Practice Essentials
Lower gastrointestinal bleeding is defined as bleeding from the bowel distal to the ligament of Treitz (see the image below). Lower GI bleeding can be acute or chronic. Massive GI bleeding will result in hemodynamic instability and decreasing hemoglobin levels, which need to be treated with transfusions. Flexible endoscopy is the gold standard for the diagnosis and treatment of GI bleeding but identifies the bleeding in only up to 40% of cases. Once bleeding is identified on endoscopy, over 90% of cases can be successfully treated. Approximately 85% of cases of lower GI bleeding can be managed by supportive treatment only. [1, 2, 3, 4, 5] Diverticulosis has been implicated as the source of bleeding in as many as 60% of cases. The diverticula are more prevalent in the left or sigmoid colon, but positive arteriographic findings for bleeding localizes the bleeding to the right colon in 60% of cases. [6]
In approximately 90% of cases, lower GI bleeds resolve spontaneously. Mortality is about 4%. Other causes of lower GI bleeding include vascular ectasia, malignancy, inflammatory bowel disease, ischemic colitis, and anorectal abnormalities. [7, 8, 9, 10]
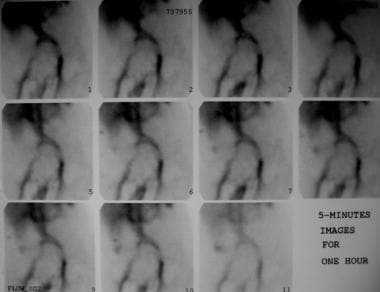 Technetium-99m (99mTc) red blood cell scan shows an abnormal focus of increasing activity in the right lower quadrant, consistent with gastrointestinal bleeding. This activity is seen to increase in intensity over time. The patient underwent angiography.
Technetium-99m (99mTc) red blood cell scan shows an abnormal focus of increasing activity in the right lower quadrant, consistent with gastrointestinal bleeding. This activity is seen to increase in intensity over time. The patient underwent angiography.
Imaging modalities
Colonoscopy after rapid oral purging has emerged as the procedure of choice for the evaluation of acute lower GI bleeding (69-90% yield), and it also provides a means for therapy. [11] This procedure involves the use of thermal contact modalities or epinephrine injections in cases of diverticula and vascular ectasia. Similarly, other causes of lower GI bleeding can be effectively treated during colonoscopy.
A meta-analysis of 6 studies found that early colonoscopy, versus delayed colonoscopy, was associated with a higher rate of detection of the bleeding source, as well as a higher rate of endoscopic intervention. [12]
Various imaging modalities may be employed to diagnose and define a source of bleeding, including colonoscopy, nuclear scintigraphy with technetium‐99m‐labeled red blood cell or 99mTc sulfur colloid, computed tomography, and transcatheter digital subtraction angiography. Because of limitations associated with colonoscopy and scintigraphy, CT is used as the diagnostic tool of choice for high sensitivity to depict arterial anatomy and help facilitate accurate catheterization. [13]
The American College of Gastroenterology guidelines for patients with acute overt lower GI bleeding include the following [6] :
-
Hematochezia associated with hemodynamic instability may be indicative of an upper GI bleeding source and thus warrants an upper endoscopy.
-
In the majority of patients, colonoscopy should be the initial diagnostic procedure and should be performed within 24 hr of patient presentation after adequate colon preparation.
-
Endoscopic hemostasis therapy should be provided to patients with high-risk endoscopic stigmata of bleeding, including active bleeding, nonbleeding visible vessel, or adherent clot.
-
The endoscopic hemostasis modality used (mechanical, thermal, injection, or combination) is most often guided by the etiology of bleeding, access to the bleeding site, and endoscopist experience with the various hemostasis modalities.
-
Repeat colonoscopy, with endoscopic hemostasis performed if indicated, should be considered for patients with evidence of recurrent bleeding.
