Practice Essentials
Hirschsprung disease (HD) is characterized by the absence of myenteric and submucosal ganglion cells (Auerbach and Meissner plexuses) along a variable length of the distal GI tract. The ganglion cells help coordinate and facilitate bowel relaxation, and if they are absent, the bowel becomes spastic and results in distal intestinal obstruction. The disease results in decreased motility in the affected bowel segment, lack of propagation of peristaltic waves into the aganglionic colon, and abnormal or absent relaxation of this segment and of the internal anal sphincter. A transition zone is present, where a marked change in caliber occurs, with the dilated normal colon above and the narrowed aganglionic colon below. [1, 2, 3]
Most cases of Hirschsprung disease are diagnosed in the newborn period. [3] Hirschsprung disease should be considered in any newborn who fails to pass meconium within 24-48 hours after birth. The first report of a patient with HD was made in 1691 by Frederick Ruysch, but it was Danish pediatrician Harald Hirschsprung who in 1888 published the classic description of congenital megacolon. [4]
As a congenital disorder, HD is manifested mostly within the first several weeks of life and is diagnosed until 5 years of age. Occasionally, patients are diagnosed during adulthood. The incidence of HD has been shown to be approximately 1 case per 5,000 live births in the United States.There seems to be a significant variance among ethnic groups, with an estimated 1.5 cases per 10,000 live births in whites, 2.1 cases per 10,000 live births in African Americans, and 2.8 cases per 10,000 live births in Asians. Males are affected more than females by a ratio of 4:1. However, for short-segment disease, the male-to-female ratio is 4.2-4.4, and for long-segment disease the female-to-male ratio 1.2-1.9. [5, 6, 7]
Preferred examination
A diagnostic evaluation should begin with plain abdominal radiography, followed by a contrast enema examination of the colon to confirm the diagnosis of HD. Occasionally, ultrasonographic findings may also suggest the diagnosis. [8, 9, 10, 11, 12] The diagnostic contrast exam has been reported to have an 83.3% sensitivity and an 82.9% specificity. for HD. Although contrast enema is useful in establishing the diagnosis, full-thickness rectal biopsy remains the criterion standard. The classic finding of Hirschsprung disease is a narrowed distal colon with proximal dilation; however, the findings are difficult to interpret in neonates (age < 1 mo) and do not demonstrate this transition zone approximately 25% of the time. Once the diagnosis is confirmed, the definitive treatment is to remove the aganglionic bowel and to restore continuity of the healthy bowel with the distal rectum, with or without an initial intestinal diversion. [13, 14, 3, 15, 16]
A radiological/ultrasonographic study alone is not a sensitive enough tool to exclude HD. Manometry, rectal mucosal biopsy, or both are required for an accurate diagnosis.
Manometry
The rectal manometry is complementary to barium enema examination and has an accuracy of 75%. It shows an absence of normal relaxation of the internal sphincter, with a reduction in the intraluminal pressure in the anal canal when the rectum is distended with a balloon. This technique is more reliable from day 12 after birth, when the normal rectoenteric reflex is present. High-resolution anorectal manometry (HRAM) for Hirschsprung disease has been reported to have greater accuracy than conventional anorectal manometry. [17]
Biopsy
The predictive value of biopsy is essentially 100% in excluding HD if ganglion cells are present. It can be performed by a rectal suction biopsy or full-thickness rectal biopsy. The first one eliminates the need for general anesthesia; however, the latter provides bigger fragments of the submucosal neural plexus for histologic examination. In HD, the biopsy reveals an absence of ganglion cells, hypertrophy and hyperplasia of nerve fibers, and an increase in acetylcholinesterase-positive nerve fibers in the lamina propria and muscularis mucosa. It must be taken well above the anal valves, since ganglion cells are normally absent in the anal canal.
(See the images below.)
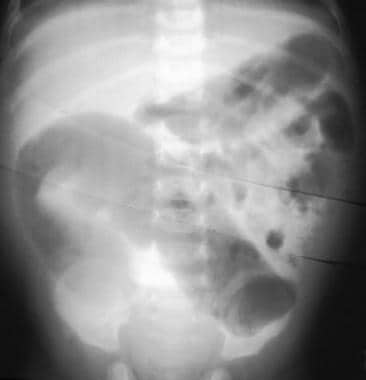 Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the bowel with no gas in the rectum.
Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the bowel with no gas in the rectum.
 Hirschsprung disease. Lateral view from a barium enema examination depicting the reduced diameter of the rectum and sigmoid.
Hirschsprung disease. Lateral view from a barium enema examination depicting the reduced diameter of the rectum and sigmoid.
 Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
Pathophysiology
The congenital absence of ganglion cells in the distal alimentary tract is the pathologic sine qua non of HD. The aganglionosis present in HD results from a failure of cells derived from the neural crest to populate the embryonic colon during development. This failure results from a fundamental defect in the microenvironment of the bowel wall that prevents ingrowth of neuroblasts. There are at least 11 genetic defects known to be associated with HD, including mutations to the endothelin-B receptor gene and the tyrosine kinase RET gene, the latter being responsible for a major role in all forms of HD susceptibility. [18] Because of the polygenic nature of HD, there is variable penetrance of the condition, leading to variable manifestations of the disease. There is variable penetrance even in families with identified genetic mutations/polymorphisms, suggesting also the presence of environmental influences and genetic modifiers.
Mortality and morbidity
The polygenic and varied penetrance gene condition of HD determines a wide range of clinical symptoms, from obstipation immediately after birth to a much milder picture associated with incomplete evacuation, leading eventually to distended abdomen, recurrent constipation, and high diaphragm.
Better diagnostic procedures, emphasis on early diagnosis, and improvements in surgical techniques have contributed to a decrease in the mortality of individuals with HD. The greatest morbidity and mortality is observed in children younger than 1 year, owing to the possible set of Hirschsprung-associated enterocolitis (HAEC), with a mean incidence of 25%, which can be fatal if not diagnosed and treated rapidly. [16]
Anatomy
HD is regarded as a neurocristopathy because there is a premature arrest of the craniocaudal migration of vagal neural crest cells in the hindgut between the fifth and twelfth week of gestation to form the enteric nervous system. As a consequence, both intramural ganglion cells in the Meissner (submucosal) and Auerbach (myenteric) plexuses are absent. The anus is always involved, and a variable length of distal intestine may also be involved. The aganglionic, aperistaltic bowel segment effectively prevents the propulsion of the fecal stream, resulting in dilation and hypertrophy of the normal proximal colon.
Patients can be classified by the extension of the aganglionosis, as follows:
-
Classic short-segment HD (75% of cases) - Aganglionic segment does not extend beyond the upper sigmoid
-
Long-segment HD (20% of cases)
-
Total colonic aganglionosis (3-12% of cases)
Some rare variants are as follows:
-
Total intestinal aganglionosis (when the whole bowel is involved)
-
Internal anal sphincter achalasia (previously referred to as ultrashort-segment HD)
Clinical details
Newborns with HD come to medical attention with the following symptoms:
-
Delayed passage of meconium (>24 hr after birth)
-
Abdominal distention that is relieved by rectal stimulation or enemas
-
Vomiting
-
Neonatal enterocolitis [16]
Older children and adult symptoms are as follows:
-
Severe constipation
-
Chronic abdominal distention
-
Vomiting
-
Failure to thrive [19]
Children presenting with abdominal distention, explosive diarrhea, vomiting, fever, lethargy, rectal bleeding, and shock may possibly have developed HAEC. The greatest risk for HAEC development occurs before the diagnosis of HD has been made or after the definitive pull-through operation. Children with Down syndrome also have increased risk for the development of HAEC.
