Practice Essentials
Celiac disease (CD) is the most common genetically related food intolerance worldwide. Celiac disease is a multifactorial autoimmune disorder that occurs in genetically susceptible individuals. [1] It is triggered by a well-identified environmental factor (gluten and related prolamins present in wheat, rye, and barley), and the autoantigen is also well known (ie, the ubiquitous enzyme tissue transglutaminase). The disease primarily affects the small intestine, where it progressively leads to flattening of the small intestinal mucosa.
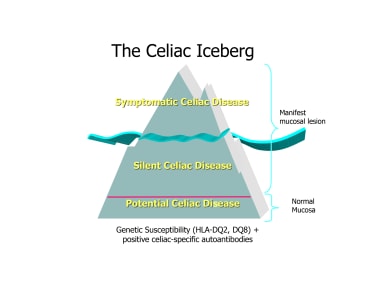
Within this definition, patients can further be defined as having silent or potential celiac disease. [2] The term silent celiac disease refers to patients showing both serologic celiac-specific autoimmunity and consistent changes of the small intestinal pathology, but presenting no symptoms. (See the figure below.) Typically, such diagnoses are made by screening asymptomatic individuals who are at increased risk for celiac disease. The term potential celiac disease describes patients who have specific serum autoantibodies and may or may not have symptoms consistent with celiac disease, but the duodenal biopsy shows no changes of the intestinal mucosa.
Signs and symptoms
Celiac disease may occur without any symptoms; asymptomatic or minimally symptomatic celiac disease is probably the most common form of the disease, especially in older children and adults.
When symptomatic, celiac disease presents with gastrointestinal (GI) and/or extraintestinal manifestations.
GI signs and symptoms characteristically appear at age 9-24 months and can begin at various times after the introduction of foods that contain gluten. Infants and young children typically present with chronic diarrhea, anorexia, abdominal distension, abdominal pain, poor weight gain or weight loss, and vomiting. GI symptoms in older children are typically less evident and include nausea, recurrent abdominal pain, bloating, constipation, and intermittent diarrhea.
The main extraintestinal manifestations of celiac disease are as follows:
-
Dermatitis herpetiformis (more common in adolescents and young adults)
-
Dental enamel hypoplasia
-
Aphthous ulcers
-
Iron-deficiency anemia
-
Short stature and delayed puberty
-
Autoimmune hepatitis and hypertransaminasemia
-
Arthritis and arthralgia
-
Osteopenia (rarely osteoporosis)
-
Neurologic problems
-
Psychiatric disorders
See Presentation for more detail.
Diagnosis
In clinical practice, it is recommended to obtain first serologic tests for celiac disease (namely the immunoglobulin A tissue transglutaminase antibody [anti-TTG-IgA] test) and then to proceed with the intestinal biopsy to diagnose the condition in positive cases. The biopsy can, however, be skipped in selected cases (see below).
See Workup for more detail.
Management
Total lifelong avoidance of gluten ingestion is the cornerstone treatment for patients with celiac disease.
See Treatment and Medication for more detail.
Background
The genetic susceptibility to celiac disease is conferred by well-identified haplotypes in the human leukocyte antigen (HLA) class II region (ie, DR3 or DR5/DR7 or HLA DR4). [3] Such haplotypes are expressed on the antigen-presenting cells of the mucosa (mostly dendritic cells); approximately 90% of patients express the DQ2 heterodimer, and approximately 7% of patients express the DQ8 heterodimer. The remaining 3% of patients possess only half of the DQ2 heterodimer.
Celiac disease can occur at any stage in life; a diagnosis is not unusual in people older than 60 years.
Data from Rubio-Tapia et al [4] showed that undiagnosed celiac disease in the United States has dramatically increased in the past half century, rising from 0.2% in the late 1940s to 0.9% 50 years later.
Pathophysiology
Pathogenesis
Celiac disease is an autoimmune disease, and the enzyme tissue transglutaminase (tTG) has been identified as the autoantigen against which the abnormal immune response is directed. Gluten is the single major environmental factor that triggers celiac disease, which has a narrow and highly specific association with class II haplotypes of HLA DQ2 (haplotypes DR-17 or DR5/7) and, to a lesser extent, DQ8 (haplotype DR-4).
Scientific knowledge on the pathogenesis of celiac disease has markedly increased in the past few years; the combined roles of innate and adaptive immunity are now better understood.
Innate immunity
Intraepithelial lymphocytes (IELs) play an important role in the destruction of epithelial cells. Through specific natural killer receptors (NKR) expressed on their surface, IELs recognize nonclassical major histocompatibility complex (MHC)-I molecules induced on the surface of enterocytes by stress and inflammation. This interaction leads to activation of these armed effector IELs to become lymphokine-activated killing cells; they cause epithelial cell death in a T-cell receptor (TCR)–independent manner. This killing is particularly enhanced through the cytokine interleukin (IL)-15, which is highly expressed in celiac mucosa. NKG2D has been found to play a crucial role in intestinal inflammation in celiac disease. [5]
Adaptive immunity
The adaptive immune response to gluten has been well described, with the identification of specific peptide sequences demonstrated in specific binding to HLA-DQ2 or DQ8 molecules and in stimulating gluten-specific CD4 T cells. These T cells express α/β TCR, and can be isolated from the lamina propria and cultivated. In vitro, they have been shown to recognize specific gluten peptides presented through interaction with DQ2 or DQ8 molecules.
