Practice Essentials
Graves disease is the most common cause of hyperthyroidism in pediatric patients. (See the images of patients with thyrotoxicosis below.) It is an immune-mediated disorder that results from the production of thyroid-stimulating immunoglobulins (TSI) by stimulated B lymphocytes. These immunoglobulins bind to the thyroid-stimulating hormone (TSH) receptor to mimic the action of TSH and stimulate thyroid growth and thyroid hormone overproduction.
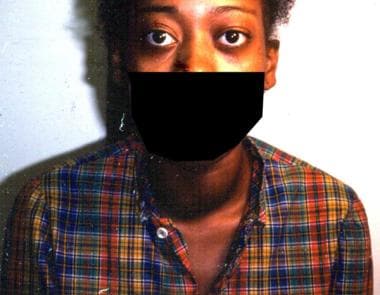 A 16-year-old girl with thyrotoxicosis for 3 years is shown. Note her thyrotoxic stare (infrequent blinking with exophthalmos) and enlarged thyroid gland (goiter).
A 16-year-old girl with thyrotoxicosis for 3 years is shown. Note her thyrotoxic stare (infrequent blinking with exophthalmos) and enlarged thyroid gland (goiter).
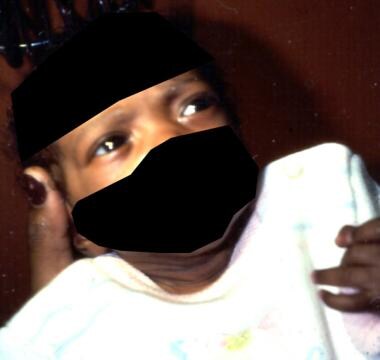 Neonate with thyrotoxicosis secondary to transplacental passage of maternal thyroid-stimulating immunoglobulins (TSI). The baby has a noteworthy stare. Upon examination, a small goiter and a rapid heart rate could be appreciated.
Neonate with thyrotoxicosis secondary to transplacental passage of maternal thyroid-stimulating immunoglobulins (TSI). The baby has a noteworthy stare. Upon examination, a small goiter and a rapid heart rate could be appreciated.
Signs and symptoms
Signs and symptoms of thyrotoxic Graves disease include the following:
-
An enlarged thyroid
-
Rapid heart rate
-
Widened pulse pressure
-
A hyperthyroid stare (infrequent blinking) or frank exophthalmos
-
Tremor
-
Sweating
-
Palpitations
-
Smooth moist skin
-
Frequent bowel movements or diarrhea
-
Sleeplessness
-
Attention problems in school
-
Irritability
-
Weight loss
See Presentation for more detail.
Diagnosis
Diagnosis requires identification of suppressed TSH (thyrotropin) levels and elevated levels of free thyroxine (FT4 ) and/or triiodothyronine (T3 ). Measurement of TSI is of interest but is not required for therapeutic evaluation.
See Workup for more detail.
Management
Treatment is directed at alleviating symptoms and reducing thyroid hormone production. Symptoms may be improved by treatment with beta-blocking drugs. Reduction of thyroid hormone is accomplished by use of drug therapy, surgical subtotal thyroidectomy, or treatment with radioactive iodine (RAI). Because circulating TSI can cross the placenta, infants born to women with a history of Graves disease may have transient neonatal Graves thyrotoxicosis and require treatment.
See Treatment and Medication for more detail.
Background
Although Perry was first to report hyperthyroidism in English, the classic description, in 1835, by Graves became most widely accepted. Europeans often prefer to recognize the description by Basedow.
The most common association with childhood Graves disease is a history of other family members with thyroid disease. On the other hand, concordance for Graves disease in identical twins is only 30-50%, indicating that genetic and environmental factors play a role in this disease.
Complications
Graves disease is potentially life threatening. The most severe manifestation of Graves disease is thyroid storm, which carries a mortality risk approaching 100% in untreated adults. Series conducted with newer treatments, including beta-adrenergic blocking agents, show a reduced risk of death near 20%. This is such a rare disorder in children that no comparable figures are available.
