Practice Essentials
Budd-Chiari syndrome (BCS) is a rare disease that is characterized by hepatic venous outflow tract obstruction (HVOTO), with an estimated incidence of 0.87 per million population per year. Most patients with Budd-Chiari syndrome have an underlying thrombotic diathesis, although in approximately one third of patients, the condition is idiopathic. Thus, it may be a primary venous problem or an intra/extrahepatic space-occupying lesion compressing/invading the venous outflow. Causes include protein C deficiency, protein S deficiency, antithrombin deficiency, factor V Leiden mutation, myeloproliferative disorders, paroxysmal nocturnal hemoglobinuria, and malignancy. [1, 2, 3, 4] The HVOTO usually occurs at the level of the inferior vena cava (IVC), the hepatic veins, and possibly at the venule level (see the images below). Obstruction of large- or small-caliber veins leads to hepatic congestion as blood flows into, but not out of, the liver. [5, 6, 7]
(See the images below.)
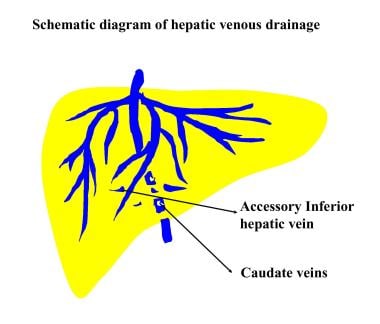 Diagram of hepatic venous drainage depicts the small veins that drain from the caudate lobe and adjacent part of the right lobe directly into the inferior vena cava. The veins tend to be spared in hepatic venous occlusion in patients with Budd-Chiari syndrome, giving rise to hypertrophy of the caudate lobe and adjacent part of the right lobe.
Diagram of hepatic venous drainage depicts the small veins that drain from the caudate lobe and adjacent part of the right lobe directly into the inferior vena cava. The veins tend to be spared in hepatic venous occlusion in patients with Budd-Chiari syndrome, giving rise to hypertrophy of the caudate lobe and adjacent part of the right lobe.
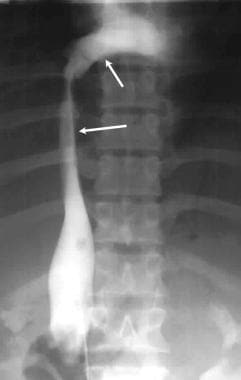 Inferior venacavogram shows compression and lateral displacement of the inferior vena cava by an enlarged caudate lobe.
Inferior venacavogram shows compression and lateral displacement of the inferior vena cava by an enlarged caudate lobe.
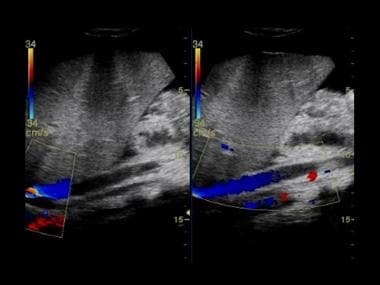 Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy who presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy who presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
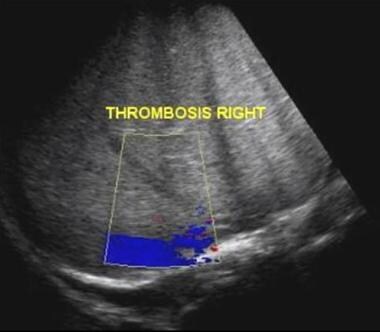 Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy that presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy that presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
Preferred examination
Digital subtraction angiography (DSA), although invasive, is the criterion standard. This examination may have to be performed particularly in patients in whom an IVC abnormality is suspected and when radiologic intervention is planned. On the whole, plain radiography has little to offer in the diagnosis of Budd-Chiari syndrome. Ultrasonography is noninvasive and has high sensitivity and specificity. Ultrasonographic images easily depict the echogenic membrane or fibrous cord in the IVC, which is a common cause of chronic BCS. Computed tomography (CT) scanning and magnetic resonance imaging (MRI) are making inroads, but diagnostic features are seen in only a minority of patients, although the cross-sectional images are superior and are vital in planning intervention. Radionuclide scanning is an elegant and noninvasive technique; however, its findings are nonspecific. [8, 9, 10, 11, 12, 13, 14, 15, 16]
The key imaging findings in BCS, including those of CT scanning, MRI, ultrasonography, and angiography, are occlusion of the hepatic veins, the IVC, or both [17] ; caudate lobe enlargement; inhomogeneous liver enhancement; and the presence of intrahepatic collateral vessels and hypervascular nodules. Awareness of these findings is important for early diagnosis and appropriate treatment. [18, 19]
All findings from cross-sectional imaging are nonspecific. False-positive and false-negative diagnoses may occur, although this limitation applies less to angiographic studies than to other tests.
Radiography
Plain radiography has little to contribute to the diagnosis of Budd-Chiari syndrome (BCS). Features of ascites, hepatosplenomegaly, and causes of BCS (eg, hepatocellular carcinoma with calcification or renal cell carcinoma with calcification) may be depicted. Calcification within a thrombosed hepatic vein is unusual.
When associated with concomitant portal vein thrombosis, calcification may be seen in the portal vein after prolonged portal hypertension. Portal vein calcification is typically linear or strandlike and lies transversely across the upper abdomen or slopes upward and obliquely toward the liver hilum.
Esophageal varices can be seen as lobulated posterior mediastinal masses in 5-8% of patients. Silhouetting of the descending aorta and an abnormal convex contour of the azygos-esophageal recess are further signs of portal hypertension. Varices, if present, can be confirmed on an upper GI barium series.
