Practice Essentials
Bronchiectasis, derived from the Greek words for stretched windpipe, is a pathologic diagnosis or clinical syndrome that results from cyclic inflammation, infection, airway destruction, and airflow limitation.
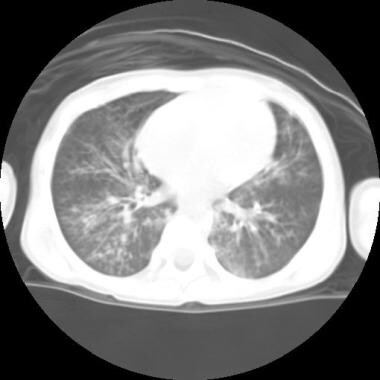
Bronchiectasis develops as a result of acute or chronic infection or inflammation, which may also be associated with an underlying anatomic airway obstruction, or congenital disease (see Etiology). When bronchiectasis occurs, it often produces recurrent cough and infectious exacerbations. When it occurs diffusely, the patient will often have additional signs and symptoms of generalized airway obstruction, reduced lung function, and may ultimately progress to respiratory failure. A computed tomography (CT) scan of the chest of a child with bronchiectasis is shown below. Bronchiectasis on imaging can be focal or diffuse.
Signs and symptoms
Bronchiectasis should be considered in children who have a daily or recurrent productive or wet cough cough for longer than 4 weeks. Recurrent cough with fetid sputum, hemoptysis, or recurrent pneumonia are important clues to early diagnosis of this disease. A high suspicion for bronchiectasis is necessary because pediatric patients do not readily expectorate. Other common symptoms and signs include the following:
-
Exertional dyspnea
-
Recurrent wheezing
-
Recurrent lung infections
-
Digital clubbing (advanced disease)
Physical examination findings in patients with bronchiectasis may include variable degrees of wheezing, crackles or coarse rhonchi and digital clubbing.
See Presentation for more detail.
Diagnosis
Laboratory studies
Laboratory evaluation of bronchiectasis may include the following tests:
-
Sweat chloride
-
With underlying asthma or cystic fibrosis (CF), evaluation for allergic bronchopulmonary aspergillosis should include immunoglobulin E (IgE) and serum precipitins for Aspergillus species , sputum culture for fungus, and an aspergillus skin test
-
Complete blood cell count
-
Serum immunoglobulin G (IgG) with IgG subclasses, immunoglobulin M (IgM), and IgA
-
Speech pathologist assessment for identification of possible dysphagia with aspiration
-
HIV test
-
Sputum culture or deep oropharyngeal swab in younger children
-
Spirometry for children older than 6 years of age
-
Testing for primary ciliary dyskinesia - genetic testing, ciliary biopsy
-
Evaluation for aspiration
-
Antinuclear antibody and rheumatoid factor
-
Vaccine antigens
-
Flexible fiberoptic bronchoscopy
Obviously, not every patient with bronchiectasis requires each of the above studies. The history and physical examination should help guide the clinician to choose the appropriate studies, especially when there is suspicion for bronchiectasis in a patient with chronic or recurrent respiratory infections.
Imaging studies
In patients with suspected bronchiectasis, a high-resolution CT (HRCT) scan is the diagnostic procedure of choice.
See Workup for more detail.
Management
In addition to the treatment of an identified underlying disorder in patients with bronchiectasis, therapy is aimed at reducing airway secretions and facilitating their removal through airway clearance techniques. Antibiotics can be used to prevent and treat recurrent infections and diminish bacterial load and the associated cycle of infection and inflammation. Thus overall goals are to interrupt the pathologic cycle, minimize exacerbations and prevent premature respiratory impairrment.
See Treatment and Medication for more detail.
Background
René Laennec, inventor of the stethoscope, first described bronchiectasis in 1819 while observing patients with tuberculosis and the sequelae of pneumonia in the pre-antibiotic era. In 1922, Jean Athanase Sicard introduced contrast bronchography, which provided imaging of the destructive changes characteristic of bronchiectasis. The term bronchiectasis is derived from the Greek bronchion, meaning windpipe, and ektasis, meaning stretched. Bronchiectasis is a pathologic term defined by the dilatation of bronchi with destruction of elastic and muscular components of their walls. Bronchiectasis is defined by the findings of bronchiole destruction on pathology or more commonly on radiologic imaging, CT scan, and a clinical syndrome of chronic wet cough, and recurrent airway infections and/or signs of airway inflammation.
