Background
Ventricular fibrillation (VF) is rare in the pediatric population; when it does occur, ventricular fibrillation is usually a degeneration of other malignant arrhythmias, such as ventricular tachycardia (VT). The period of arrhythmia may not be extensive, but ventricular fibrillation that occurs without a few initial beats of ventricular tachycardia is unusual. In adults, ventricular fibrillation is preceded by ventricular tachycardia in approximately 80% of cases. [1]
Primary ventricular fibrillation is uncommon in children. In a study of pediatric out-of-hospital arrests, ventricular fibrillation was the initial recorded rhythm in 19% of cardiac arrests. [2] Causes of ventricular fibrillation varied and included medical illness, overdose, drowning, and trauma; only 2 of 29 patients had congenital heart disease. Thus, ventricular fibrillation as a terminal rhythm in cardiac arrest may result from various causes. [3]
The outcome in patients with ventricular fibrillation is better than in patients with asystole or pulseless electrical activity (PEA), and outcome may be further improved by prompt recognition and treatment of ventricular fibrillation. In a population of patients with known ventricular arrhythmias, individuals who had ventricular fibrillation were more likely to have underlying significant heart disease (eg, cardiac tumors, long QT syndrome, structural congenital heart disease) than patients with ventricular tachycardia. [4]
After initial resuscitation, therapy in patients with ventricular fibrillation is primarily focused on preventing the antecedent ventricular tachycardias. However, technologic advances in both implantable and external automated defibrillators have made these devices an important part in the management of malignant ventricular arrhythmias. [5]
Pathophysiology
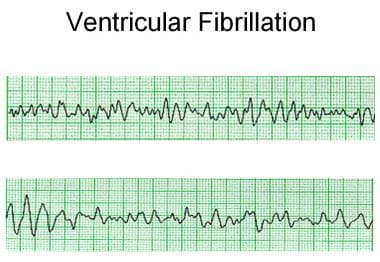
The electrical activity in ventricular fibrillation is characterized by chaotic depolarization of cells throughout the ventricular myocardium. The lack of coordinated depolarization prevents effective contraction of the myocardium and, thus, ejection of blood from the heart. Surface ECG demonstrates no identifiable QRS complexes, although a wide range of amplitude of electrical activity is present, from sine-wave ventricular flutter to fine ventricular fibrillation, which may be difficult to distinguish from asystole (see image below). This arrhythmia is maintained by multiple re-entrant circuits because portions of the myocardium are constantly depolarizing. Ventricular fibrillation may be initiated when an area of myocardium has refractory and conducting portions, and, as in any reentrant circuit, this combination promotes a self-sustaining rhythm. [6]
Etiology
Various factors can lead to the lowering of the ventricular fibrillation threshold and, thus, increase the likelihood of an arrhythmia proceeding to ventricular fibrillation. These precipitating factors include electrolyte abnormalities, proarrhythmic medications, alterations in the sympathetic-parasympathetic balance (particularly increased catecholamines), hypothermia or hyperthermia, primary electrical disease (eg, long QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia), and hypoxia/ischemia. These variables may influence myocardial susceptibility to an R-on-T phenomenon, causing depolarization of partially repolarized tissue, potentially initiating ventricular fibrillation.
Ventricular tachycardias
Because ventricular fibrillation is usually a degeneration of ventricular tachycardias, the role ventricular tachycardias play in the evolution of the rhythm disturbance must be considered.
The triggers for ventricular tachycardia are diverse. For more information, see Ventricular Tachycardia and Pediatric Hypertrophic Cardiomyopathy.
Briefly, the triggers for ventricular tachycardia include a long QT interval (eg, congenital, acquired), drug use (eg, digoxin, antiarrhythmics, antidepressants, phenothiazines, terfenadine, erythromycin), alcohol intake, metabolic imbalance (eg, electrolytes, hypoxia, acidemia), coronary artery disease, myocarditis, cardiomyopathy (eg, idiopathic, hypertrophic cardiomyopathy, Chagas disease), mitral valve prolapse(possibly), intracardiac tumors, and congenital heart disease, especially postsurgical interventions. [7, 8]
Wolff-Parkinson-White syndrome
Atrial fibrillation (AF) in the presence of an accessory pathway (bypass tract) that allows extremely rapid antegrade stimulation of the ventricle (>300 beats per minute [bpm]) presents a potential risk for degeneration to ventricular fibrillation in patients with Wolff-Parkinson-White (WPW) syndrome. The risk of rapidly conducting AF depends on the conduction and refractory characteristics of the accessory pathway. These electrophysiologic properties may vary during the day (eg, with catecholamine state associated with exertion, anxiety), by age, and with other clinical variables.