Most plain radiographic findings have low sensitivity and are nondiagnostic. The causes of ascites, portal hypertension, and hepatosplenomegaly are legion and cannot be separated on the basis of plain radiographic findings.
Computed Tomography
Contrast-enhanced CT scan findings in patients with Budd-Chiari syndrome (BCS) include an inhomogeneous mottled liver with delayed enhancement in the periphery of the liver and around the hepatic veins. [8]
The peripheral zones of the liver may appear hypoattenuating because of reversed portal venous blood flow, which results from increased postsinusoidal pressure produced by hepatic venous obstruction.
The caudate lobe is enlarged (caudate lobe hypertrophy) and demonstrates increased contrast enhancement as compared to the rest of the liver. Identification of hepatic veins may fail. Thrombosis within the hepatic veins and the IVC can be identified in 18-53% of patients. [20, 21]
(CT scans demonstrating the features of BCS appear below.)
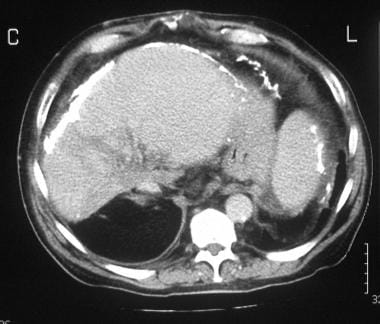 A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, a contrast-enhanced CT scan of the upper abdomen was performed and shows extensive liver/splenic capsular and peritoneal calcification and patchy attenuation within the liver. The left lobe/caudate lobe appears hypertrophied.
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, a contrast-enhanced CT scan of the upper abdomen was performed and shows extensive liver/splenic capsular and peritoneal calcification and patchy attenuation within the liver. The left lobe/caudate lobe appears hypertrophied.
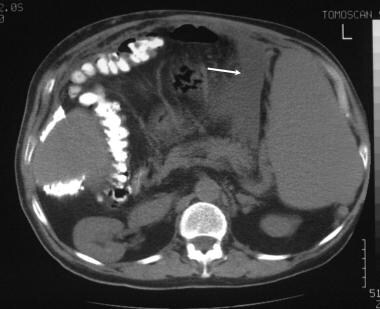 A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, unenhanced CT scan was performed and shows splenomegaly and a small ascites medial to the spleen (arrow).
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, unenhanced CT scan was performed and shows splenomegaly and a small ascites medial to the spleen (arrow).
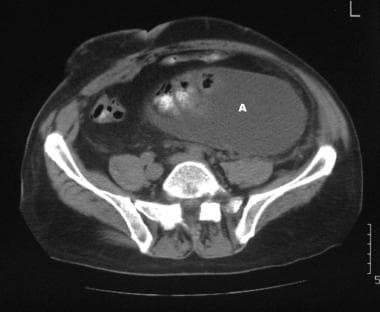 A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, unenhanced CT scan of the pelvis was performed and shows a loculated ascites, which was responsible for a displaced bladder and right ureter.
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, unenhanced CT scan of the pelvis was performed and shows a loculated ascites, which was responsible for a displaced bladder and right ureter.
Degree of confidence
Although CT scan appearances of BCS are nonspecific, certain features, such as thrombosis within the hepatic veins and the IVC, are diagnostic of BCS when they are seen in the appropriate clinical setting. [20, 21]
When spiral CT scans are being interpreted, it is important not to confuse nonopacified hepatic veins and IVC with hepatic venous occlusion, especially in patients with portal hypertension, in whom enhancement of hepatic veins may be delayed.
Causes of an enlarged caudate lobe (caudate lobe hypertrophy) include the following:
-
Cirrhosis
-
Hepatic venous occlusion in which venous drainage from the caudate lobe to the IVC is maintained by emissary veins
-
Focal mass lesions within the caudate lobe
Cirrhosis of any kind can result in what appears to be an enlarged caudate lobe (caudate lobe hypertrophy). Cirrhosis often has companion findings of right or left lobar atrophy, irregular lobular liver contour, ascites, and varices. In cirrhosis and other causes of a shrunken liver, relative sparing of the caudate lobe often occurs, so that the caudate lobe looks large in relation to the remaining liver.
The caudate lobe is spared in cases of hepatic venous occlusion in which venous drainage from the caudate lobe to the IVC is maintained by emissary veins. An enlarged caudate lobe may narrow the intrahepatic portion of the IVC.
Lesions such as cysts, abscess, or neoplasm may enlarge the caudate lobe. Fatty infiltration confined to the caudate lobe may mimic a tumor. Traumatic fractures of the caudate lobe may occur but are rare. Inhomogeneous contrast enhancement may occur in cirrhosis, diffuse liver metastasis, and hepatitis.
Magnetic Resonance Imaging
On MRI scans, findings of Budd-Chiari syndrome (BCS) may manifest themselves as regional differences in signal intensity because of varying perfusion, atrophy, hypertrophy, necrosis, and differences in the amount of intracellular fat or iron
(See the images below.)
