Practice Essentials
Hemolytic-uremic syndrome (HUS) was first described by Gasser in a German publication in 1955. Hemolytic-uremic syndrome consists of the triad of microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure. [1] Since 1955, thousands of cases have been reported, and hemolytic-uremic syndrome is recognized as the most common cause of acute renal failure in the pediatric population.
The clinical course of hemolytic-uremic syndrome can vary from subclinical to life-threatening. Observations over time have revealed distinct subgroups of hemolytic-uremic syndrome and have identified several etiologies for the disease. Nomenclature for the various types of hemolytic-uremic syndrome varies throughout the literature. For consistency, this article uses the following set of terms throughout this review:
-
STEC-HUS is used to describe hemolytic-uremic syndrome mediated by Shiga toxin (Stx)–producing Escherichia coli. This is also called classic, typical, Stx, diarrhea-positive, or D+ hemolytic-uremic syndrome.
-
Atypical HUS (aHUS) is used to describe hemolytic-uremic syndrome not mediated by Shiga toxin. This is also called complement-mediated, diarrhea-negative, non–diarrhea-associated, or D- hemolytic-uremic syndrome. This disease is usually mediated by abnormalities of the complement system or other heritable factors.
-
Pneumococcal-associated HUS is a subtype of atypical hemolytic-uremic syndrome, mediated by neuraminidase in the presence of infection with Streptococcus pneumoniae. This is also called neuraminidase-associated hemolytic-uremic syndrome.
The distinction is important because the clinical courses, treatments, and prognoses differ for each category. The first reported cases were aHUS; however, STEC-HUS is now much more common. Classification based solely on the presence of diarrhea can be misleading, as a significant percentage of patients with aHUS may have diarrhea.
Hemolytic-uremic syndrome shares many features with thrombotic thrombocytopenic purpura (TTP). For more information, see the Medscape Reference article Thrombotic Thrombocytopenic Purpura. Both diseases include multiorgan dysfunction due to thrombotic microangiopathy, with active hemolysis and thrombocytopenia. The traditional classification describes patients with predominantly renal disease as having hemolytic-uremic syndrome, and patients with predominantly CNS disease as having TTP. However, hemolytic-uremic syndrome can include severe neurologic impairment, and TTP can involve severe renal failure. Involvement of other organ systems also overlaps.
Whether these are, in fact, separate diseases remains controversial; some authors describe "hemolytic-uremic syndrome–TTP" as a single disease entity with a diverse spectrum of presentations. In many cases, both nephrologists and hematologists collaborate on the care of patients with these complex illnesses. Many authors now consider ADAMTS13 activity in distinguishing aHUS from TTP. Patients with very low ADAMTS13 activity, generally less than 10%, are considered to have TTP, whereas higher levels of activity point to a diagnosis of HUS.
Pathophysiology
STEC-HUS is usually preceded by a colitis caused by Shiga toxin–producing Escherichia coli (STEC). Subsequent inflammation of the colon facilitates systemic absorption of the Stx and lipopolysaccharide from the GI tract. The major toxins that cause hemolytic-uremic syndrome, Shiga toxin 1 (Stx1) and Shiga toxin 2 (Stx2), are similar in structure to the classic Stx. These toxins bind to globotriaosylceramide (Gb3), a glycolipid receptor molecule on the surface of endothelial cells in the gut, kidney, and, occasionally, other organs. Differential expression of Gb3 on glomerular capillaries compared with other endothelial cells may explain the predominance of renal injury. Damaged endothelial cells of the glomerular capillaries release vasoactive and platelet-aggregating substances. The endothelial cells swell, and fibrin is deposited on the injured vessel walls.
Swelling and microthrombi formation within the glomerular capillaries produce a localized intravascular coagulopathy. The glomerular filtration rate is reduced, and renal insufficiency ensues. Erythrocytes are damaged and fragmented as they traverse the narrowed glomerular capillaries. This leads to the characteristic microangiopathic hemolytic anemia. Hemolysis may also be a result of lipid peroxidation. See the image below.
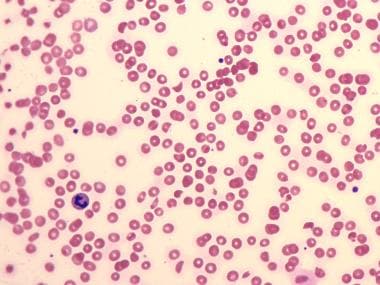 Peripheral blood smear in hemolytic-uremic syndrome (HUS) showing many schistocytes and RBC fragments due to hemolysis, and relatively few platelets reflective of thrombocytopenia.
Peripheral blood smear in hemolytic-uremic syndrome (HUS) showing many schistocytes and RBC fragments due to hemolysis, and relatively few platelets reflective of thrombocytopenia.
Thrombocytopenia is believed to result from a combination of platelet destruction, increased consumption, sequestration in the liver and spleen, and intrarenal aggregation. Platelets are damaged as they pass through the affected glomerular capillaries. Remaining platelets circulate in a degranulated form and show impaired aggregation. Stx also binds to activated platelets.
