Practice Essentials
Prematurity is a term for the broad category of neonates born at less than 37 weeks' gestation. Preterm birth is the leading cause of neonatal mortality and the most common reason for antenatal hospitalization. [1] For premature infants born with a weight below 1000 g, the three primary causes of mortality are respiratory failure, infection, and congenital malformation.
Preventing premature birth is a challenge for obstetricians. Specialized centers with a maternal-fetal medicine specialist can play a significant role in managing high risk pregnancies.
Signs and symptoms
Confirmation of gestational age is based on physical and neurologic characteristics. The Ballard Scoring System remains the main tool clinicians use after delivery to confirm gestational age by means of physical examination. [2] The major parts of the anatomy used in determining gestational age include the following:
-
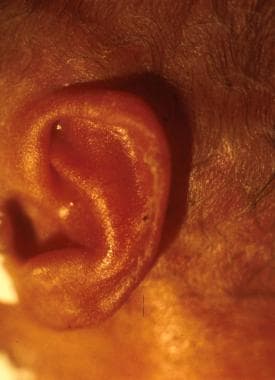
Ear cartilage (eg, a preterm infant at 28 weeks' gestation has a small amount of ear cartilage and/or a flattened pinna) (See the following image.)
-
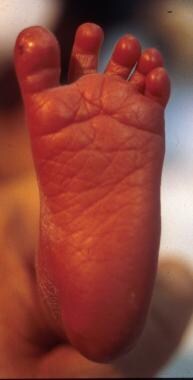
Sole (eg, a preterm infant at 33 weeks' gestation has only an anterior crease on the sole of the foot) (See the image below.)
-
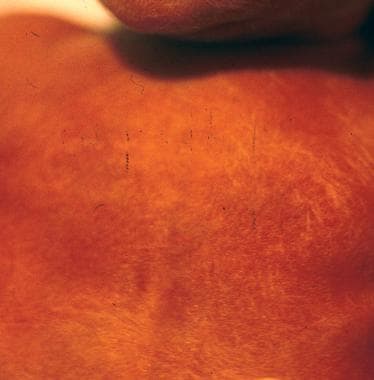
Breast tissue (eg, a preterm infant at 28 weeks' gestation has no breast tissue, and the areolae are barely visible) (See the following image.)
-
Genitalia
See Presentation for more detail.
Diagnosis
Laboratory studies
Initial laboratory studies in cases of prematurity are performed to identify issues that, if corrected, improve the patient's outcome. Such tests include the following:
-
Frequent blood glucose measurement: This is essential because premature infants are prone to hypoglycemia and hyperglycemia.
-
Complete blood count (CBC): Anemia or polycythemia may be revealed that is not clinically apparent and thrombocytopenia will be known form CBC.
-
White blood cell (WBC) count: A high or low WBC count and numerous immature neutrophil types may be found; an abnormal WBC count may suggest subtle infection or due to maternal preeclampsia. [3]
-
Blood type and antibody testing (Coombs test): These studies are performed to detect blood-group incompatibilities between the mother and infant and to identify antibodies against fetal red blood cells (RBCs); such incompatibilities increase the risk for jaundice and kernicterus.
-
Serum electrolyte levels: Frequent determination of serum sodium, potassium, calcium, and glucose levels, in conjunction with monitoring of daily weight and urine output in extremely low birth weight (ELBW) infants, assist the clinician in managing fluid and electrolytes.
Imaging studies
Imaging studies are specific to the organ system affected. Chest radiography is performed to assess the lung parenchyma and heart size in newborns with respiratory distress. Cranial ultrasonography is performed to detect occult intracranial hemorrhage in premature infants.
Lumbar puncture
Lumbar puncture is performed in premature infants with positive blood cultures and in those who have clinical signs of central nervous system infection. The decision to perform lumbar puncture in ELBW premature infants can sometimes be a difficult one because of their size and the surrounding clinical circumstances. However, when feasible, lumbar puncture should be performed; this will help in determining the duration of antibiotic therapy if CNS infection is suspected.
See Workup for more detail.
Management
Stabilization in the delivery room with prompt respiratory and thermal management is crucial to the immediate and long-term outcome of premature infants, particularly extremely premature infants.