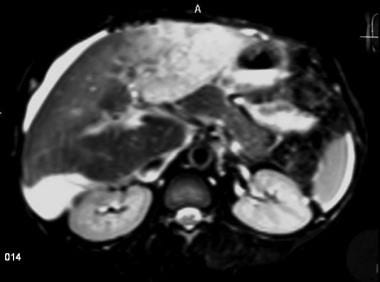 Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
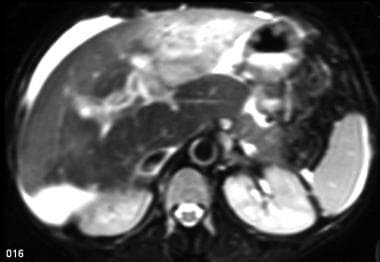 Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
 Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
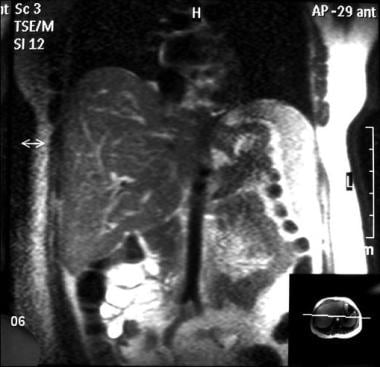
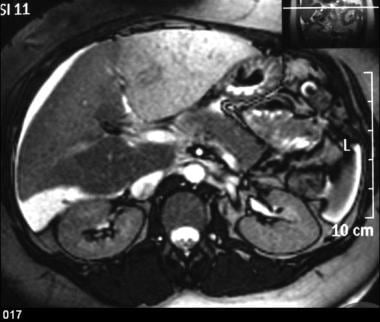 Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
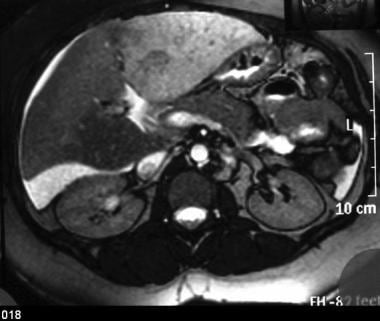 Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
 Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
The liver parenchyma appears inhomogeneous in 64% of patients. The atrophic peripheral liver typically demonstrates low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.
After the administration of an MRI contrast agent, enhancement of the liver periphery is variable. In patients with long-standing disease, nodular regenerative hyperplasia is seen as large nodules with increased signal intensity on T1-weighted images and low to intermediate signal intensity on T2-weighted images.
In acute BCS, the peripheral part of the liver often demonstrates decreased enhancement. Standard MRI findings reported in patients with BCS include hepatic vein thrombosis, hepatic vein occlusion and narrowing, hepatomegaly, atrophy of the right lobe of the liver, and enlargement of the caudate lobe. IVC abnormalities shown on MRI include diffuse narrowing and focal thrombosis.
Venous collaterals are readily shown. Comma-shaped intrahepatic varices are a characteristic finding that is commonly seen on coronal scans but is not readily appreciated by other modalities.
Ren et al used magnetic resonance angiography (MRA) with an FBI (fresh blood imaging) sequence to evaluate FBI's validity in the preoperative assessment of 50 patients with suspected BCS after they had been checked by B-ultrasonography. [9] The FBI results were the following: a correct diagnosis for 38 patients, an incorrect diagnosis for 1 patient, and 3 missed diagnoses. The diagnostic sensitivity of the FBI in this study was 93%; the specificity, 89%; and the accuracy, 92%.
In a meta-analysis of the diagnostic performance of MRA in patients with BCS, using digital subtraction angiography as reference, the pooled MRA estimates had a sensitivity of 97.6% [95% confidence interval (CI), 95.1-99.0%], a specificity of 70.7% (95% CI, 54.5-83.9%), a positive likelihood ratio (LR+) of 3.163 (95% CI, 2.03-4.94), and an LR- of 0.045 (95% CI, 0.02-0.09). The overall diagnostic odds ratio was 94.053 (95% CI, 32.71-270.41). The area under the curve was 0.972. [14]
This Ren et al study showed that FBI can identify a membranous stenosis and an obstruction and thrombosis of the IVC. It can also demonstrate thickening of the flexural hepatic vein and development of intrahepatic compensatory branches with slow blood flow. FBI can therefore guide the puncturing and opening of the hepatic vein involved in an interventional therapy for BCS patients. [9]
MRI examinations in 22 patients with pathologically confirmed BCS showed the following [22] :
-
Thrombosis of 3 hepatic veins in 19 patients (86%) and 2 hepatic veins in 3 patients (14%)
-
Spontaneous intrahepatic anastomoses in 5 patients (23%)
-
Ascites in 15 patients (68%)
-
Thrombosis of the IVC by an enlarged caudate lobe in 6 patients (27%) and external compression of the IVC by an enlarged caudate lobe in 5 patients (23%)
-
Prominent azygos and hemiazygos veins in 7 patients (32%), 6 of whom had thrombosis of the IVC
-
Hepatomegaly in all patients and enlarged caudate lobe in 18 patients (82%)
-
Inhomogeneous signal intensity of hepatic parenchyma in 14 patients, revealed on T1- and T2-weighted MRI scans (64%)
-
Homogeneous signal intensity of hepatic parenchyma in 8 patients (36%), revealed on T1- and T2-weighted MRI scans
Degree of confidence
MRI techniques are noninvasive and provide excellent multiplanar imaging. Blood vessels can be depicted with or without the administration of contrast agents. However, unlike ultrasonography, MRI has limited availability, and the procedure is expensive. The exact sensitivity and specificity of MRI in the diagnosis of BCS are unknown.
Primary BCS must be distinguished from cirrhosis and pulmonary heart disease. In severe congestive heart failure and right-sided heart failure, patchy enhancement and congestive liver changes similar to those depicted in BCS may occur; however, the hepatic veins are enlarged rather than attenuated.