Gluten is a complex macromolecule that contains abundant proline and glutamine residues, rendering it largely indigestible. Under usual circumstances, gluten is left (in part) unabsorbed by the GI tract. Gluten is composed of glutenins and gliadins, the alcohol-water soluble fraction. These gliadins are further divided into alpha, gamma, and omega fractions based on electrodensity. [6]
Among these fractions, one particular peptide fragment is the alpha gliadin 33-mer, which contains an immunodominant peptide fragment. This fragment is deamidated by tTG. tTG is a ubiquitous enzyme and is known to deamidate glutamine to glutamic acid, creating a strong negative charge within the peptide. This modification is crucial in increasing selection to the positive charges within the binding pocket of HLA-DQ2 or DQ8 molecules on antigen-presenting cells in the lamina propria. When conveyed to gluten specific CD4+ T cell, it induces proliferation and induction of a Th1 cytokine response, primarily with the release of interferon-γ.
B cells receive signals through this HLA interaction, leading to tTG autoantibody production. The role of these autoantibodies is still unclear; they have been shown to be deposited along the subepithelial region even in normal-appearing intestinal biopsy findings prior to positive serology and without the onset of overt epithelial cell damage.
Relevant anatomy
Celiac disease primarily affects the small intestine. This organ is schematically divided into three areas: the duodenum (which begins beyond the pylorus), the jejunum, and the ileum (ending at the ileocecal junction, where the large intestine begins). These three parts share similar tissue architecture and are responsible for most of the body's nutrient absorption. The intestinal wall has four layers, which (from the lumen inward) are termed the mucosa, submucosa, muscularis, and serosa. The two main functions of the mucosa are to accomplish all digestive-absorptive processes for nutrients and electrolytes and to provide a barrier function by excluding foreign antigens and toxins.
Celiac disease affects the mucosal layer: here, a cascade of immune events leads to the changes that can be documented by histology.
Pathology
The classic celiac lesion occurs in the proximal small intestine with typical histological changes of villous atrophy, crypt hyperplasia, and increased intraepithelial lymphocytosis. Three distinctive and progressive histological stages have been described and are termed the Marsh classification. [7] The histological changes of celiac disease are classified as follows:
-
Type 0 or preinfiltrative stage (normal)
-
Type 1 or infiltrative lesion (increased intraepithelial lymphocytes)
-
Type 2 or hyperplastic lesion (type 1 plus hyperplastic crypts)
-
Type 3 or destructive lesion (type 2 plus villous atrophy of progressively more severe degrees [termed 3a, 3b, and 3c])
Etiology
Birth modalities appear to influence the onset of celiac disease in genetically predisposed individuals, likely due to the abnormal microbiota in babies born via a cesarean delivery. [8]
Breastfeeding, previously thought to have a protective role, [9] does not in fact appear to play any role. Additionally, early (age ≤3 mo) first exposure to gluten may favor the onset of celiac disease in predisposed individuals. Large amounts of gluten at weaning and for the first 2-3 years of life are associated with an increased risk for developing celiac disease, as is now well documented. [10] Finally, repeated rotavirus infections, [11] but also other infections, [12] in infancy appear to be associated with a higher risk of developing celiac disease autoimmunity in genetically predisposed individuals.
No role for vaccinations in favoring celiac disease has been demonstrated. [13]
Epidemiology
United States statistics
The availability of sensitive and specific serologic tests has made it possible to assess the true prevalence of celiac disease by detecting minimally symptomatic or even asymptomatic cases with typical mucosal changes. [14] Screening studies have shown that celiac disease has a very high prevalence, occurring in almost 1% of the general population throughout North America. [15, 16]
International statistics
Celiac disease is as common in Europe as it is in North America, but it has now been detected in populations from many other parts of the world, including African and Middle Eastern countries, and in Asia, with the highest prevalence worldwide in Saharawi children. [17]
Furthermore, the prevalence of celiac disease appears to be increasing quite dramatically during the past few decades. [15, 18, 19, 16] In Northern Sweden, an epidemiological investigation using a combined serological/endoscopic approach in an unselected population of 1000 adults found a prevalence of almost 2%. [20]
Epidemiological data do document worldwide a true increase in prevalence, with rates doubling approximately every 20 years. A concomitance of environmental factors are likely responsible for this, but most of them are still unclear. Among the hypotheses to explain such increase are: the hygiene hypothesis, [21] increased rates of births through elective cesarean delivery, [22] changes in infant feeding practices as dramatically documented by the so-called Swedish epidemic, [23] and repeated infections—by rotavirus but also generic, nongastrointestinal infections in early infancy. [12]
An investigation in Sweden proved that early vaccinations are not risk factors for the development of celiac disease. [13]
A study reported that children living in socioeconomically deprived areas in the UK are less likely to be diagnosed with CD. The study added that increased implementation of diagnostic guidelines could result in better case identification in more-deprived areas. [24, 25]
Race-, sex-, and age-related demographics
In some ethnicities, such as in the Saharawi population, celiac disease has been found in as many as 5% of the population. As mentioned, celiac disease is considered extremely rare or nonexistent in people of African, Chinese, or Japanese descent.