HD occurs as an isolated trait in 70% of patients; it is associated with a chromosomal abnormality in 12% of cases (>90% trisomy 21) and with additional congenital anomalies in 18% of cases. [19]
Some associated syndromes are as follows:
-
Down syndrome
-
Multiple endocrine neoplasia type 2 (MEN2)
-
Cat eye syndrome
-
Waardenburg syndrome
-
Bardet-Biedl syndrome
Differentials
The differential diagnosis includes the following:
-
Intestinal Neuronal Dysplasia
Intervention
The treatment is surgical and is based on the removal or bypass of the poorly functioning aganglionic bowel, with anastomosis of normally innervated bowel just above the anus, at a level that prevents further functional obstruction but at the same time preserves fecal continence. [20] This can be done using a preliminary colostomy followed by a definitive pull-through procedure or a definitive single-stage procedure using the 3 common operations: the Soave pull-through, the Duhamel procedure, and the Swenson procedure. In current practice, the repair can be done transanally or with the assistance of laparoscopy.
In general, the treatment plan varies according to the extent of aganglionosis and the age of the patient. In most cases, this restores nearly normal motility and enables most affected individuals to have normal bowel function.
All children with HD are at risk for postoperative incontinence, enterocolitis, and obstructive symptoms, regardless of which operation is performed. Every child should therefore be followed up on a regular basis until at least age 5 years, or longer if they are still having problems at that point. [21, 16]
Radiography
Radiographs of the neonatal abdomen with Hirschsprung disease (HD) may show multiple loops of dilated small bowel with air-fluid levels that can usually be determined to be a distal bowel obstruction. An empty rectum is a common finding. The classic image is a dilated proximal colon with the aganglionic cone narrowing toward the distal gut. [19] A cutoff sign in the rectosigmoid region with an absence of air distally is a useful finding in Hirschsprung-associated enterocolitis (HAEC). [16]
(See the images below.)
 Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the small bowel with no gas in the rectum.
Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the small bowel with no gas in the rectum.
 Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the bowel with no gas in the rectum. In the sitting position, air-fluid levels in the large bowel are seen.
Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the bowel with no gas in the rectum. In the sitting position, air-fluid levels in the large bowel are seen.
 Hirschsprung disease. Lateral abdominal radiograph shows a very enlarged, stool-filled sigmoid. No air or stool content is seen in the rectum.
Hirschsprung disease. Lateral abdominal radiograph shows a very enlarged, stool-filled sigmoid. No air or stool content is seen in the rectum.
 Hirschsprung disease. Plain abdominal radiograph showing dilatation of the transverse colon and mucosal edema (toxic megacolon).
Hirschsprung disease. Plain abdominal radiograph showing dilatation of the transverse colon and mucosal edema (toxic megacolon).
HD is more definitively diagnosed by means of contrast enema examination, which can show the presence of a transition zone, [14] irregular contractions, mucosal irregularity, and delayed evacuation of contrast material, among other findings. Contrast enemas should be avoided in patients with enterocolitis because of the risk of perforation. [22]
(See the images below.)
 Hirschsprung disease. Lateral view from a barium enema examination depicting the reduced diameter of the rectum and sigmoid.
Hirschsprung disease. Lateral view from a barium enema examination depicting the reduced diameter of the rectum and sigmoid.
 Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
 Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
 Hirschsprung disease. A 24-hour-delayed radiograph obtained after a barium enema examination shows retention of barium and stool in the rectum. This is associated with a dilated stool-filled sigmoid.
Hirschsprung disease. A 24-hour-delayed radiograph obtained after a barium enema examination shows retention of barium and stool in the rectum. This is associated with a dilated stool-filled sigmoid.
 Hirschsprung disease. Barium enema showing reduced caliber and length of the large bowel, with no clear transition zone (total colonic aganglionosis).
Hirschsprung disease. Barium enema showing reduced caliber and length of the large bowel, with no clear transition zone (total colonic aganglionosis).
 Hirschsprung disease. Barium enema showing a reduced-caliber rectum and dilated large-bowel loops with an irregular mucosal contour (dyskinesia).
Hirschsprung disease. Barium enema showing a reduced-caliber rectum and dilated large-bowel loops with an irregular mucosal contour (dyskinesia).