-
Radiographic interventions (tagged red blood cell scintigraphy, computed tomographic angiography, and angiography) should be considered in high-risk patients with ongoing bleeding who do not respond adequately to resuscitation and who are unlikely to tolerate bowel preparation and colonoscopy.
The British Society of Gastroenterology (BSG) has published guidelines that include the following [5, 14, 15] :
-
Patients presenting with a minor self-terminating bleed (such as those with an Oakland score ≤8 points), with no other indications for hospital admission can be discharged for urgent outpatient investigation.
-
Patients with a major bleed should be admitted to hospital for colonoscopy.
-
If a patient is hemodynamically unstable or has a shock index (heart rate/systolic BP) of >1 after initial resuscitation and/or active bleeding is suspected, CT angiography provides the fastest and least invasive means to localize the site of blood loss before planning endoscopic or radiological therapy.
-
Because lower GI bleeding associated with hemodynamic instability may be indicative of an upper GI bleeding source, an upper endoscopy should be performed immediately if no source is identified by initial CT angiography (CTA). If the patient stabilizes after initial resuscitation, gastroscopy may be the first investigation.
-
Where indicated, catheter angiography with a view to embolization should be performed as soon as possible after a positive CTA to maximize chances of success. In centers with a 24/7 interventional radiology service, this should be available within 60 min for hemodynamically unstable patients.
-
No patient should proceed to emergency laparotomy unless every effort has been made to localize bleeding by radiologic and/or endoscopic modalities, except under exceptional circumstances.
The European Society of Gastroenterology (ESGE) has published the following guidelines [16, 17, 18] :
-
Patients with hemodynamic instability and suspected ongoing bleeding should undergo CTA before endoscopic or radiologic treatment to locate the site of bleeding.
-
Red blood cell scintigraphy is not recommended in the setting of acute lower GI bleeding because of its limited accuracy in identifying the location of the bleeding site and logistical constraints.
-
Transcatheter arterial embolization should be reserved for the treatment of acute, potentially life-threatening, lower GI bleeding either in hemodynamically unstable patients with active bleeding as demonstrated by CTA or in patients with brisk and ongoing bleeding not amenable to, or not effectively treated by, endoscopic interventions.
However, the 2 widely used diagnostic tests for lower GI bleeding are nuclear scanning (see the image above) during episodes of bleeding or angiography (see the images below). [19, 20] In cases in which colonoscopy is unsuccessful, scanning during episodes of bleeding and angiography are considered to be next imaging tests to determine the cause of the bleeding. Catheter-directed angiography remains the best option in a patient in unstable condition and should be performed in cases of massive bleeding. [21]
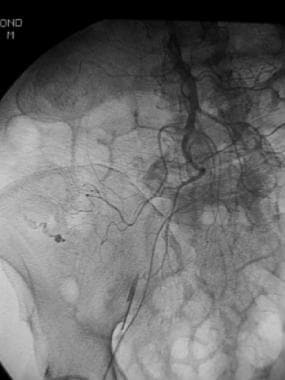 Superior mesenteric arterial arteriogram shows extravasation of contrast material from the right colic branch in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
Superior mesenteric arterial arteriogram shows extravasation of contrast material from the right colic branch in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
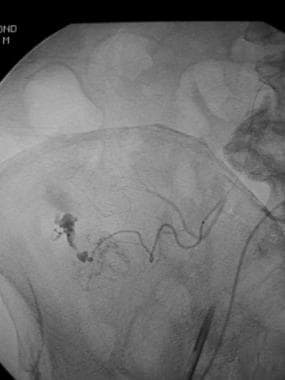 Selective arteriogram through a microcatheter further localizes the point of bleeding in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
Selective arteriogram through a microcatheter further localizes the point of bleeding in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
An advantage of catheter-directed angiography over colonoscopy or scintigraphy is that no special preparation is needed. Thus, it can be performed in a relatively short period; angiography also provides a means for immediate treatment. However, patients in stable condition can undergo scintigraphy to guide and increase the yield of angiography.