Ultrasonography
Color-flow Doppler ultrasonography
Color-flow Doppler (CFD) images demonstrate abnormality of the anatomy or flow, such as the following:
-
Part of or the entire right hepatic vein (regardless of apparent intraluminal echoes), with no flow or inappropriately directed flow
-
No depiction of part or all of a hepatic vein using either conventional ultrasonography or CFD of the right side of the liver
-
Discontinuity between the main hepatic vein and the IVC
-
Reversed flow in hepatic veins (ie, bicolor hepatic veins)
-
Intrahepatic collaterals
-
Portal vein changes
-
IVC changes
Regarding intrahepatic collaterals, these include intrahepatic veins that communicate with systemic vessels via subcapsular collaterals, collateral vessels that shunt blood from occluded veins to nonoccluded veins or to enlarged inferior hepatic veins or caudate lobe veins, and large collaterals that drain directly into the IVC. Collateralization is suggested when reversed flow is found, which may be seen before and after shunting.
When portal vein changes occur, the flow may be hepatopetal or hepatofugal; portal flow dynamics may change between examinations.
When IVC changes occur, there may be no flow, reduced flow, very slow flow, balanced bidirectional flow, thrombus or tumor within the IVC, compression of the caudate lobe, long or localized segmental narrowing, or echogenic membrane or fibrous cord in the IVC (easily depicted by CFD), a common cause of chronic Budd-Chiari syndrome (BCS).
(For color ultrasonographic scans of BCS, see the images below.)
 Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy who presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy who presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
 Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy that presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy that presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
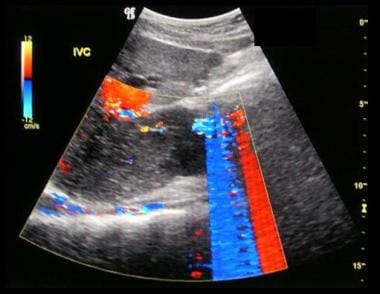 Budd-Chiari syndrome: Six ultrasound images (see the next 5 images) of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
Budd-Chiari syndrome: Six ultrasound images (see the next 5 images) of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
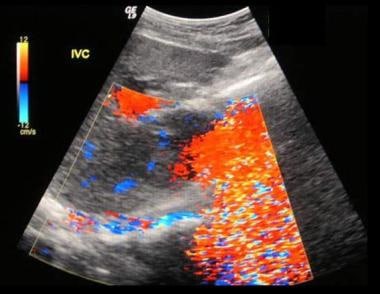 Budd-Chiari syndrome: The second of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
Budd-Chiari syndrome: The second of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
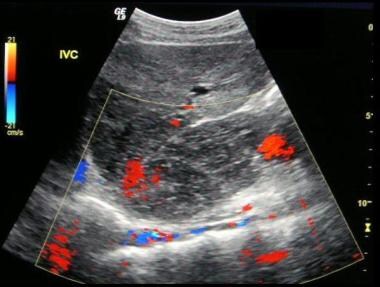 Budd-Chiari syndrome: The third of six ultrasound images of a 28-year-old female that presented who a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
Budd-Chiari syndrome: The third of six ultrasound images of a 28-year-old female that presented who a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
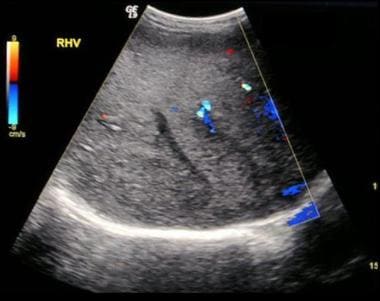 Budd-Chiari syndrome: The fourth of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
Budd-Chiari syndrome: The fourth of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
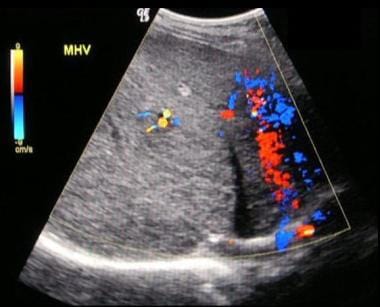 Budd Chiari Syndrome: The fifth of six ultrasound images on a 28-year old female that presented with a non-specific illness and abnormal liver function tests. The Ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly and varices at the splenic hilum.
Budd Chiari Syndrome: The fifth of six ultrasound images on a 28-year old female that presented with a non-specific illness and abnormal liver function tests. The Ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly and varices at the splenic hilum.
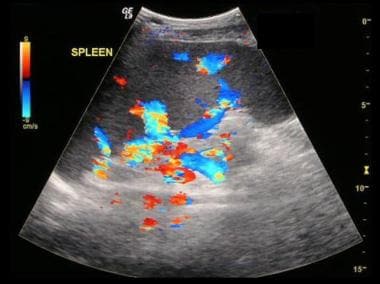 Budd-Chiari syndrome: The last of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
Budd-Chiari syndrome: The last of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
Conventional ultrasonography
Conventional ultrasonography may show gallbladder wall thickening, ascites, a patchy liver echo pattern, splenomegaly, and a hypertrophied caudate lobe.
Veno-occlusive disease
Veno-occlusive disease of the liver may be regarded as a variant of BCS. Unlike classic BCS, occlusion of the IVC or major hepatic veins does not occur. The basic pathophysiology is related to occlusion of the postsinusoidal venules by an inflammatory process.
Earlier research suggested that it would be possible to establish a diagnosis using ultrasonography, because the modality demonstrates an increase in the resistive index in the hepatic artery and a decrease or reversal of flow in the portal vein. However, the results of later research were disappointing, reflecting the nonspecificity of the signs mentioned above. Thus, veno-occlusive disease remains a clinical or histologic diagnosis.
Transjugular intrahepatic portosystemic shunts
Transjugular intrahepatic portosystemic shunts (TIPS) are placed percutaneously via the jugular vein. TIPS placement is becoming popular as a definitive procedure for decompressing the portal venous system or as prelude to liver transplantation.