Commotio cordis
Commotio cordis is an uncommon syndrome of abrupt ventricular fibrillation following blunt chest wall trauma that typically occurs in young participants in sports (notably, ice hockey, lacrosse, baseball, and softball). [9, 10, 11]
Commotio cordis is unusual and appears to particularly affect individuals aged 5-15 years; boys are affected more often than girls. Whether this is secondary to increased participation of boys in higher-risk sports activities or differences inherent to each sex is unclear.
Commotio cordis is characterized by a relatively low-energy impact that does not cause structural damage to the chest wall, myocardium, coronary arteries, or elsewhere within the thorax. Ventricular fibrillation is the most common rhythm recorded after an event, although complete heart block and idioventricular rhythms have also been observed. Commotio cordis is notably difficult to convert to sinus rhythm, and survival rates from this type of arrhythmic event are unfortunately quite low.
In an animal study by Link et al, timing of low-energy chest wall impact was coordinated with the cardiac cycle. [10] They found that ventricular fibrillation could be produced when impact occurred at 15-30 milliseconds before the peak of the T wave on ECG; it was not produced at any other time during the cardiac cycle. Ventricular fibrillation was initiated at the time of impact and was not preceded by ventricular ectopy, ischemic changes in ECG, or heart block.
Whether individual susceptibility to commotio cordis occurs or whether it is solely an issue of an electrical timing vulnerability remains unclear. Regardless, survivors of commotio cordis are recommended to wear adequate chest protection during future contact sports participation.
Epidemiology
United States data
The incidence of ventricular fibrillation from all causes is very low in the pediatric population. In studies of pediatric cardiac arrests, ventricular fibrillation was the first identified rhythm in 6-19% of patients, with asystole or PEA as the most frequent rhythm identified first. [12, 13] Overall incidence is likely to be higher because cardiac rhythms frequently change during an arrest, and ventricular fibrillation may have preceded asystole in some patients.
Race-, sex-, and age-related demographics
Although some predisposing factors may demonstrate genetic trends, ventricular fibrillation can be observed throughout all populations.
Vulnerability to ventricular fibrillation is not significantly different between males and females, although, at least in adults, torsade de pointes is more commonly observed in females than in males. In preadolescent children, this sex difference in QT interval range and propensity to torsade de pointes is not evident.
Sudden cardiac death is unusual in pediatric populations, even in children with known cardiac disease; however, patients with congenital heart disease may encounter increasing risk of arrhythmias with or without surgical intervention and as they age. Although terminal rhythms are not often documented in sudden death populations, ventricular fibrillation may represent a final common pathway for these patients.
Various forms of congenital heart disease have been associated with an increased incidence of late sudden death, including tetralogy of Fallot, aortic stenosis, and the atrial switch operations for D-transposition of the great arteries. This may represent an increased incidence of both ventricular tachycardia and ventricular fibrillation vulnerability in this population because the sudden death is presumed to be of arrhythmic etiology. [14]
Prognosis
The short-term prognosis of a patient with ventricular fibrillation is primarily dictated by time to defibrillation, and long-term issues are modulated by any underlying conditions that may have led to the ventricular fibrillation event.
Survival with good outcomes has improved considerably in recent years. A retrospective study of pediatric patients who experienced in-hospital recurrent cardiac arrest events from 2010 to 2018 reported 50% survival to discharge; all survivors had a good neurologic outcome. [15] A study from several decades ago demonstrated a good outcome in 17% of patients presenting with cardiac arrest and ventricular fibrillation, all of whom had early defibrillation. [16] Early defibrillation is essential to a good outcome.
Morbidity/mortality
Without prompt and aggressive therapy, sustained ventricular fibrillation is uniformly lethal. Polymorphic ventricular tachycardia (eg, torsade de pointes) may be sustained or nonsustained, and morbidity is related to the duration of the arrhythmia and to the cardiac output. Some ventricular arrhythmias allow adequate cardiac ejection for a limited period, but once a rhythm degenerates to ventricular fibrillation, ejection is minimal. Until the rhythm is converted, cardiac output is not effective; thus, patients are extremely vulnerable to ischemia and death. [3]
Patient Education
Families of patients with life-threatening arrhythmias, such as ventricular fibrillation, must be competent in bystander CPR and must be aware of the need for early defibrillation.
Any patient who has averted sudden death or has been identified as at risk for such an event is likely to require psychological support and counseling, as may family members.
Driving can be an issue in adolescents with life-threatening arrhythmias. Individual states have restrictions that must be followed that are often based on a certain length of time that the driver has been free of an event.
In patients with long QT syndrome, Brugada syndrome, arrhythmogenic right ventricular dysplasia, familial dilated or hypertrophic cardiomyopathy, and other inherited arrhythmia disorders, family members should be evaluated for the presence of the disease.
For patient education resources, see Heart Health Center, as well as Atrial Fibrillation (A fib) and Cardiopulmonary Resuscitation (CPR).
-
Ventricular fibrillation with polymorphic morphology and cycle lengths varying from 80-280 milliseconds.