Respiratory management
-
Recruitment and maintenance of optimal lung volume in infants with respiratory distress: This step can be accomplished with early use of continuous positive airway pressure (CPAP) given nasally, by nasal mask, or by using an endotracheal tube when mechanical ventilation and/or surfactant is administered.
-
Avoidance of hyperoxia or hypoxia with the aid of pulse oximetry: Always use blended oxygen with an oxygen saturation target range (SaO2) of 90-95%. The lower limits of the pulse oximeter alarm should be set closer to the lower saturation limit, and the upper alarm limit should be no more than 95%. [4]
-
Prevention or minimization of barotrauma or volutrauma should always be the goal when using a mechanical ventilator. Normal tidal volume is 4-7 mL/kg.
-
Early administration (age < 2 hours) of surfactant is recommended when indicated. Routine use of prophylactic surfactant solely for prematurity is not advisable.
Thermoregulation
It is very important to maintain normal temperature in any newborn, but this is particularly important for premature infants. Use radiant warmers with skin probes to regulate the desired temperature (in general, a normal body temperature of 36.5º-37.5ºC [97.7º-99.5ºF] [5] ). A heated and humidified isolette is ideal for ELBW infants. Food-grade plastic wrap/sheets can also be very helpful immediately after birth to control humidity and prevent heat loss in ELBW infants. Warm newborn hat is also a must to prevent hypothermia in premature infant. The environmental temperature should be maintained to at least 25ºC (77º F). [5]
Fluid and electrolyte management
Preterm infants require close monitoring of their fluid and electrolyte levels for several reasons (eg, immature skin increases transepidermal water loss; immature kidney function [6] ; the use of radiant warmer, phototherapy, mechanical ventilation). The degree of prematurity dictates the initial fluid management. The following are general principles of fluid and electrolyte management when caring for premature infants:
-
The initial fluid should be a solution of glucose and water.
-
Calcium may be added in the initial fluid.
-
Total parenteral nutrition should be started as early as possible, especially for ELBW infants, some centers do start starter TPN with basic ingredients to prevent negative nitrogen balance.
Close monitoring of glucose and electrolyte levels as well as acid-base balance is the key when managing ELBW infants. Strict monitoring of input and output is crucial. Thus, urine output, serum electrolyte levels, and daily weight are critical in handling fluids and electrolytes in premature infants.
See Treatment and Medication for more detail.
Background
Prematurity refers to the broad category of neonates born at less than 37 weeks' gestation. Preterm birth is the leading cause of neonatal mortality and the most common reason for antenatal hospitalization. [1] Although the estimated date of confinement (EDC) is 40 weeks' gestation, the World Health Organization (WHO) broadened the range of full term to include 37-42 weeks' gestation.
Premature newborns have many physiologic challenges when adapting to the extrauterine environment. The near-term neonate (34-36 weeks' gestation) has issues of prematurity that include feeding immaturity, temperature instability, and prolonged jaundice. Serious morbidities occur in extremely low birth weight (ELBW) infants. Articles in the Neonatology section discuss in detail the most serious of these problems.
For more information, see Extremely Low Birth Weight Infant, Pediatric Acute Respiratory Distress Syndrome, Bronchopulmonary Dysplasia, Intraventricular Hemorrhage in the Preterm Infant.
Pathophysiology
Before birth, the placenta serves three major roles for the fetus: provision of all the nutrients for growth, elimination of fetal waste products, and synthesis of hormones that promote fetal growth.
With the exception of most electrolytes, the maternal circulation contains more substrate (eg, blood glucose) than the fetal circulation. In addition, the placenta is metabolically active and consumes glucose. Waste products of fetal metabolism (eg, heat, urea, bilirubin, carbon dioxide) are transferred across the placenta and eliminated by the mother's excretory organs (ie, liver, lung, kidneys, skin).
In addition, the placenta acts as a barrier to infection through mucosal macrophages and by allowing transfer of maternal immunoglobulins (immunoglobulins such as immunoglobulin G [IgG]) to the fetus beginning at 32-34 weeks' gestation. Placental dysfunction is involved in the transfer of IgG. Antibacterial activity of the amniotic fluid improves as gestational age advances.