Pathophysiology
The pathophysiology of bronchiectasis is based on the theory of an extended vicious cycle of infection, inflammation and airway destruction. Bronchiectasis exists on a continuum beginning with an acute respiratory infection progressing to protracted bacterial bronchitis then chronic suppurative lung disease and eventually bronchiectasis. Bronchiectasis is more directly the product of obstruction and/or inflammation of the airway; however, it is generally the result of an intricate interaction between the host, pathogens and the environment. The obstruction and inflammation may be due to any of the underlying disorders listed above or to infection, including acute tuberculosis, adenovirus, measles, Mycobacteriumavium, or Aspergillus fumigatus.
Mucus clearance is reduced in the setting of bronchiectasis due to airflow limitation, abnormal quantity and quality of mucus produced, and specific bacterial characteristics that contribute to ciliary dyskinesia. Bronchiectasis associated with bronchial obstruction may have a focal distribution distal to the site of obstruction. Bronchiectasis associated with underlying disease is more likely to be diffuse.
Regardless of the etiology, there is an impairment in the mucociliary clearance ability of the lungs, which leads to a diminished ability to clear the airway of the purulent and inflammatory material, which in turn leads to increased bacterial colonization and infection. [1] Cole proposed a “vicious cycle” of infection and dysregulated airway inflammation, leading to progressive destruction of bronchial walls resulting in dilatation and airflow obstruction. [2]
Infection leads to recruitment of neutrophils, T lymphocytes, and monocyte-derived cytokines. Multiple studies have evaluated the inflammatory patterns seen in samples from bronchoscopic and sputum analysis. These studies have found increased interleukin-8 expression, infiltration by neutrophils, T lymphocytes and mucous gland hypertrophy on mucosal biopsies and sputum and bronchoalveolar lavage fluid with increased concentrations of inflammatory mediators such as neutrophil elastase, interleukin-8, tumor necrosis factor-alpha and prostanoids. [3] The release of inflammatory mediators, elastases, and collagenases leads to inflammation and destruction of elastic and muscular components of bronchial walls. In addition, the outward elastic recoil forces of surrounding lung parenchyma exert traction, which causes expansion of airway diameter. Two different types of bronchiectasis are noted: cylindrical, which is presumably more readily reversible if the underlying disorder can be controlled, and saccular, which is less readily reversible even if the underlying disorder is controlled.
These changes may be accompanied by bronchial arterial proliferation, which predisposes to hemoptysis. Hemoptysis may also occur as a result of the dilating airways impinging on the accompanying blood vessels.
Etiology
Bronchiectasis may result from multiple etiologies including most commonly infection, congenital or genetic disorders, or idiopathic. A systematic review (12 studies involving 989 children) found 63% had an underlying cause. [4] Previous pneumonia (19%), primary immunodeficiency (17%), recurrent aspiration, including an inhaled foreign body (10%), and primary ciliary dyskinesia (7%) were implicated most commonly. It has been estimated that approximately 30% of cases of bronchiectasis are idiopathic. [5]
Common theory suggests that a single severe acute lower respiratory tract infection or multiple lower respiratory tract infections early in life can lead to bronchiectasis. More specifically, a correlation has been found between the overall number of pneumonias rather than the site of pneumonia and bronchiectasis. Although, infections remain the most common etiology of bronchiectasis, there has been a reduction in post-infectious bronchiectasis due to the widespread use of vaccinations and antibiotics.
All causes share the same pathophysiologic pathway: ineffective pulmonary toilet and chronic or recurrent infection and inflammation.
