqueduct. As a secondary finding, the measurement of aqueductal CSF stroke volume had a perfect sensitivity and specificity (100%) for the diagnosis of NPH, as compared to healthy controls and patients with brain atrophy. [31]
Siasios et al found that fractional anisotropy and mean diffusivity could help differentiate idiopathic NPH from Alzheimer or Parkinson disease. [32]
The diagnosis of NPH is complex because of clinical and imaging characteristics similar to those of other degenerative diseases. An accurate diagnosis is fundamental, as the treatment is quite invasive, requiring intracranial procedures such as ventriculoperitoneal shunting. Imaging research has been mostly devoted to trying to identify factors that can predict response to shunting.
Yamada et al correlated 3D MRI-obtained volume ratios with a tap-positive test as a less invasive way to assess responsiveness to shunt treatment. In a study of 24 patients with a tap-positive study, 25 with a tap-negative study, and 23 matched controls, the researchers found that there was a correlation between the volume of the ventricles, the CSF volume of the SA space, the parietal convexity, and the upper to lower SA space ratio to a tap-positive test. [33]
Tullberg and colleagues differentiated between periventricular and deep white-matter hyperintensity as seen on T2-weighted images and found that neither was predictive of the outcome of CSF shunting. [34] Thus, the authors cautioned that findings compatible with microvascular white-matter disease do not predict a poor outcome of CSF shunting. [35] These findings included CSF flow void sign, periventricular increase signal on T2-weighted images, and corpus callosal thinning. The authors found that only the CSF flow void sign may be predictive of shunt responsiveness and that periventricular signal hyperintensity and corpus callosal morphology are not predictive of positive treatment results.
Bradley and colleagues assessed the predictive value of the presence of a CSF void for shunt responsiveness and found a significant correlation. [36] However, in a later study, the researchers did not find a statistically significant relationship between responsiveness to CSF shunting and aqueductal flow void score, but MRI assessment of CSF flow stroke volume was found to be predictive of shunt responsiveness. [37] Marmarou and colleagues concluded that neither MRI CSF flow void sign nor quantitative CSF flow velocity seems to have significant diagnostic value, and they questioned whether stroke volume may have some benefit. [38] However, Kahlon suggested that cine phase-contrast MRI measurements of stroke volume in the cerebral aqueduct are not useful in predicting patient response to CSF shunt surgery. [39] Ragunathan and Pipe showed the degree of bias in CSF flow quantification as a result of radiofrequency saturation effects using 2-dimensional cine phase contrast MRI. [40]
Phase-contrast magnetic resonance (PC-MR) is widely used in patients with idiopathic normal pressure hydrocephalus (iNPH). In a study by He et al of 46 patients with definite iNPH, PC-MR was used to evaluate CSF peak velocity (PV), average velocity, aqueductal stroke volume (ASV), net ASV, and net flow to determine effectiveness of preoperative PC-MR CSF flow measurement in predicting clinical response. No CSF parameters significantly differed between shunt improvement and non-improvement groups, according to the authors. [41]
Studies by Kizu and colleagues using proton chemical shift imaging have suggested that intraventricular lactate measurements may be useful in discriminating patients with NPH from those with other forms of dementia. [42]
In another study, 9 of 9 patients with clinically diagnosed NPH exhibited ventricular lactate peaks by way of proton chemical shift imaging. No lactate peaks were found in the 5 control subjects or in the 6 patients with other diagnosed dementias, including Alzheimer disease, Pick disease, and frontotemporal dementia. [42]
Kazui and colleagues reviewed 71 NPH patients who had undergone surgery [43] and did not find that any neuroimaging studies that predicted the disappearance of symptoms after shunt surgery. McGirt et al found that corpus callosum distention had some predictive value. [44] Exclusive of imaging parameters, the former group found that younger age was a predictor of gait improvement, and the latter group found that gait as the primary symptom was predictive.
A CSF hydrodynamics study, [45] which is not a routinely used MRI tool, looked at a cohort of 20 patients deemed to be shunt responsive (N=14) versus not responsive (N=6), using a mean threshold velocity of CSF through the aqueduct of Sylvius greater than 26 mm/sec. To predict responsiveness, they found a sensitivity of 50%, specificity of 83.3%, positive predictive value of 87.5%, and accuracy of 70%. Thus, this study stresses the ongoing difficulty in identifying patients who might benefit from surgery; 6 of 20 patients did not benefit.