Scintigraphy and/or angiography also play important roles in diagnosis and embolization when colonoscopy reveals negative findings or when it is not feasible. Some investigators also advocate an upper GI endoscopy after colonoscopy yields negative results, [22] as about 10% of cases are ultimately found to involve an upper GI source of bleeding. [23]
Computed tomography angiography (CTA) is increasingly becoming an accepted alternative to catheter-directed angiography and may ultimately become the favored alternative in patients with suspected GI bleeding. [24]
CTA can provide immediate noninvasive diagnosis by visualizing the entire GI tract. In a retrospective analysis of lower endoscopy versus CTA by Clerc et al, median time to CTA was 3 hours for CTA versus 22 hours for lower endoscopy; active bleeding was identified in 31% with CTA versus 15% with lower endoscoy; and a nonactively bleeding source was found by CTA in 22% versus 31% for lower endoscopy. The authors noted that for postinterventional bleeding, urgent lower endoscopy is advocated, but for other causes of lower GI bleeding, CTA was efficient and more available than colonoscopy. [25]
Radiologic intervention
Two transcatheter methods for the treatment of GI bleeding include vasopressin infusion and embolization. Superselective embolization has become more accepted. [26, 27, 28, 29]
Vasopressin infusion causes vasoconstriction of the small arteries, arterioles, and capillaries, and it has been used for both upper and lower GI bleeds. [28] The rate of successful control of bleeding has been reported to be 70-90%. The repeat bleeding rate is reported to be 17-27%.
The infusion is started with the catheter in the main trunk of the mesenteric artery that is the cause of bleeding. The initial rate is 0.2 U/min. A follow-up angiogram is obtained after about 30 minutes. In cases of active hemorrhage, the rate is increased to 0.4 U/min. Higher rates are not recommended, because the potential complications from vasoconstriction can exceed the benefits. The infusion is tapered at 6- to 12-hour intervals and then stopped if no further bleeding ensues.
Complications of vasopressin infusion include myocardial ischemia, arrhythmia, hypertension, bowel ischemia, peripheral vascular ischemia, and antidiuretic effects. Because of significant rebleeding, variable success, the need for ICU monitoring, and the aforementioned adverse effects of the infusion, there has been renewed interest in embolization therapy. In fact, embolization has now become the preferred transcatheter therapy. Embolization also achieves immediate control of the bleeding, and ICU monitoring to the degree required with vasopressin infusion is avoided.
The aim of embolization is to decrease the arterial inflow so that the pressure at the bleeding site is decreased and hemostasis occurs. The important issue is to avoid devascularization of the tissues, which leads to ischemia and infarction. [30]
Bookstein first described transcatheter embolization by using an autologous clot. [31] Since then, various investigators have described small series of transcatheter embolization for lower GI bleeds, with varying success. The potential complication of bowel ischemia and infarction initially limited use of this technique. Earlier groups had described postembolization bowel infarction rates ranging from 0 to 23%. However, some authors claim that the high rate of significant ischemia may have been related to proximal embolization sites in relation to the marginal artery, as lack of large vascular collaterals in the large bowel may jeopardize significant areas of colon.
With this issue in mind, further studies with superselective catheterization techniques and embolization were performed using polyvinyl alcohol (PVA) particles and Gelfoam, although most of the studies have used microcoils (platinum coils), either alone or in conjunction with Gelfoam or PVA.