Each of the immature organs of a premature infant has functional limitations. The tasks of caregivers in neonatal intensive care units (NICUs) are to recognize and monitor the needs of each infant and to provide appropriate support until functional maturity can be achieved.
Etiology
Premature delivery can be the result of preterm labor and preterm premature rupture of the membranes (PPROM), or it can be due to maternal indications (eg, pregnancy-induced hypertension).
Chorioamnionitis
Maternal fever (>38ºC [>100.4ºF]), fetal tachycardia, maternal leukocytosis (>15,000-18,000/mm3 [15-18 × 109/L]), and uterine tenderness are universally accepted signs and symptoms of chorioamnionitis. Amniocentesis that demonstrates bacteria, white blood cells (WBCs), and a low glucose concentration confirms the diagnosis of chorioamnionitis and is an indication for delivery.
A decrease in the biophysical score or profile in association with chorioamnionitis is associated with fetal infection.
Rates of perinatal mortality, neonatal infection, and respiratory distress syndrome (RDS) increase in the presence of maternal fever and chorioamnionitis.
Intrauterine growth restriction (10th percentile for birth weight)
Intrauterine growth restriction is significantly associated with perinatal mortality and long-term morbidity.
Low socioeconomic status
Programs offering additional social support for at-risk pregnant women have not been demonstrated to reduce the numbers of extremely low birth weight (ELBW) or preterm infants.
Maternal diabetes
Pregnancies complicated by diabetes and poor glycemic control are associated with a high incidence of prematurity, macrosomia, malformation, fetal death, and neonatal death. [7]
The rate of preterm birth (< 37 weeks' gestation) is 20-22% of persons with insulin-dependent diabetes.
In women with diabetes diagnosed before pregnancy, the frequency of preeclampsia is increased as the severity of diabetes increases. [8]
Multiple gestation pregnancies
Women with multiple gestation pregnancies are at high risk of preterm labor and delivery and account for an increasing percentage of preterm births and ELBW infants. Preterm birth rate for twins increased from 40.9% in 1981 to 55% in 1997. [9]
Multiple births related to infertility treatment have dramatically increased. [10] With advances in assisted reproductive technology, multiple gestation pregnancies have increased. [11] Prepregnancy counseling of prospective parents regarding the risks related to multiple gestations is important.
Preterm birth (< 35 weeks' gestation) occurs in 26% of twins compared with 3% of singletons.
Triplet pregnancies are associated with an increased incidence of preterm labor and delivery at a decreased gestational age and birth weight, compared with singletons and twins. [12] When the data are controlled for gestational age, outcomes are similar for singletons, twins, and triplets.
Maternal age
In women aged 13-15 years, the rate of preterm birth is 5.9%. [13] This rate declines to 1.7% in women aged 18-19 years and 1.1% in women aged 20-24 years.
The rate of preterm births increases in pregnancies in which the mother is older than 40 years. The scoring system for the risk of preterm delivery uses a criterion of age older than 40 years.
Tobacco or marijuana use
Approximately 15-20% of pregnant women smoke tobacco. Tobacco use is a risk factor for placental abruption and accounts as a factor for 15% of preterm births and 20-30% of ELBW infants. [14, 15]
Maternal marijuana use appears to be associated with an increased risk of neonatal morbidity (eg, infection and neurologic morbidity) or death. [16]
Epidemiology
United States data
In the general population, an estimated 10.5% of infants are born prematurely, [17] and about 50% of preterm births are preceded by preterm labor. [1, 18]
International data
Exact international numbers are not available because different countries use different definitions of birth (eg, survival after birth, survival after 1 month). Chawanpaiboon et al examined the national civil registration and vital statistics databases of 194 WHO Member States from 1990 to 2014. They estimated the global preterm birth rate for 2014 was 10.6%, of which more than 80% of preterm births were in south Asia and Sub-Saharan Africa. [19, 20]
Race- and sex-related demographics
Premature infants are born to women of every race. Extremely low birth weight (ELBW) infants are most commonly born to women of low socioeconomic status, black women, teenage females, and women older than 40 years. Women at highest risk of premature delivery can be assessed by using a scoring system that reviews their socioeconomic status, history, daily habits, and current pregnancy events. [21] About 30% of women with a high-risk score deliver prematurely compared to 2.5% of women with a low-risk score.