In a study of 108 patients with idiopathic normal pressure hydrocephalus who underwent preoperative MRI, clinical evaluation 12 months after surgery showed that a small callosal angle, wide temporal horns, and occurrence of disproportionately enlarged subarachnoid space hydrocephalus were significant predictors of a positive shunt outcome. [46]
In a study of CSF pressure gradients in patients with idiopathic normal hydrocephalus versus gradients in healthy controls, 4-dimensional phase-contrast MRI showed that the pressure gradients of patients with normal pressure hydrocephalus was 3.2 times greater than that in controls. [47]
Kamiya et al studied diffusion MRI as a means of distinguishing reversible and irreversible microstructural changes in idiopathic NPH that can aid in determining treatment outcomes. [48]
Postoperative failure of shunt placement has been associated with decreased deep gray-matter stiffness, as well as increased temporal stiffness, using MR elastography, suggesting a plausible application to this method. [17]
Nuclear Imaging
In nuclear medicine studies, some of the nonspecific signs seen in iNPH are the heart-shaped appearance of lateral ventricles (normally shaped like a trident); persistence of tracer more than 24-48 hours in the ventricular system because of impaired absorption; an absence of an extension of tracer onto the superior aspect of lateral ventricles; and backflow of CSF into lateral ventricles. [3]
Traditionally, isotope cisternography and CT cisternography have been used in NPH to assess for disturbances in CSF dynamics, such as reversal of flow. This study is likely to be an unreliable predictor of NPH, despite its historical popularity. [49, 50]
Using single-photon emission computed tomography (SPECT) and statistical brain mapping, Sasaki found regional cerebral blood flow reduction with frontal dominance and severe hypoperfusion around the corpus callosum. [51] This finding was consistent with some of the regions of brain dysfunction that clinical assessment indicated were involved.
Isotope cisternography and CT cisternography appear to be unreliable in helping to predict whether patients with possible NPH will respond to CSF shunting. [49, 50] One of the likely difficulties in identifying responsiveness to shunting is that many individuals in the age group can have overlapping diagnoses, such as Alzheimer disease and subcortical microangiopathic disease, in which the latter can cause both dementia and gait disturbance identical to that seen in NPH. Incontinence, part of the NPH diagnostic triad, is generally common in both men and women. Thus, recent advances in neuroimaging for conditions such as Alzheimer disease might help differentiate these other confounding conditions.
There have been advances in positron emission tomography (PET) scanning for NPH evaluation. For example, although clinical outcomes were not a focus of the study, Leinonen et al [52] looked at [(18)F]flutemetamol PET scanning in patients with possible NPH. In concept, this could help establish the total burden of amyloid-beta pathology and, more specifically, the burden of Alzheimer disease that would not be corrected by invasive surgical treatment of NPH such as ventriculoperitoneal shunting.
-
Axial nonenhanced computed tomography (CT) scan of the head of a patient with normal pressure hydrocephalus at the level of the middle cranial fossa. Note the disproportionately enlarged temporal horns of the lateral ventricles compared with the relatively normal sulcal size.
-
Axial nonenhanced computed tomography (CT) scan at the level of the basal ganglia in a patient with normal pressure hydrocephalus. Note the prominent lateral ventricles, which are disproportionately dilated in comparison with the mild sulcal widening.
-
Axial T2-weighted magnetic resonance image of the brain in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system, especially the atria of the lateral ventricles (V), which is out of proportion with sulcal atrophy.
-
Axial T2-weighted magnetic resonance image through the level of the superior colliculi in a patient with normal pressure hydrocephalus. Note the enlarged temporal horns of the lateral ventricles (V). Also, note the cerebrospinal fluid (CSF) flow void in the cerebral aqueduct (arrow). This flow void lacks signal and appears black, while nonturbulent CSF, as imaged in the ventricles, is hyperintense on T2-weighted images.
-
Midline sagittal T1-weighted magnetic resonance image in a patient with normal pressure hydrocephalus. Note the enlarged ventricular system (V), which is out of proportion with sulcal atrophy. Also note the thinned corpus callosum (arrow).
-
Axial T2-weighted magnetic resonance image of the brain in a patient with hydrocephalus ex vacuo. Note the enlarged ventricular system and noticeable sulcal atrophy.