A study by Evangelista and Hallisey supported the use of superselective embolization as the most effective procedure in reducing complication rates. [32] The investigators reported an 88% success rate and argued against the use of PVA as the sole embolization agent, as the small particles may reach intramural circulation and thus occlude the submucosal plexus beyond the level of collateralization, leading to significant bowel ischemia. An advantage of coils is that they are visible and, therefore, more controllable. [32]
Bandi et al also supported superselective embolization as a feasible, safe, and effective technique for treating acute lower GI hemorrhage. [26] They performed embolization in only those patients in whom they could successfully catheterize the arteria recta leading to the bleeding point. PVA alone (150-500 µm) was used in 28 of these procedures. In 25 patients who underwent objective follow-up with colonoscopy (n = 12), surgery (n = 9), or both (n = 4), mucosal ischemia was demonstrated in 6 (24%) of these patients, but they remained asymptomatic without clinical sequela. No clinically significant bowel ischemia was seen. [26]
Digital subtraction angiography‐guided interventional embolization appears to be most useful in patients who do not respond to conservative treatment, patients who are too high risk for surgery, or patients who are unlikely to tolerate bowel preparation and urgent colonoscopy. [13]
Computed Tomography
Junquera et al used CT angiography (CTA) to evaluate suspected colonic angiodysplasia. [33] The sensitivity of CTA in the detection of colonic angiodysplasia was 70%, its specificity was 100%, and it had 100% positive predictive values, compared with findings of angiography or colonoscopy.
A meta-analysis of 672 patients from 22 studies demonstrated a sensitivity of 85.2% and a specificity of 92.1% for CTA in detecting active acute GI hemorrhage. [34]
In a study by Awais et al, CT with GI bleed protocol was found to be more accurate in detecting and localizing the source of acute lower GI bleeding than technetium-labeled RBC scintigraphy. [35]
CTA can provide immediate noninvasive diagnosis by visualizing the entire GI tract. In a retrospective analysis of lower endoscopy versus CTA, by Clerc et al, median time to CTA was 3 hours for CTA versus 22 hours for lower endoscopy; active bleeding was identified in 31% with CTA versus 15% with lower endoscoy; and a nonactively bleeding source was found by CTA in 22% versus 31% for lower endoscopy. The authors noted that for postinterventional bleeding, urgent lower endoscopy is advocated, but for other causes of lower GI bleeding, CTA was efficient and more available than colonoscopy. [25]
Magnetic Resonance Imaging
In an animal study, Hilfiker et al evaluated the use of 3-dimensional (3-D) MRI. [36] The investigators compared technetium-99m (99mTc)-labeled red blood cell (RBC) scintigraphy with 3-D MRI after the intravascular administration of a contrast agent. MRI had 100% sensitivity and specificity, compared with 78% sensitivity and 72% specificity for scintigraphy. [36]
Hanna et al suggested that magnetic resonance angiography (MRA) may serve as an alternative technique for detecting acute lower GI bleeding when nuclear scintigraphy is unavailable or in younger, radiosensitive patients. [37]
Nuclear Imaging
Scintigraphic bleeding scans are performed by intravenously injecting radiotracers (eg, red blood cells, sulfur colloid) tagged with radioisotopes (eg, technetium-99m) and acquiring images for usually between 60 and 90 minutes. If the findings are positive, 2-D planar images will demonstrate a focus of radiotracer in an abnormal location, which, over time, will change in position because of bowel peristalsis.
The main advantage of this examination is its ability to detect bleeding rates as low as 0.05-0.1 mL/min, as compared to 0.5-1.0 mL/min with angiography. [38] It is highly sensitive and specific, with reported rates of 93% and 95%, respectively. Additionally, it is noninvasive, and no special patient preparation is required. [39, 40, 41]
As with other radiologic examinations, its disadvantages are that detection is dependent on active bleeding. Furthermore, there is a controversy regarding the accuracy of scintigraphic scans at localizing sites of bleeding. One group reported inaccurate localization leading to a surgical error rate of 42%. However, some authors claim that most of the available reports in the literature base their conclusions on results obtained with older techniques. The newer techniques involve greater dynamic imaging (frequent acquisition of data), extra-large field-of-view gamma cameras, and cine scintigraphy or movie-mode displays. Some studies have shown that RBC scans have an accuracy of nearly 90% in the localization of the bleeding site.