Primarily because of the increased incidence of preterm infants, the overall neonatal mortality rate in black populations in the United States is 2.3 times that of white populations. Improvements in socioeconomic status and perinatal care have not improved the rate of prematurity and infant mortality rate in this population.
The results from a study (N = 2549 neonates) noted that male infants born prematurely have a higher risk of grade III/IV intraventricular hemorrhage, sepsis, and major surgery than premature females. A greater risk of mortality and poorer long-term neurologic outcome were also noted; however, sex-related differences for these appeared to lose significance at 27 weeks' gestation. [22]
Female sex is associated with increased rates of survival of newborns born at 22-25 weeks' gestation.
Prognosis
Mortality and morbidity are inversely proportional to gestational age and birth weight. Infants with extremely low birth weight (ELBW) who are born at tertiary care centers have outcomes more favorable than those who are born at level I or II centers and then transferred.
Roberts et al found that children born at 22-27 weeks' gestation have high rates of adverse neurodevelopmental outcome at age 8 years. [23] Assessment of a regional cohort of 144 survivors of preterm birth showed that, relative to matched term controls, the preterm cohort had substantially higher rates of blindness, deafness, cerebral palsy, and intellectual impairment and disabilities caused by these impairments. Comparison of preterm children born in 1997 with those born in 1991-1992 showed that the rates of moderate or severe disability were similar in the two cohorts (19%), but the rate of mild impairment was greater in 1997 (40% vs 24%); disability rates in control groups showed virtually no change over time. [23]
Infants born at born at 23-25 weeks of gestation who receive antenatal exposure to corticosteroids appear to have a lower rate of mortality and complications compared with those who do not have such exposure. [24] Infants born at 34-36 weeks' gestation with antenatal exposure to corticosteroids between 24 and 34 weeks of gestation also appear to have a lower incidence of respiratory disorders. [25]
Morbidity/mortality
Preterm births account for approximately 70% of neonatal deaths and 36% of infant deaths, as well as 25-50% of cases of long-term neurologic impairment in children. [1]
The mortality rate is high in developing countries, especially those of Sub-Saharan Africa. The perinatal mortality rate is 70 deaths per 1000 births; the neonatal mortality rate is 45 deaths per 1000 live births. Preterm birth is the strongest independent predictor of mortality in the United States. Preterm delivery accounts for 75-80% of all neonatal morbidity and mortality.
Since the early 1960s, survival rates of premature infants substantially increased because of technological advances. From 1989-1990, infants with birth weights less than 751 g had a survival rate of 39% (range among centers, 23-48%). In 1992, the US Food and Drug Administration (FDA) approved exogenous surfactant therapy for respiratory distress syndrome (RDS), leading to a considerable improvement in survival rates. Since the FDA approved the use of surfactant and since the subsequent introduction of numerous natural surfactants, the mortality rate attributed to surfactant deficiency has been markedly reduced. See Respiratory Distress Syndrome.
Data from the Vermont Oxford Network in 1994-1996 indicated that the survival rate of infants born weighing less than 1000 g was 74.9%. [26] Survival of infants born weighing less than 1000 g and requiring cardiopulmonary resuscitation in the delivery room was substantially decreased (53.8%). The changes in obstetric and neonatal care in the first half of the decade of 1990s decreased mortality and morbidity for ELBW infants. No additional improvements in mortality and morbidity were observed at the end of the decade.
Obstetric and pediatric personnel must be familiar with their own institutional data in addition to national benchmarks related to gestational age and mortality rates. These data are essential for proper prenatal counseling of parents and/or caregivers regarding survival and resuscitation plans.
The three primary causes of mortality in infants born with a weight of less than 1000 g are respiratory failure, infection, and congenital malformation. Infection of the amniotic fluid leading to pneumonia is the major cause of mortality. [27] In infants who weigh less than 500 g at birth, immaturity is listed as the only cause of mortality. See Ethical Issues in Neonatal Care.