Common infectious causes include the following:
Infectious:
-
Varying severity of pneumonia - viral and bacterial
-
Mycobacterial and Fungal infections
-
Measles, tuberculosis, pertussis, adenovirus, Mycobacterium avium, and Aspergillus fumigatus
-
HIV infection: HIV-related Lymphocytic interstitial pneumonitis is associated with subsequent development of bronchiectasis
Congenital/Genetic disorders:
-
Cystic Fibrosis
-
Young syndrome
-
Primary Ciliary dyskinesia
-
Alpha-1 antitrypsin deficiency
-
Congenital absence of bronchial muscle (Mounier-Kuhn syndrome) or cartilage (Williams-Campbell syndromes)
-
Usher Syndrome
Immune deficiency:
-
Immunoglobulin A (IgA) and G (IgG) deficiencies and IgG subclass deficiencies, especially IgG2 deficiency
-
Allergic bronchopulmonary aspergillosis (ABPA)/ allergic bronchopulmonary mycoses
Acquired disorders associated with bronchiectasis include the following:
-
Intrinsic airway luminal obstruction by a retained bronchial foreign body [6] or extrinsic compression by mass
-
Chronic aspiration, which is associated with swallowing dysfunction, gastroesophageal reflux disease, or tracheoesophageal fistula
-
Connective tissue disorders, including rheumatoid arthritis and systemic lupus erythematosus
-
Extrinsic airway narrowing (vascular ring, adenopathy, compressive mass)
-
Endobronchial mass or tumor
-
Tracheal stenosis with impaired mucociliary clearance
-
Severe tracheomalacia or bronchomalacia with impairment of mucociliary clearance
-
Fibrosing lung diseases associated with sarcoidosis or idiopathic pulmonary fibrosis
Epidemiology
United States statistics
The true prevalence of bronchiectasis in children has been difficult to determine due to the frequent delay in diagnosis, difference in prevalence among various populations, physician awareness, and the availability of high resolution CT scans as the diagnostic modality of choice. Current population-based estimates of occurrence are not available. In 1963, Clark estimated an incidence of 1.06 cases per 10,000 population. [7] The incidence of bronchiectasis associated with underlying systemic disease reflects the incidence of the particular disease. The most common genetic disease associated with bronchiectasis is cystic fibrosis (CF). One study estimates that 110,000 people in the United States have bronchiectasis, including adults. [8] The incidence of non-cystic fibrosis bronchiectasis in childhood has been estimated to range from 0.2 to 4.2 per 100,000 in the United States.
In most high-resource countries, the overall prevalence of childhood bronchiectasis has significantly declined over the last 4 decades due to earlier detection and treatment and public health measures including broader immunization programs, improved control and treatment of infectious diseases, sanitation, reduced crowding, improved nutrition, and easier access to medical care. The exception to this is indigenous populations and disadvantaged groups where the prevalence has not decreased as dramatically.
Indigenous populations within North America have been reported to have the highest incidence of pediatric bronchiectasis. The incidence among Alaskan Native children in the Yuskon-Kuskokwim region is about 140 cases per 10,000 population. [9] The incidence of bronchiectasis in southwest Alaskan Natives is 16 cases per 1000 population. [10]
International statistics
In high resource countries, the frequency is similar to that in the United States with bronchiectasis being more common among indigenous populations and disadvantaged groups. Similarly to the United States, the most clinically significant cause of bronchiectasis in developed affluent countries is cystic fibrosis. However, throughout the world, bronchiectasis is predominantly associated with non-CF related conditions rather than CF.
The frequency is higher in the resource limited countries, where measles, adenovirus infection, pneumonia, tuberculosis, and HIV infection are highly prevalent and are associated with bronchiectasis.
In a study from the United Kingdom that started in 1949, Field studied children with bronchiectasis for almost 2 decades and documented a fall in the annual hospitalization rate for bronchiectasis in 5 British hospitals. During the study period, as broad-spectrum antibiotics became widely available, the hospitalization rate decreased from approximately 48 cases per 10,000 population to 10 cases per 10,000 population. [11] More recent estimates of annual incidence of pediatric bronchiectasis in the United Kingdom are appoximately 15 per 100,000. [12] In high-income countries the annual incidence ranges from 0.2 per 100,000 to 15.0 per 100,000. [12, 13, 14]
In New Zealand, Twiss and colleagues reported the incidence of bronchiectasis in children younger than 15 years at 3.7 cases per 100,000 population in 2001-2002. [15] The incidence was highest among Pacific children, at 17.8 cases per 100,000 population. The incidence was 4.8 cases per 100,000 population in Maori children and 1.5 cases per 100,000 in New Zealand overall, compared with 2.4 cases per 100,000 in other Pacific regions.