Doppler ultrasonography is a sensitive and relatively specific means used to evaluate TIPS malfunction. Ultrasonographic evaluation of the shunt usually is performed within 24 hours after shunt placement to establish baseline velocities within the portal vein, hepatic vein, and shunt. Follow-up studies usually are performed every 3 months unless the clinical setting dictates a more emergent examination. The main object of Doppler study of a TIPS is to document flow in the shunt and to search for stenosis. [23]
The accuracy of Doppler ultrasonography in depicting shunt malfunction depends on several ultrasonographic parameters, which include changes in peak shunt velocity, distal shunt velocity, and portal vein velocity, as well as the presence of antegrade flow in the left and right portal veins. Flow velocities in the portal vein may double, compared to preoperative velocities, with successful TIPS placement. Direct observation of shunt thrombosis is possible on color duplex Doppler images. Echo-enhanced color Doppler ultrasonography also can be helpful in the assessment of TIPS.
Complications of TIPS that are detectable on ultrasonographic images include early complications and delayed complications.
Early complications include the following:
-
Intraperitoneal hemorrhage
-
Shunt thrombosis
-
Neck hematoma
-
Compromise of hepatic blood supply
-
Portal vein thrombosis
-
Hepatic artery occlusion
-
Hepatic infarction
-
Failure of stent deployment
-
Inadequate stent expansion
-
Stent retraction
-
Stent fracture
-
Biliary obstruction
Delayed complications (shunt stenosis) include pseudointimal hyperplasia and hepatic vein stenosis.
Degree of confidence
Ultrasonography is noninvasive and has high sensitivity and specificity. Ultrasonographic images easily depict the echogenic membrane or fibrous cord in the IVC, which is a common cause of chronic BCS.
Ultrasonography is not an accurate means of measuring the pressure gradient in the IVC, but because the choice of an appropriate decompressive shunt (mesoatrial vs portocaval or mesocaval) depends on the presence of a pressure gradient within the IVC, venography is likely to remain an important part of the evaluation of BCS in any patient in whom surgical decompression is considered.
Duplex ultrasonography of the hepatic veins may be useful for studying liver disease associated with fibrosis and steatosis. [24] In patients with well-compensated liver disease, flattening of the Doppler waveform suggests the presence of cirrhosis.
The differential diagnosis of hepatofugal portal venous flow includes BCS, portal hypertension, side-to-side portocaval shunts, surgical/spontaneous splenorenal shunts with cirrhosis, tricuspid regurgitation (tricuspid flow reversal), and severe congestive cardiac failure.
Passive hepatic congestion due to compromise of liver venous drainage causes passive hepatic venous congestion, which is not an uncommon complication of congestive heart failure and constrictive pericarditis, in which the elevated central venous pressure is transmitted from the right atrium to the hepatic veins. Passive hepatic congestion often is accompanied by hepatomegaly. Hepatocytes are extremely sensitive to ischemic injury, even over short periods. A variety of cardiac-related circulatory disorders may cause ischemic injury in the liver.
On ultrasonographic images, the IVC is depicted as distended and associated with dilated hepatic veins. The normal variation of IVC caliber during respiration is lost. Ascites may occur. The portosplenic veins may show variable degrees of dilatation. Hepatomegaly may occasionally be associated with mild to moderate splenomegaly.
Echogenicity of the liver varies from echo poor to bright. In acute venous congestion, the starry-sky appearance may occur. Pleural and/or pericardial effusions are often present.
Cardiomegaly and cardiac rhythm abnormalities may be depicted on liver scans.
Nuclear Imaging
As a result of retained venous drainage and, hence, comparatively normal hepatic function of the caudate lobe, technetium-99m (99mTc) sulfur colloid uptake is increased (ie, hot) in the caudate lobe at the expense of the rest of the liver, in which uptake may be normal, reduced, absent, or patchy. Often, colloid shifts to the spleen and bone marrow. Wedge-shaped focal peripheral defects are occasionally identified.
(See the images below.)
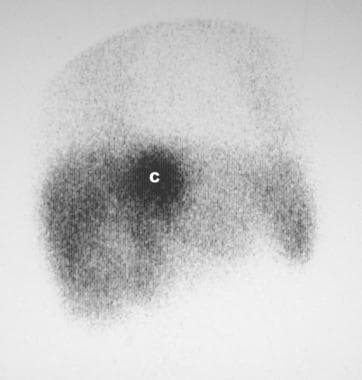 Technetium-99m sulfur colloid scan in a 53-year-old man who presented with nonspecific symptoms and a mild swelling of the legs. The patient had no features of heart failure. Scan shows patchy radionuclide uptake in most of the liver but intense activity in the region of the caudate lobe. Analysis of a sample from percutaneous liver biopsy revealed histologic features of Budd-Chiari syndrome.
Technetium-99m sulfur colloid scan in a 53-year-old man who presented with nonspecific symptoms and a mild swelling of the legs. The patient had no features of heart failure. Scan shows patchy radionuclide uptake in most of the liver but intense activity in the region of the caudate lobe. Analysis of a sample from percutaneous liver biopsy revealed histologic features of Budd-Chiari syndrome.
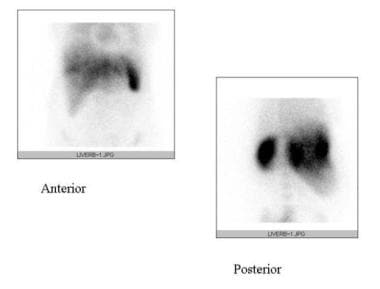 Budd-Chiari syndrome. Technetium-99M sulfur colloid scan of the liver shows peripheral area's diminished uptake due to liver atrophy and intense activity in the caudate lobe due to hypertrophy in a patient with BCS.