Women who have an intrauterine infection do not respond to tocolytics. Preterm premature rupture of membranes (PPROM) is associated with 30-40% of premature deliveries. See Premature Rupture of Membranes. Mortality of the premature infant increases with coexisting PPROM but depends on gestational age and the expertise of the maternal-fetal monitoring team. Postnatal findings of periventricular leukomalacia (PVL) on cranial ultrasonography are highly correlated with chorioamnionitis.
In premature infants with a congenital heart defect (CHD), excluding isolated patent ductus arteriosus, the actuarial survival rate is 51% at 10 years, whereas infants with both CHD and prematurity have substantially worsened outcomes than infants who only have one of these conditions. [28] The survival rate improved as the study period (1976-1999) progressed. Congenital anomalies are an independent risk factor for mortality and morbidity in preterm birth.
In a longitudinal study of 1279 extremely premature children, (gestational age ≤28 week; birth weight < 1250 g), Robertson et al found permanent hearing loss in 3.1% and severe-to-profound loss in 1.9%. [29] Among affected children, hearing loss was delayed in onset in 10% and progressive in 28%. Prolonged supplemental oxygen use was the most important marker for predicting hearing loss.
In a retrospective analysis of data from 20,231 live births recorded between 1995 and 2003, Werner et al found that very premature infants who are delivered vaginally have fewer breathing problems than do those delivered by cesarean section. [30, 31] All of the study's infants were born after 24-34 weeks' gestation, with 69.3% of them delivered vaginally. In comparison with the vaginally delivered infants, those delivered by cesarean section were more likely to be born in respiratory distress (39.2% compared with 25.6% for vaginal delivery). Infants in the study who underwent cesarean delivery were also more likely than vaginally delivered infants to have a 5-minute Apgar score of less than 7 (10.7% vs 5.8%, respectively). [30, 31]
Complications
Premature infants are prone to have complications. These complications are gestational age dependent. Complications are inversely proportionate to gestational age. [20] These complications can range from devastating intracranial hemorrhage, sepsis, severe bronchopulmonary dysplasia (chronic lung disease), and necrotizing enterocolitis totalis to minor feeding related issues. Fortunately, not every premature infant experiences complications. Long-term consequences of prematurity can affect the cardiovascular and central nervous systems as well as the pulmonary, renal, and endocrine systems. [20]
Patient Education
Communicate clearly to the expectant parents regarding potential adverse outcomes. Clear up any inappropriate expectations on the part of the family. The smallest and most immature infants are at greatest risk of adverse neurodevelopmental outcomes.
When the premature infant is stabilized, parents or caregivers should participate in providing daily routine care. These kind of opportunities help foster a strong bond between the infant and the caregiver. Premature infants require social interaction on a regular basis to enhance the neurodevelopmental outcome. Book reading, storytelling, and singing with a soft voice can be part of routine social interaction with growing premature infants.
Discharge education
Discharge teaching of caretakers of the premature infant includes the following:
-
Basic infant care: Bathing, skin care, taking a temperature
-
Infant feeding: Feeding cues, support of breastfeeding
-
Infant safety: Use of car seats, avoiding exposure to a smoky environment
-
Back to sleep: Strategies to help preterm infants return to sleep
-
Illness prevention (handwashing, crowd avoidance, prophylaxis against infection with respiratory syncytial virus [RSV] as indicated, compliance with immunization schedule)
-
When to call healthcare provider: Poor feeding, signs of illness, change in behavior, respiratory distress
-
Specifics related to chronic conditions (eg, use of a nasal canula, home oxygen therapy)
-
Preterm infant at 28 weeks' gestation. Note the small amount of ear cartilage and/or flattened pinna.
-
Preterm infant at 33 weeks' gestation. Note the increased cartilage, recoil, and outer ridge curving inward.
-
A term infant has well-developed cartilage with instant recoil.
-
Preterm infant at 28 weeks' gestation. Note the flat sole.
-
Preterm infant at 33 weeks' gestation. Note the presence of only an anterior crease.
-
Term gestation. Note the multiple creases.
-
Preterm infant at 28 weeks' gestation. No breast tissue is present, and the areolae are barely visible.
-
Preterm infant at 33 weeks' gestation. The breast tissue is less than 1 cm, and the areolae are raised and/or pigmented.