Even children and adolescents with less severe manifestations of Graves disease can display long-term consequences of this disorder, including problems with schooling and chronic loss of bone mineral.
Patient education
Instruct patients treated with antithyroid drugs as to possible adverse effects and the need for close follow-up. [1] Patients treated with surgery and RAI must understand the rationale for the development of hypothyroidism and the need for close follow-up.
For patient education information, see the Thyroid and Metabolism Center, as well as Thyroid Problems.
Pathophysiology
The reasons for the development of Graves disease are presently unknown. Patients likely have defective immune tolerance, leading to the development of specific autoantibodies directed against various thyroid antigens and against proteins with putatively similar antigenic sites in other tissues, notably the subcutaneous tissues and extraocular muscles. The TSH receptor is the most significant thyroid autoantigen in this disorder. However, children with Graves disease also produce immunoglobulins directed against thyroperoxidase (anti-TPO) and thyroglobulin, as well as TSH receptor–blocking antibodies, as may be found in chronic lymphocytic thyroiditis (Hashimoto thyroiditis).
One study conducted a genome-wide association study in 1536 individuals with Graves disease and further evaluated a group of associated single nucleotide polymorphisms (SNPs) in a second set of 3994 cases. The data confirmed previously reported loci (TSHR, CTLA4, and FCRL3), and identified 2 new susceptibility loci (RNASET2-FGFR1OP-CCR6 region at 6q27), and an intergenic region at 4p14. These newly associated SNPs were associated with the expression levels of RNASET2 at 6q27, of CHRNA9, and of a previously uncharacterized gene at 4p14, respectively. Strong associations of TSHR and major histocompatibility complex class II variants with persistently TRAb-positive Graves disease were also identified. [2]
Because other antibodies can coexist with TSI, not all children with Graves disease are thyrotoxic. However, thyrotoxicosis is the hallmark of most cases of Graves disease. In general, thyrotoxic Graves disease is considered in this article. Onset of Graves disease in susceptible individuals has variously been attributed to acute infections and to physical and emotional stress.
The thyroid is enlarged because of constant TSH receptor stimulation and the presence of activated T lymphocytes and plasma cells in pseudofollicular patterns. The thyroid often has a firm, rubbery consistency when palpated, and the pyramidal lobe may be prominent. When overstimulated by TSI, the thyroid becomes quite vascular, and an audible bruit is not uncommon. If the thyroid becomes very large, it may cause pressure symptoms and signs, including difficulty swallowing and hoarseness. Rarely, children may report associated pain.
Thyroid hormone excess, as a result of thyroid hyperstimulation, affects all organ systems. Patients with thyroid hyperstimulation are irritable and restless, have poor sleep habits, and often report daytime tiredness associated with nocturnal insomnia. Inability to concentrate and tremor translate in children into scholastic inattention, poor handwriting, and deteriorating school performance. Neuropsychiatric symptoms can mimic attention deficit hyperactivity disorder (ADHD), yet few children with ADHD are actually discovered to be thyrotoxic. ADHD and thyrotoxicosis are usually easily distinguished by thyroid examination and measurement of pulse and blood pressure (BP).
Cardiovascular stimulation by thyroid hormone leads to a rapid pulse rate and a dynamic precordium. Patients sometimes subjectively report palpitations. The patient typically shows a widened pulse pressure. Hypermetabolism usually leads to weight loss with increased appetite. Heat intolerance is often subtle.
Muscle wasting is present, with decreased muscle strength. Typically, atrophy of the thenar and hypothenar eminences may be observed. The hair becomes fine, and temporal hair loss often occurs. Rare genetically determined individuals may develop thyrotoxic periodic paralysis. Darkening of the skin may occur, most noticeably in darker-skinned individuals, and intense pruritus may also occur. The skin is typically very fine and moist. Sweating is increased. Thickening of the skin (localized myxedema) is almost never observed in childhood Graves disease.