Budd-Chiari syndrome. Technetium-99M sulfur colloid scan of the liver shows peripheral area's diminished uptake due to liver atrophy and intense activity in the caudate lobe due to hypertrophy in a patient with BCS.
Tc-99m sulfur colloid scanning is an elegant, noninvasive technique in which findings may not only suggest the diagnosis of Budd-Chiari syndrome (BCS) but also provide a rough index of liver function and the presence or absence of splenomegaly.
Diehl and associates describe a patient with primary hepatic lymphoma imaged with CT, MRI, ultrasound, and fluorodeoxyglucose (FDG) positron emission tomography (PET) images, which characterized primary hepatic lymphoma and demonstrated hepatic vein thrombus that extended into the inferior vena cava—a feature not previously described. Recognition of this imaging pattern may help in the differential diagnosis of future such cases. FDG-PET was instrumental in confirming the diagnosis, staging, and treatment response. [25]
False positives/negatives
Hot spots depicted on 99mTc sulfur colloid scans may appear within healthy liver segments in patients with diffuse liver disease resulting from any cause.
Superior vena caval obstruction (not associated with BCS) is a far less frequent cause of a hot spot within the liver and is produced by collateral flow through the intercostal to the paraumbilical vein, hepatic veins, and right atrium.
Between the paraumbilical and hepatic veins, radionuclide uptake may occur in Kupffer cells within a localized area of the liver.
Increased activity within focal nodular hyperplasia is a well-known phenomenon and exploited diagnostically.
Angiography
Hepatic and celiac-axis angiograms reveal hepatosplenomegaly; intrahepatic arteries are markedly stretched and bowed by the edematous liver. In patients with long-standing disease, the hepatic arteries may dilate, and arteriovenous shunting may develop.
The hepatogram phase may be intense and prolonged and may demonstrate a mottled appearance. Large lakes of sinusoidal contrast accumulation are occasionally seen. Progression of contrast material through the liver may be slow, often with outflow through the portal vein, which has been termed the cul-de-sac phenomenon.
Opacification of splenic and portal veins is usually faint, while being nonetheless adequate to demonstrate portal vein patency and hepatopetal flow. Flow may become bidirectional or centrifugal in chronic disease. Portography may demonstrate central hepatic enhancement of the liver with normal hepatopetal flow. Splenoportographic findings depend on the duration of disease. In early stages, when the flow within the portal vein is centripetal, the portal vein radicles may be stretched, and emptying is delayed. In later stages, portal venous flow reverses, and the splenic and portal veins may not fill.
Generally, arteriography is not recommended in patients with Budd-Chiari syndrome (BCS) and usually is performed to investigate hepatosplenomegaly and/or portal hypertension in patients in whom the diagnosis of BCS is not suspected.
Major vascular findings in Budd-Chiari syndrome
Major vascular findings in patients with BCS occur on inferior venacavography and hepatic venography. The IVC may demonstrate occlusion by a thrombus or tumor at the renal or hepatic level. This appearance usually is adequate for a diagnosis. If the IVC is occluded, the hepatic veins may be catheterized via the jugular/axillary veins through the right atrium.
Inferior venacavograms show a variety of changes: (1) in the acute form, liver swelling usually gives rise to constriction of the intrahepatic IVC; (2) on the anteroposterior view, the IVC may appear as a contrast-filled, thin, dense, stringlike structure reaching the diaphragm; (3) IVC obstruction by a web may be observed; and (4) IVC obstruction from a tumor or thrombus may be seen.
A hepatic venogram obtained with selective catheterization of a hepatic vein may show the following changes:
-
Fine-mesh spiderweb appearance - Resulting from the many collateral channels that develop between hepatic venules and systemic veins.
-
Coarse-mesh spiderweb pattern - When changes as above are seen, but an intrahepatic network of collaterals cover larger spaces of 1-2 cm in diameter.
-
Hepatic vein stenosis - Usually occurs near the orifice of the major hepatic veins; collaterals can be seen coursing around the stenosis.
-
Hepatic vein occlusion - Caused by tumor, which is most commonly associated with hepatocellular carcinoma.
-
Veno-occlusive disease (Senecio) pattern - Pattern from contrast injection; similar to that found in the coarse-mesh spiderweb pattern, except the major hepatic veins are patent. In the veno-occlusive disease pattern, the use of contrast enhancement shows a patent, but narrowed, hepatic vein.
Direct-puncture hepatogram
If the roots to the hepatic veins are occluded, direct-puncture hepatogram may be performed, in which the liver is accessed directly by needle puncture and contrast is injected into the liver parenchyma. The contrast material usually breaks through into the proximal hepatic venules, subsequently filling the collateral channels and resulting in the spiderweb pattern.
(Angiograms of BCS are shown below.)
 Inferior venacavogram shows compression and lateral displacement of the inferior vena cava by an enlarged caudate lobe.
Inferior venacavogram shows compression and lateral displacement of the inferior vena cava by an enlarged caudate lobe.
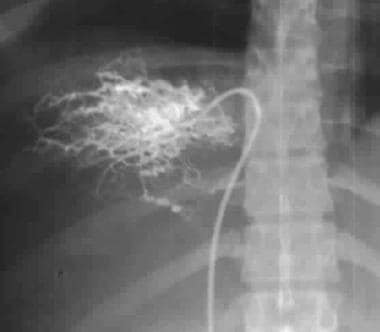 Wedged hepatic venogram shows a coarse-mesh spiderweb pattern resulting from an intrahepatic network of collateral vessels.
Wedged hepatic venogram shows a coarse-mesh spiderweb pattern resulting from an intrahepatic network of collateral vessels.