Transition zone is the term applied to the region in which a marked change in caliber occurs, with the dilated, normal colon above and the narrowed, aganglionic colon below; although this is a highly reliable sign of HD, failure to visualize a transition zone does not rule out the presence of the disease. [23, 14]
The hallmark of the diagnosis is demonstration of the transition zone from the dilated bowel to the reduced-caliber bowel. Obviously, finding more than 1 sign increases the accuracy in diagnosis. Signs of HD after barium enema administration include the following [11, 12] :
-
Transition zone (often subtle during the first week of life)
-
Abnormal, irregular contractions of aganglionic segment (rare)
-
Thickening and nodularity of colonic mucosa proximal to transition zone (rare)
-
Delayed evacuation of barium
-
Mixed barium-stool pattern on delayed radiographs
-
Distended bowel loops on plain radiographs that almost fill after contrast enema
-
Question mark–shaped colon in total colonic aganglionosis [24]
According to the results of one study, the use of the rectosigmoid index (widest diameter of the rectum divided by the widest diameter of the sigmoid colon < 1 in HD) can in some cases help identify HD in patients when the diagnosis would have been missed by looking at the transition zone alone. [25]
In another study, of 192 children suspected of having HD, the sensitivity and specificity for diagnosing HD by the presence of a radiologic transition zone were 86.9% and 92.1%, respectively. [14]
Degree of confidence
Sensitivity and specificity of a contrast enema in the diagnosis of HD are reported as being 76% and 97%, respectively, [26] but may be extremely difficult in total colonic aganglionosis, with a transition zone only being accurately determined in 25% or less of all colonic aganglionosis patients. [27] Barium enema is not as sensitive or reliable as rectal suction biopsy in ruling out HD. [28]
Many studies have documented that a maximum of 10% of neonates with HD do not have a transition zone on contrast enema. [29] In addition, older children with a very short aganglionic segment may not demonstrate a transition zone on contrast enema, particularly if the catheter has been placed above the transition zone in the rectum. False-positive rates can be as high as 48.5% on contrast enema and higher in female patients and children younger than 30 days. [30] Finally, the contrast study is not always completely accurate in identifying the location of the pathologic transition zone, with 12% of cases having a pathologic transition zone, which is different from the radiologic transition zone. [31]
Ultrasonography
Although ultrasonography is not the first-choice imaging tool for diagnosing Hirschsprung disease (HD), diagnosis is possible with real-time ultrasonography. [32] Oestreich reported a case of unsuspected HD in a 1-month-old baby who was taken to a pediatrician for a check-up. A distended abdomen was noted. Ultrasonography revealed the same pattern that is observed in a barium enema examination, that is, a dilated sigmoid narrowing to a narrow rectum. [33] Ultrasonography may also help in determining the dynamic or adynamic state of fluid- or solid-filled bowel loops. The degree of confidence is low because gas-filled bowel loops can make the diagnosis of HD difficult.
-
Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the bowel with no gas in the rectum.
-
Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the small bowel with no gas in the rectum.
-
Hirschsprung disease. Frontal abdominal radiograph showing marked dilatation of the bowel with no gas in the rectum. In the sitting position, air-fluid levels in the large bowel are seen.
-
Hirschsprung disease. Lateral abdominal radiograph shows a very enlarged, stool-filled sigmoid. No air or stool content is seen in the rectum.
-
Hirschsprung disease. Barium enema technique shows slow contrast-material infusion.
-
Hirschsprung disease. Lateral view from a barium enema examination depicting the reduced diameter of the rectum and sigmoid.
-
Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
-
Hirschsprung disease. Barium enema showing reduced caliber of the rectum, followed by a transition zone to an enlarged-caliber sigmoid.
-
Hirschsprung disease. A 24-hour-delayed radiograph obtained after a barium enema examination shows retention of barium and stool in the rectum. This is associated with a dilated stool-filled sigmoid.
-
Hirschsprung disease. Barium enema showing reduced caliber and length of the large bowel, with no clear transition zone (total colonic aganglionosis).
-
Hirschsprung disease. Barium enema showing a reduced-caliber rectum and dilated large-bowel loops with an irregular mucosal contour (dyskinesia).
-
Hirschsprung disease. Plain abdominal radiograph showing dilatation of the transverse colon and mucosal edema (toxic megacolon).