Most studies indicate a prevalence for the female sex, ranging from 1.5:1 to 3:1.
Celiac disease can occur at any stage in life; a diagnosis is not unusual in people older than 60 years. Classic GI pediatric cases usually appear in children aged 9-18 months. Celiac disease may also occur in adults and is usually precipitated by an infectious diarrheal episode or other intestinal disease.
Prognosis
The prognosis for celiac disease is excellent; the disorder is fully reversible if trigger foods are avoided.
Morbidity/mortality
The morbidity rate of celiac disease can be high. Its complications range from osteopenia, osteoporosis, or both to infertility in women, short stature, delayed puberty, anemia, and even malignancies (mostly related to the GI tract [eg, intestinal T-cell lymphoma]). As a result, the overall mortality in patients with untreated celiac disease is increased.
Evidence also suggests that the risk of mortality is increased in proportion to the diagnostic delay and clearly depends on the diet; subjects who do not follow a gluten-free diet have an increased risk of mortality, as high as 6 times that of the general population. The increased death rates are most commonly due to intestinal malignancies that occur within 3 years of diagnosis. [26, 27] Some indirect epidemiological evidence suggests that intestinal malignancies can be a cause of death in patients with undiagnosed celiac disease. [28]
Complications
Celiac disease is fully reversible—in the majority of patients—if trigger foods are avoided. However, when compliance is suboptimal, complications may occur. The level of gluten that is safe to consume widely varies among people with celiac disease; hence, a zero-tolerance policy must be enforced. Available evidence suggests that although almost no individuals with celiac disease show signs or symptoms of relapse while ingesting as much as 10-20 mg of gliadin per day, most react to ingestion of more than 100 mg/d. [29]
Complications in noncompliant patients include the following:
-
Osteopenia (true osteoporosis is more common in adults)
-
Adverse effects during pregnancy, including miscarriages
-
Ulcerative jejunitis, colitis, refractory celiac disease (thought to be a low-grade intestinal lymphoma, but not reported in pediatric patients)
-
GI malignancies, most commonly an enteropathy-associated T-cell lymphoma (EATL), also not reported in pediatric patients
-
A population-based study by Canova et al found no evidence of an increased risk of fractures for pediatric celiac disease patients (overall hazard ratio, 0.87) [30]
Children with celiac disease typically respond well to a gluten-free diet, with rapid improvement of most symptoms, especially GI symptoms. [31] Systemic and extraintestinal symptoms improve less quickly. [32]
However, the occurrence of so-called "nonresponsive celiac disease" (NRCD, defined as the lack of a clinical and/or laboratory response to a gluten-free diet after 12 months) has also been well documented and is thought to affect about 15% of newly diagnosed children. [33] Of interest, about half of such patients have either ongoing gluten ingestion (usually involuntary) or a functional GI disorder such as constipation or irritable bowel syndrome. [33] In some cases in which no other causes can be found, temporary use of budesonide accompanied by a particularly rigorous, medically supervised diet has been suggested. [34]
The most serious complications of celiac disease, namely refractory celiac disease and GI cancers of various types and particularly enteropathy-associated T-cell lymphoma (EATL), have not been reported in pediatric patients.
Patient Education
A consensus report by Ludvigsson et al stated that in adolescence, patients with celiac disease should gradually assume exclusive responsibility for their own care, learning how to follow a gluten-free diet and the consequences of not following it. [35]
In modern society, living a life without gluten is not easy. Educating patients and their families about how to select and properly maintain such a diet is a major, ongoing task.
The role of support groups can never be overestimated. The physician has a duty to care for patients with celiac disease and to adequately inform the family about how to connect with such groups.
Several university-associated centers that provide excellent materials for patient education are now available in the United States (eg, the University of Chicago Celiac Disease Center) and in Europe. In the United States, the American Celiac Disease Alliance (ACDA) offers patient education as well as links to other centers.
For excellent patient education resources, see the Digestive Disorders Center and Skin Conditions & Beauty Center. Also, see the patient education articles Celiac Disease, Anatomy of the Digestive System, and Canker Sores.
-
Potbelly and muscle wasting in a child with celiac disease.
-
The celiac iceberg.
-
Presentations of celiac disease.
-
Extraintestinal manifestations of celiac disease.
-
GI signs and symptoms of celiac disease.
-
Approximate prevalence of celiac disease in other autoimmune disorders.