Abnormalities of anti–platelet-aggregating agents (eg, prostaglandin I2 [PGI2]), platelet-aggregating agents (thromboxane A2 [TXA2]) and von Willebrand factor (vWF) multimers are also important factors that contribute to thrombocytopenia. A decrease in PGI2 during the early stages of hemolytic-uremic syndrome has been noted. Defective PGI2 production is believed to play a role in STEC-HUS; abnormal PGI2 synthesis is believed to play a role in aHUS.
TXA2 levels are increased during the acute stage of hemolytic-uremic syndrome, leading to increased platelet aggregation. Another possible cause for increased platelet aggregation is large vWF multimers. In vitro, these large multimers have a greater ability to aggregate platelets than the normal, smaller multimers. Normal plasma contains a vWF-cleaving metalloproteinase (ADAMTS13) that rapidly degrades large vWF multimers. Many cases of TTP are associated with deficient function of ADAMTS13.
Abnormalities of ADAMTS13 may take the form of decreased quantity or absence of the enzyme, a mutation resulting in normal quantity of a defective enzyme, or an antibody inhibitor of the enzyme. Genetic or acquired defects in this protease have also been reported in patients with aHUS, but less frequently than in patients with TTP. Alterations in ADAMTS13 are not involved in the pathogenesis of STEC-HUS. The role of ADAMTS13 in both TTP and, less commonly, aHUS, remains incompletely understood. Most current authors define a thrombotic microangiopathy with ≤10% ADAMTS13 activity as TTP and not aHUS.
White blood cell (WBC) counts are usually elevated in the blood of patients with hemolytic-uremic syndrome. Activated neutrophils are believed to damage endothelial cells by releasing elastase (a catabolic enzyme that promotes endothelial cell detachment) and by producing free radicals. Monocytes may be stimulated to release cytokines (ie, interleukin 1 and tumor necrosis factor [TNF]) that also damage endothelial cells.
aHUS frequently occurs in patients with genetic abnormalities of the alternative pathway of the complement system. Genetically mediated cases are often not preceded by diarrheal illness, often manifest a recurrent course, and are associated with a less favorable long-term prognosis. Mutations causing aHUS have been identified in the genes coding for:
-
Complement factor H (CFH)
-
Complement factor I (CFI)
-
Complement factor B (CFB)
-
Complement C3 (C3)
-
CD46, also known as membrance cofactor protein (MCP)
-
DGKE
-
Thrombomodulin (TBHD)
-
Diacylglycerol kinase (DGKE)
This is not an exclusive list, and it continues to grow as new genetic knowledge develops. Up to 50% of patients with aHUS do not have an identifiable mutation. Patients may also develop aHUS due to an acquired antibody inhibitor of factor H or other complement factors.
Pneumococcal-associated hemolytic-uremic syndrome constitutes a distinct subgroup of aHUS. This variant occurs with infections caused by S pneumoniae, usually pneumonia. [2] It is actually a distinct entity that has little relation to the aHUS associated with complement factor mutations. The bacterial toxin neuraminidase damages endothelial cells and initiates hemolytic-uremic syndrome in this setting.
As a toxin-mediated disease, pneumococcal-associated hemolytic-uremic syndrome has much in common with STEC-HUS mediated by Stx. Bacteria with neuraminidase remove N-acetylneuraminic acid from cell-surface glycoproteins and expose the normally hidden T antigen (Thomsen-Friedenreich antigen) on erythrocytes, platelets, and glomeruli. Serum contains anti-T immunoglobulin M (IgM), which can react with the antigen and cause damage to RBCs and the kidneys.
Etiology
STEC-HUS
GI tract infection with Stx–producing E coli (STEC) precedes most cases of STEC-HUS. Stx1 is identical to the Stx produced by Shigella dysenteriae. Stx2 has a 55-60% amino acid homology with Stx. They injure the gut and lead to hemorrhagic colitis. Most cases worldwide are associated with STEC 0157:H7 infection. This organism is very resilient; viable bacteria have been reported in environments up to 10 months following initial contamination. Aside from Stx production, this bacteria produces virulence factors that mediate tight adherence to the host cell, facilitating transluminal transport of the toxins into the systemic circulation. Cattle are the major reservoir for human infection. The use of antimotility agents, antidiarrheal agents, and antibiotics has been reported to increase the risk of developing hemolytic-uremic syndrome. E coli O104:H4 was responsible for a large outbreak of hemolytic-uremic syndrome in Germany.
Other causes of hemolytic-uremic syndrome include infection by the following:
-
S dysenteriae (established as an etiologic agent)
-
Salmonella typhi (established as an etiologic agent)
-
Campylobacter jejuni (established as an etiologic agent)
-
Bacteroides species
-
Entamoeba histolytica
-
Aeromonas hydrophilia
-
Organisms of the class Microtatobiotes
aHUS
Causes of aHUS include the following:
-
Inherited (eg, mutations in the gene for factor H, a complement regulatory protein)
-
S pneumoniae (neuraminidase-associated)
-
Portillo virus
-
Coxsackie virus
-
Pregnancy: Hemolytic-uremic syndrome or thrombotic thrombocytopenic purpura (TTP) are associated with pregnancy; preeclampsia and HELLP syndrome also have features in common and should be part of the differential diagnosis.