In individuals with severe hypermetabolism, abnormal liver function may be observed with elevations of serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT). Increases in gut motility result in diarrhea and frequent bowel movements. Graves disease with thyrotoxicosis leads to loss of bone mineral, decreased bone density, and resultant hypercalcuria. Hypercalcuria, as well as hyposthenuria, as a direct effect of thyroid hormone on the renal tubule, leads to nocturia, and, in some susceptible children, it leads to nocturnal enuresis. Nocturnal enuresis occasionally is the first finding noted in children with Graves disease.
Growth in height may be enhanced by hypermetabolism, and bone age may be advanced. [3] Puberty may be affected. Girls with Graves disease may have irregular, sparse menses, and boys may have excess estrogen effect because of increased metabolism of steroids to estrogen. Symptoms of gynecomastia and decreased libido in older adolescents are not uncommon.
Etiology
Graves disease is a humorally mediated autoimmune disorder in which hyperthyroidism is induced by TSH receptor–stimulating antibodies. In most children and adults, these antibodies are endogenous; however, transplacental passage of immunoglobulin G (IgG) antibodies from women with Graves disease to their infants may lead to the development of neonatal Graves disease. This is a self-limited disorder that resolves when the immunoglobulins are cleared by the neonate and may be followed by transient hypothyroidism if fetal pituitary TSH remains suppressed.
The presence of a long-acting thyroid stimulator (LATS) was postulated by Adams and Purves in 1956 and was confirmed by the identification of the stimulatory immunoglobulins some years later. These immunoglobulins bind to the TSH receptor and mimic TSH action.
Almost all patients producing TSI also produce other immunoglobulins more commonly associated with chronic lymphocytic thyroiditis, such as antibodies directed against thyroperoxidase and thyroglobulin. This suggests a close relation between Graves disease and chronic lymphocytic thyroiditis. Indeed, many individuals have thyrotoxic components to their chronic lymphocytic thyroiditis, and the natural history of untreated Graves disease is that a percentage of individuals with Graves eventually become hypothyroid. Moreover, lymphocytic infiltrates similar to those of chronic lymphocytic thyroiditis are found in the thyroids of patients with Graves disease.
Immunoglobulins produced in this disorder may be measured by numerous in vitro assays. Because these assays may measure different aspects of immunoglobulin function, results in different assays may be discrepant. For instance, TSH-receptor binding is measured in assays of thyroid-binding immunoglobulins (TBI), whereas TSH-receptor activation (eg, increased activation of adenyl cyclase) is measured by TSI or thyroid-stimulating antibodies (TSAb).
Some immunoglobulins may bind to the TSH receptor without stimulating it and may actually block the action of TSH (so-called blocking antibodies). These may be produced in individuals with Graves disease or chronic lymphocytic thyroiditis, further complicating the picture occasionally in individuals with these disorders.
The mechanism of the failure of immune tolerance that leads to the development of Graves disease is not entirely understood, and competing hypotheses have not yet been definitively evaluated.
Nonetheless, histocompatibility antigen haplotypes commonly associated with other autoimmune disorders (B8, DR3) have also been linked to Graves disease.
An infectious etiology of thyrotoxicosis has been postulated based on occurrence frequency in unrelated family members. An association with Yersinia enterocolitica infection has been described but has not been fully confirmed.
Case-controlled studies have suggested an association with recent life stress. The familial occurrence of thyrotoxicosis, as well as other autoimmune thyroid disorders, suggests a genetic link that may be more powerful than that of the histocompatibility antigen association.
Epidemiology
Occurrence in the United States
The prevalence of Graves disease in childhood in the United States has not been quantitated. An incidence of 0.2-0.4% has been speculated but is probably an overestimation. Approximately 10% of infants born to women with Graves disease have elevations of thyroid hormone levels, but only 1-2% have clinical symptoms of thyrotoxicosis.