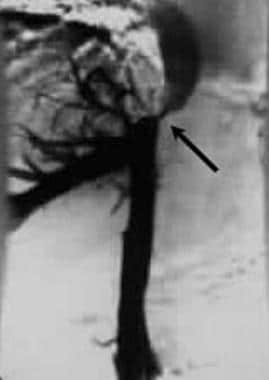 Inferior venacavogram shows an upper inferior vena cava stenosis with reflux of the contrast into the hepatic venous circulation due to partial obstruction of the distal inferior vena cava.
Inferior venacavogram shows an upper inferior vena cava stenosis with reflux of the contrast into the hepatic venous circulation due to partial obstruction of the distal inferior vena cava.
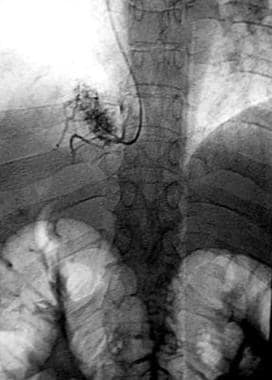 Conventional wedged hepatic venogram showing absence of the right hepatic vein and a spiderweb pattern of collaterals and recanalized veins.
Conventional wedged hepatic venogram showing absence of the right hepatic vein and a spiderweb pattern of collaterals and recanalized veins.
Degree of confidence
Arteriographic and splenoportographic findings may demonstrate the cause of hepatic venous occlusion. Inferior venacavography and hepatic venography findings may be specific for BCS. The greatest benefit of these procedures is accrued with the diagnosis of a web across the hepatic vein or IVC, because no other modality can diagnose this disorder with confidence.
The examination is safe. A transient elevation of serum enzyme levels has been reported after wedged hepatic venography, but the evaluation appears to be of no consequence. Ultrasonography is making inroads in the diagnosis of BCS, but venography is likely to remain an important part of the evaluation of BCS in any patient in whom surgical decompression is considered.
The arterial pattern in BCS is similar to that in alcohol-induced hepatitis.
The cul-de-sac phenomenon also may be seen in patients with heart failure; however, this can be excluded on clinical grounds.
Marked narrowing of the IVC may occur in patients with cirrhosis; however, unlike the predominantly side-to-side compression found in BCS, the narrowing is usually circumferential.
Unilobar BCS is a rare occurrence, in which hepatic venous occlusion is asymmetrical and incomplete.
Occlusion gives rise to intrahepatic flow competition, and portoportal and hepatic vein–portal shunts give rise to unusual contrast parenchymal patterns, leading to difficulties in diagnosis.
-
Diagram of hepatic venous drainage depicts the small veins that drain from the caudate lobe and adjacent part of the right lobe directly into the inferior vena cava. The veins tend to be spared in hepatic venous occlusion in patients with Budd-Chiari syndrome, giving rise to hypertrophy of the caudate lobe and adjacent part of the right lobe.
-
Inferior venacavogram shows compression and lateral displacement of the inferior vena cava by an enlarged caudate lobe.
-
Wedged hepatic venogram shows a coarse-mesh spiderweb pattern resulting from an intrahepatic network of collateral vessels.
-
Inferior venacavogram shows an upper inferior vena cava stenosis with reflux of the contrast into the hepatic venous circulation due to partial obstruction of the distal inferior vena cava.
-
Following placement of a wall stent in the upper inferior vena cava, a wide patency of the inferior vena cava is seen. Note the lack of reflux of contrast into the hepatic veins.
-
A 53-year-old man presented with nonspecific symptoms and a mild swelling of the legs. He had no features of heart failure. Abdominal B-mode sonogram shows a distended inferior vena cava with intraluminal filling defects suggestive of a thrombus within the inferior vena cava. On a thorough search, no hepatic veins were identified, but the liver presented a heterogeneous echo pattern, although no discrete mass was identified.
-
Technetium-99m sulfur colloid scan in a 53-year-old man who presented with nonspecific symptoms and a mild swelling of the legs. The patient had no features of heart failure. Scan shows patchy radionuclide uptake in most of the liver but intense activity in the region of the caudate lobe. Analysis of a sample from percutaneous liver biopsy revealed histologic features of Budd-Chiari syndrome.
-
A 42-year-old man presented with an intractable ascites of unknown cause before CT scanning and gray-scale ultrasonography were available. The patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. At one stage, the symptoms were urological, and an intravenous urogram was performed, which shows right hydronephrosis and displacement of the right lower ureter and bladder to the right. Note the unusual linear calcification in the right upper quadrant. Many years after the initial symptoms appeared, imaging techniques had advanced.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, sagittal ultrasound was performed in the patient's liver and shows 2 hepatic veins, which appeared pruned, with no ramifications.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, a contrast-enhanced CT scan of the upper abdomen was performed and shows extensive liver/splenic capsular and peritoneal calcification and patchy attenuation within the liver. The left lobe/caudate lobe appears hypertrophied.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, unenhanced CT scan was performed and shows splenomegaly and a small ascites medial to the spleen (arrow).
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, unenhanced CT scan of the pelvis was performed and shows a loculated ascites, which was responsible for a displaced bladder and right ureter.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, T1-weighted axial MRI was performed through the liver and shows a low signal within the right liver. The left lobe/caudate lobe is hypertrophied. Note the misshapen inferior vena cava.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, T2-weighted axial MRI was performed through the liver and shows a high signal within the right liver. The left lobe/caudate lobe is hypertrophied. Note the misshapen inferior vena cava.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, axial short-tau inversion recovery MRI was performed through the liver and shows a high signal (edema) within the right liver. The left lobe/caudate lobe is hypertrophied. Note the misshapen inferior vena cava.