-
Drugs (eg, chemotherapy, oral contraceptives, cyclosporine, tacrolimus)
-
Malignancy
-
Idiopathic
-
Glomerulonephritis, especially membranoproliferative glomerulonephritis
-
Malignant hypertension
Epidemiology
United States statistics
Between 1982 and 2002, 354 E coli O157:H7–associated hemolytic-uremic syndrome cases were reported. Transmission route was highest among swimming outbreaks, followed by person-to-person, unknown, animal contact, foodborne, and drinking water–related outbreaks. Daycare centers were the most common person-to-person outbreak setting. Although contaminated ground beef was the most common cause of foodborne outbreaks, produce-associated outbreaks are also common (ie, lettuce, sprouts, cabbage, apple cider, apple juice). These have been attributed to fecal contamination of produce in the fields, from wild animals or from fertilization containing human or animal fecal matter.
Incidence is increased during the summer and early fall. Outbreaks of diarrhea followed by hemolytic-uremic syndrome have been reported in institutions, boarding schools, and daycare centers. Seasonal variation is not observed in aHUS. STEC-HUS is much more common than aHUS.
International statistics
Hemolytic-uremic syndrome occurs worldwide but has a higher incidence in South Africa, Holland, and Argentina. The largest outbreak to date occurred in Germany in 2011.
Race-, sex-, and age-related demographics
Hemolytic-uremic syndrome occurs in all races. Race is a purely social construct, based on self-identification. It has no biologic basis, and should not be used in determining diagnosis or treatment.
Males and females are affected in equal numbers.
A large majority of cases of STEC-HUS occur in children aged 7 months to 6 years. STEC-HUS is much less common in adults, although the disease may occur at any age. A large outbreak in Germany due to a novel strain of E. coli 0104:H4 forms a notable exception, in which 88% of patients were adults. [3] No age predilection is noted for aHUS. Genetically mediated forms may present as early as birth or the neonatal period.
Prognosis
STEC-HUS
Most patients who receive the appropriate treatment have a good recovery. Recurrence is very rare. Poor prognostic indicators include the following:
-
Elevated WBC count at diagnosis
-
Prolonged anuria
-
Severe prodromal illness
-
Severe hemorrhagic colitis with rectal prolapse or colonic gangrene
-
Severe multisystemic involvement
-
Persistent proteinuria
-
Genetic abnormalities in complement regulatory factors
The long-term prognosis for survivors of childhood STEC-HUS remains unknown. A 5-year follow-up of a cohort of patients showed no difference in blood pressure and slightly higher rates of microalbuminuria compared with controls. [4] The patients also had lower glomerular filtration rates (GFRs) as measured by cystatin C but not as measured by serum creatinine levels. Other studies have shown similar findings. Continued long-term follow-up studies are needed to help determine whether survivors have residual subclinical renal injury that could manifest itself later in life. At present, patients should be counseled on avoiding risk factors for renal disease (eg, tobacco use, obesity, hypertension) and the importance of continued medical follow-up.
aHUS
The prognosis is more guarded than for STEC-HUS. Patients with aHUS are at risk for relapses and a higher risk of progression to end-stage renal disease (ESRD). Ongoing treatment with eculizumab reduces this risk.
Morbidity/mortality
Mortality rates have decreased progressively from near universal fatality in 1955 to only 3-5% during the 1990s. This improvement is attributed to better management during the acute stage of the disease, with aggressive management of hypertension, fluid overload, electrolyte disturbances, and nutrition, often requiring dialysis. The mortality rate in underdeveloped countries remains as high as 72%. Patients with hereditary hemolytic-uremic syndrome may have a worse prognosis due to the risk of recurrent episodes.
Complications
Renal system complications are as follows:
-
Renal insufficiency
-
Renal failure
-
Hypertension
CNS complications are as follows:
-
Intellectual disability
-
Seizures
-
Focal motor deficit
-
Optic atrophy
-
Cortical blindness
-
Learning disability
Endocrine system complications are as follows:
-
Diabetes mellitus
-
Pancreatic exocrine insufficiency
GI system complications can include intestinal necrosis.
Cardiac system complications can include congestive heart failure.
Patient Education
Diet concerns are as follows:
-
Low-salt diet to decrease risk of hypertension
-
Diet high in iron and folic acid content to help recover from anemia
-
High-energy diet to help patient regain lost weight
Social worker or psychologist consultation can help the family cope with the illness.
-
Peripheral blood smear in hemolytic-uremic syndrome (HUS) showing many schistocytes and RBC fragments due to hemolysis, and relatively few platelets reflective of thrombocytopenia.
-
Micrograph of a glomerulus in hemolytic-uremic syndrome, showing thrombi and red blood cell fragments in the capillary space. Courtesy of Xin J (Joseph) Zhou, MD, Renal Path Diagnostics, Pathologists BioMedical Labs.