Examples of scintigraphic agents include technetium-99m-labeled RBCs and, less commonly, 99mTc sulfur colloid. [42, 43, 44, 45, 46] Although both agents have similar bleeding detection rates, [38] ] 99mTc RBC scanning is felt to be superior to 99mTc sulfur colloid for the following reasons [] [47] :
-
99mTc-labeled RBCs persist longer in the intravascular compartment. improving bleeding site localization
-
Repeat imaging can be performed with 99mTc RBC scanning after an interval of as long as 24 hours
-
99mTc sulfur colloid scans may potentially mask bleeding in the upper abdomen because of activity in the liver and spleen
-
99mTc sulfur colloid scans deliver a larger absorbed dose to organs in the upper abdomen
(The following image is an example of a 99mTc RBC scan.)
 Technetium-99m (99mTc) red blood cell scan shows an abnormal focus of increasing activity in the right lower quadrant, consistent with gastrointestinal bleeding. This activity is seen to increase in intensity over time. The patient underwent angiography.
Technetium-99m (99mTc) red blood cell scan shows an abnormal focus of increasing activity in the right lower quadrant, consistent with gastrointestinal bleeding. This activity is seen to increase in intensity over time. The patient underwent angiography.
Degree of confidence
Scintigraphic bleeding scans are highly sensitive and specific at detecting the presence of active gastrointestinal bleeding, with reported rates of 93% and 95%, respectively. For this reason, scintigraphic bleeding scans are commonly used as preliminary examinations prior to angiography.
As scintigraphic bleeding scans detect lower rates of bleeding, if a scintigraphic scan is negative, an invasive angiographic procedure may potentially be avoided. If the preliminary scan is positive, then studies have shown improved angiographic bleeding detection following positive scintigraphic scans. [47] This was illustrated by Gunderman et al, who described that, without scintigraphic screening, angiograms depicted bleeding at a rate of 22%, which increased to 53% after scintigraphic screening. [48]
In a study of patients with suspected lower GI bleeding, SPECT/CT fusion imaging with 99mTc-labeled red blood cells had a diagnostic sensitivity of 92.9% (52/56) and a positional accuracy value of 92.3% (48/52). [41]
Angiography
The earliest description of the angiographic demonstration of GI bleeding was in 1963. Since then, angiography has been used in the evaluation of GI bleeding, although with varying frequency over time and with the advent of colonoscopy and scintigraphy. [49, 50, 51, 52, 53, 54, 37]
Screen-film arteriography can demonstrate bleeding at rates as low as 0.5 mL/min in dogs, although some authors claim that the actual detectable rate of bleeding in clinical conditions may be in the range of 1.0-1.5 mL/min. Digital subtraction angiography (DSA) has been reported to be more sensitive than conventional screen-film angiography. Rees et al showed that DSA tended to be more sensitive than conventional angiography in depicted simulated extravasation in vitro; however, in the clinical study, DSA was severely limited in evaluation of the lower GI tract because of misregistration artifact caused by bowel motion. [55] In another study, Kruger et al showed similar findings. [56] The investigators reported that DSA was superior to conventional angiography, provided that it is performed with adequate parasympathicolysis and suspended respiration.
On angiograms, hemorrhage is identified as the extravasation of contrast material into the lumen of the bowel (see the following images). The contrast material extravasation can be free or pooling, and it persists during or even after the injection. The angiodysplasia has been described to have a characteristic appearance. It appears as a vascular tuft, along with an early and persistent draining vein.
 Superior mesenteric arterial arteriogram shows extravasation of contrast material from the right colic branch in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
Superior mesenteric arterial arteriogram shows extravasation of contrast material from the right colic branch in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
 Selective arteriogram through a microcatheter further localizes the point of bleeding in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
Selective arteriogram through a microcatheter further localizes the point of bleeding in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
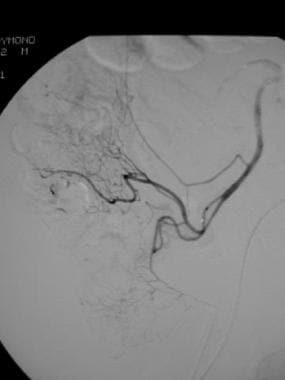 Postembolization selective angiogram in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant. No further extravasation of contrast material is seen. Polyvinyl alcohol particles were used.