International statistics
A Danish study identified a national incidence density for thyrotoxicosis of 0.79 cases per 100,000 person-years in children aged 0-14 years. [4] Incidence density increases during childhood, with a peak incidence of 0.48 cases per 100,000 persons for boys and 3.01 cases per 100,000 persons for girls, aged 10-14 years.
Race-, sex-, and age-related demographics
No race predilection for Graves disease is apparent. It has been reported in every population studied. In whites, Graves disease is associated with certain histocompatibility antigens (ie, DR3, DR1) that have previously been linked to other autoimmune disorders. The link between histocompatibility antigen subtypes and Graves disease identified in Whites is weaker in Blacks.
At any age, Graves disease is much more common in girls than in boys. The female preponderance has been estimated to be 4-7 girls for every boy affected.
The incidence of Graves disease increases with age, reaching a childhood peak during adolescence. Graves disease is a very rare cause of thyrotoxicosis in children younger than age 5 years.
Prognosis
Graves disease is a chronic illness without a true cure. None of the management options for this disorder actually remove the underlying immunologic disorder. Therefore, the prognosis of the disorder greatly depends on the form of therapy chosen.
Antithyroid drug therapy
In one review, 46.8% of patients had a permanent remission following drug treatment for a variable number of years, and 29% had a relapse. Of this population of 651 children, culled from a number of reports, 5.6% of patients developed granulocytopenia, 2.3% had arthritis, 1.9% had liver disease, and 8% developed a skin rash. The likelihood of remission is greater if the thyroid gland is smaller, the radioactive iodine uptake (RAIU) is relatively low, and TSI levels are lower.
A statistical analysis of children who had long-term drug treatment suggested that approximately 25% of children have remission every 2 years. This remission rate is generous, and it is lower than the remission rate observed in adults.
Subtotal thyroidectomy
A review of outcomes in 555 children, taken from several large series, suggested that 42% of patients undergoing subtotal thyroidectomy become hypothyroid and that 10% have recurrence. In this combined series, 2% of patients had hypoparathyroidism, 1.2% had vocal cord paralysis, 0.2% had bleeding, 1.7% had keloid formation, and 1.5% were discovered to have papillary cancer by histology.
Radioactive iodine therapy
In a review of outcomes of 555 children, taken from several large series, 69% of children who underwent radioactive iodine therapy became hypothyroid, 98% experienced cure of hyperthyroidism, 12% required retreatment, and 4.4% had histologically benign nodules. The practice today in most centers is to aim for hypothyroidism, which would change these figures.
Complications
Hyperthyroidism leads to hypercalciuria and loss of bone mineral during childhood and adolescence. In severely thyrotoxic individuals, assessment of bone mineral by dual energy radiographic absorptiometry (DRA) may be advisable.
Thyroid storm is the most severe form of thyrotoxicosis and can be provoked by surgical or medical stress in an undiagnosed thyrotoxic individual.
Other autoimmune disorders can be associated with Graves disease, including type 1 diabetes mellitus, Addison disease, vitiligo, alopecia, and lupus.
Treatment complications include the following:
-
Severe drug reaction to methimazole or propylthiouracil (PTU), including liver disease, lupus, and agranulocytosis [5]
-
Surgical complications, including hypoparathyroidism or recurrent laryngeal nerve damage
-
Rare induction of hypoparathyroidism post-RAI therapy and a questionable slight increase in the risk of thyroid cancer
-
Fetus with thyroid ablation in women treated with RAI during pregnancy
-
A 16-year-old girl with thyrotoxicosis for 3 years is shown. Note her thyrotoxic stare (infrequent blinking with exophthalmos) and enlarged thyroid gland (goiter).
-
Neonate with thyrotoxicosis secondary to transplacental passage of maternal thyroid-stimulating immunoglobulins (TSI). The baby has a noteworthy stare. Upon examination, a small goiter and a rapid heart rate could be appreciated.