Twiss and colleagues noted that the incidence of bronchiectasis in New Zealand children was nearly twice the rate of CF and 7 times that of bronchiectasis in Finland, which is the only other country reporting a childhood national rate. They further noted that in central Australian Aboriginal children, the incidence is 14 cases per 1,000 population, compared with 0.1 cases per 1,000 in Scotland and 4.9 cases per 1,000,000 in Finnish children. [15] More recently, extrapolated data from Janu et al. found the incidence of pediatric bronchiectasis among the indigenous population of Australia to be as high as 410 per 100,000. [5] [16]
Ethnicity-, sex-, and age-related demographics
Bronchiectasis is more common in patients of Polynesian and Alaskan Native ancestry. A study in Turkey suggests possible genetic predisposition in some populations and found that 43% of children with bronchiectasis had parents who were first-degree or second-degree relatives but presumably without any other known underlying disorder. [17]
Non-CF bronchiectasis is more common and more virulent in women. The differences may result from inflammatory-immune, environmental, anatomic, or other genetic factors. [18]
Prognosis
Overall, the prognosis is good for a child with non-cystic fibrosis bronchiectasis. There is very limited data on the prognosis of non-CF pediatric bronchiectasis, yet with increasing earlier detections and multi-disciplinary management, the lung function in children with bronchiectasis often stabilizes and patients have an overall good prognosis. A few studies have found evidence of reversibility and normalization on imaging however many studies have found that while clinical symptoms improve there is a persistence of signs of bronchiectasis on CT scan and improvement in lung function tests without complete normalization. [19, 20, 21, 22] Reversibility is most likely associated with early diagnosis and management.
The prognosis of non-CF bronchiectasis primarily depends on the underlying cause and whether that etiology is an acute or chronic condition. The key to a successful outcome is determining whether the cause of the damage is ongoing (eg, chronic aspiration) and then treating the underlying problem. In the absence of an underlying condition, children with isolated or localized bronchiectasis often have a better prognosis compared to those with more diffuse disease.
Growth of new pulmonary tissue in children proceeds rapidly until about age 6 years and then tapers off through childhood. Injury at an early age may be compensated for in part by growth of normal healthy lung parenchyma in the absence of ongoing damage.
Progressive bronchiectasis from underlying disease (eg, CF) or ongoing pulmonary insult (eg, aspiration syndromes) causes a progressive obstructive defect and, ultimately, respiratory compromise. In these cases bronchiectasis is an irreversible process associated with progressive and persistent lung damage. The progression of the lung damage that occurs with bronchiectasis is associated with significant morbidity as it usually manifests as recurrent infectious exacerbations and progressive obstructive lung disease. Respiratory compromise may manifest as dyspnea at rest or with exercise or sleep-disordered breathing. Ultimately, patients may experience chronic hypoxemia, pulmonary hypertension, cor pulmonale, hypercarbia, respiratory failure, and death.
Progressive focal disease may lead to progressive infection with fever and abnormal growth. The area may contribute enough ventilation/perfusion mismatch to cause hypoxemia with exercise. Although not yet proven, infected secretions from the abnormal portion of the lung could spill over to other portions of the lung, causing more widespread infection.
Limited mortality data are available. In Field's original group, who were studied at the beginning of the antibiotic era, 4% of children with medically treated bronchiectasis died (mostly from infection), and 3% of children who were surgically treated died (many immediately following or as a late result of surgery) in the ensuing two decades. [23] In the countries of England, New Zealand, and India between 0.2% to 11% of pediatric subjects within population stiudies on pediatric bronchiectasis died. [24, 25]
A poorer prognosis is associated with the presence of asthma, bilateral lung involvement, and saccular bronchiectasis. Akalin and colleagues reported decreased left ventricular function and exercise capacity in bronchiectasis, which has subsequently been found to present late in the disease course. [17]
Complications
Complications that can develop solely from bronchiectasis range from mild such as focal atelectasis to severely life threatening such as massive hemoptysis. Other complications associated with disease progression consist of heart related morbidity and respiratory failure. In those with more diffuse involvement, significant impairment in lung function may affect physical activity and quality of life.
Additional morbidity occurs from side effects of medications used in the management of bronchiectasis, predominantly related to the well-documented side effects of antibiotics.
-
Posteroanterior chest radiograph of a child with bronchiectasis due to chronic aspiration.
-
CT scan of the chest of a child with bronchiectasis due to chronic aspiration.
-
Chest radiograph of a child with severe adenoviral pneumonia as an infant. The child has persistent symptoms of cough, congestion, and wheezing.
-
Bronchoscopic bronchogram of the left lower lobe on a patient with history of adenoviral pneumonia, demonstrating cylindrical and varicose types of bronchiectasis.
-
Bronchoscopic bronchogram of the right upper lobe of a patient with a history of adenoviral pneumonia, demonstrating saccular bronchiectasis.