-
A 42-year-old man presented with an intractable ascites of unknown cause many years ago. At the time, the patient had repeated juguloperitoneal shunt placements for relief of the ascites. The ascites diminished, but the patient experienced persistent vague abdominal symptoms. When imaging techniques evolved, a wedged hepatic venogram was performed and shows a fine-mesh spiderweb pattern resulting from an intrahepatic network of collateral vessels. At this stage, direct questioning elicited that the patient had been accidentally exposed to radiation in the late 1950s. Analysis of a biopsy specimen confirmed Budd-Chiari syndrome. The cause of the capsular/peritoneal calcification could not be determined, but 2 factors may have been the cause: radiation damage or the repeated placement of jugular peritoneal shunts.
-
Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy who presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
-
Budd-Chiari syndrome: Two ultrasound images from a 13-year old boy that presented with jaundice, abdominal distention, and features of hepatic encephalopathy and sepsis. Ultrasound showed bilateral pleural effusions, ascites, and no flow within the hepatic veins but a patent IVC.
-
Budd-Chiari syndrome: Six ultrasound images (see the next 5 images) of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
-
Budd-Chiari syndrome: The second of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
-
Budd-Chiari syndrome: The third of six ultrasound images of a 28-year-old female that presented who a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
-
Budd-Chiari syndrome: The fourth of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
-
Budd Chiari Syndrome: The fifth of six ultrasound images on a 28-year old female that presented with a non-specific illness and abnormal liver function tests. The Ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly and varices at the splenic hilum.
-
Budd-Chiari syndrome: The last of six ultrasound images of a 28-year-old female who presented with a nonspecific illness and abnormal liver function tests. The ultrasound scans show no flow in hepatic veins, compressed IVC, enlarged caudate lobe, splenomegaly, and varices at the splenic hilum.
-
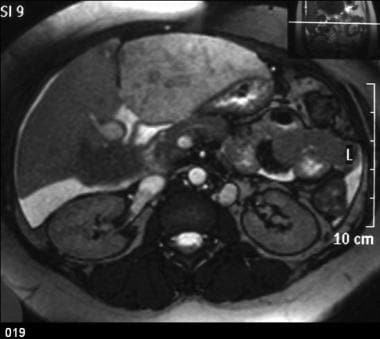
Budd-Chiari syndrome: Contrast-enhanced axial CT scan showing a large ascites, patchy enhancement of the liver, an enlarged caudate lobe, attenuated hepatic veins, hepatic vein–portal vein shunts, and a thrombus within the intrahepatic IVC.
-
Budd-Chiari syndrome: Contrast-enhanced axial CT scan showing a large ascites, patchy enhancement of the liver, an enlarged caudate lobe, attenuated hepatic veins, hepatic vein–portal vein shunts, and a thrombus within the intrahepatic IVC.
-
Budd-Chiari syndrome: Contrast-enhanced axial CT scan showing a large ascites, patchy enhancement of the liver, an enlarged caudate lobe, attenuated hepatic veins, hepatic vein–portal vein shunts, and a thrombus within the intrahepatic IVC.
-
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
-
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
-
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
-
Acute Budd-Chiari syndrome with recovery. A 36-year-old female patient with known Behcet's disease presented with abdominal distention, leg edema, and abnormal liver function tests. Nonenhanced in-phase T2WI shows heterogeneity of the liver parenchyma. Note the thrombus in the IVC and the ascites.
-
Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
-
Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
-
Gadolinium-enhanced out-of-phase T2WIs demonstrate the inhomogeneous perfusion of the liver, which is characteristic of the disease. The IVC thrombosis is extending to the right renal vein.
-
Post-gadolinium in-phase T2WI shows the attenuation of the hepatic veins with the typical late peripheral enhancement of the liver.
-
Post-gadolinium in-phase T1WI showing the hypertrophied caudate lobe compressing the intra-hepatic IVC
-
Follow-up T2WI scans 10 months later, showing homogeneous liver parenchyma and partial recanalization of the IVC. The caudate lobe is almost back to its normal size, and the compression on the IVC is significantly reduced. The ascites is no longer seen.
-
Follow-up T2WI scans 10 months later, showing homogeneous liver parenchyma and partial recanalization of the IVC. The caudate lobe is almost back to its normal size, and the compression on the IVC is significantly reduced. The ascites is no longer seen.
-
Follow-up T2WI scans 10 months later, showing homogeneous liver parenchyma and partial recanalization of the IVC. The caudate lobe is almost back to its normal size, and the compression on the IVC is significantly reduced. The ascites is no longer seen.
-
Follow-up T2WI scans 10 months later, showing homogeneous liver parenchyma and partial recanalization of the IVC. The caudate lobe is almost back to its normal size, and the compression on the IVC is significantly reduced. The ascites is no longer seen.
-
Follow-up T2WI scans 10 months later, showing homogeneous liver parenchyma and partial recanalization of the IVC. The caudate lobe is almost back to its normal size, and the compression on the IVC is significantly reduced. The ascites is no longer seen.
-
Budd-Chiari syndrome. Technetium-99M sulfur colloid scan of the liver shows peripheral area's diminished uptake due to liver atrophy and intense activity in the caudate lobe due to hypertrophy in a patient with BCS.
-
Conventional wedged hepatic venogram showing absence of the right hepatic vein and a spiderweb pattern of collaterals and recanalized veins.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.
-
A 26-year-old man with known Budd-Chiari syndrome palliated with a transjugular intrahepatic portosystemic shunt (TIPS) procedure. He had 2 revisions following hepatic vein angioplasty. The images provided are procedural and contrast-enhanced CT scans following the TIPS procedure. Note the inhomogeneous enhancement, mimicking metastases.