Postembolization selective angiogram in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant. No further extravasation of contrast material is seen. Polyvinyl alcohol particles were used.
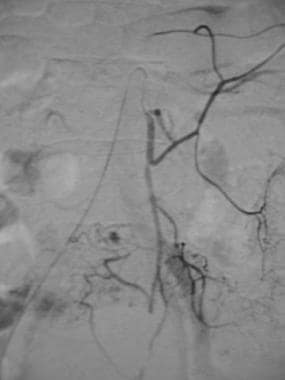 Inferior mesenteric arteriogram in a patient with acute lower gastrointestinal bleeding shows extravasation of contrast material in the sigmoid colon.
Inferior mesenteric arteriogram in a patient with acute lower gastrointestinal bleeding shows extravasation of contrast material in the sigmoid colon.
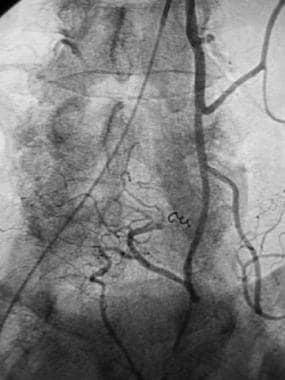 Postembolization arteriogram in a patient with acute lower gastrointestinal bleeding shows no further bleeding (same patient as in previous image). Microcoils and polyvinyl alcohol were used.
Postembolization arteriogram in a patient with acute lower gastrointestinal bleeding shows no further bleeding (same patient as in previous image). Microcoils and polyvinyl alcohol were used.
Degree of confidence
The clinical sensitivity of angiography has been reported variably in different studies. [57] The typical values are around 60%. Attempts to identify predictors for positive angiographic findings have shown mixed results. In a retrospective study, Pennoyer et al did not identify any single useful predictor to increase the likelihood of obtaining a positive angiographic result. [49] Evaluated factors included a history of previous GI bleeding, transfusions, orthostatic hypotension, and tachycardia. However, Nicholson et al found a perfect correlation between a systolic blood pressure of less than 100 mm Hg and a positive arteriographic result. [58] Therefore, some clinicians advocate immediate arteriography rather than nuclear medicine imaging in hemodynamically unstable patients.
Pharmacologic techniques have been used to increase the diagnostic yield of arteriography. These include the use of heparin, vasodilators, and thrombolytics. The reported studies show a 33-65% increase in the yield of angiography. These small studies have not shown any significant complications, although larger studies are needed to prove safety of this method.
Digital subtraction angiography‐guided interventional embolization appears to be most useful in patients who do not respond to conservative treatment, patients who are too high risk for surgery, or patients who are unlikely to tolerate bowel preparation and urgent colonoscopy. [13]
-
Technetium-99m (99mTc) red blood cell scan shows an abnormal focus of increasing activity in the right lower quadrant, consistent with gastrointestinal bleeding. This activity is seen to increase in intensity over time. The patient underwent angiography.
-
Superior mesenteric arterial arteriogram shows extravasation of contrast material from the right colic branch in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
-
Selective arteriogram through a microcatheter further localizes the point of bleeding in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant.
-
Postembolization selective angiogram in a patient whose technetium-99m (99mTc) red blood cell scan showed an abnormal focus of increasing activity in the right lower quadrant. No further extravasation of contrast material is seen. Polyvinyl alcohol particles were used.
-
Inferior mesenteric arteriogram in a patient with acute lower gastrointestinal bleeding shows extravasation of contrast material in the sigmoid colon.
-
Postembolization arteriogram in a patient with acute lower gastrointestinal bleeding shows no further bleeding (same patient as in previous image). Microcoils and polyvinyl alcohol were used.








