Practice Essentials
Oliguria is defined as a urine output that is less than 1 mL/kg/h in infants, less than 0.5 mL/kg/h in children, and less than 400 mL daily in adults. It is one of the clinical hallmarks of renal failure and has been used as a criterion for diagnosing and staging acute kidney injury (AKI), previously referred to as acute renal failure. At onset, oliguria is frequently acute. It is often the earliest sign of impaired renal function and poses a diagnostic and management challenge to the clinician. (See Presentation and Workup.) [1, 2]
Background
A standardized definition of AKI has been proposed by the Kidney Disease: Improving Global Outcomes (KDIGO) AKI working group, which identifies and stages AKI based on changes in serum creatinine from baseline or a decrease in urine output (oliguria) as shown below. [3]
KDIGO Staging of AKI (Open Table in a new window)
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 1 | Increase by 1.5-1.9 times baseline within 7 days OR Increase by 0.3 mg/dL (26.5 µmol/L) or more within 48 hours |
Less than 0.5 mL/kg/h for 6-12 hours |
| 2 | Increase by 2-2.9 times baseline | Less than 0.5 mL/kg/h for 12 hours or longer |
| 3 | Increase by 3 times baseline or greater OR Increase to 4 mg/dL (353.6 µmol/L) or greater OR Renal replacement therapy initiation OR In patients younger than 18 years, decrease in estimated GFR to less than 35 mL/min/1.73m2 |
Less than 0.3 mL/kg/h for 24 hours or longer OR Anuria for 12 hours or longer |
In a cohort study of adults in an intensive care unit, Bianchi et al found that more than 77% of the patients met KDIGO criteria for AKI. The use of urinary output criteria enabled the researchers to detect AKI in patients who did not meet the serum creatinine criteria. Those patients whose AKI was identified solely by urinary output criteria, with no increase in serum creatinine, had a higher 90-day mortality than those without AKI. In addition, oliguria that lasted more than 12 hours (KDIGO stage 2 or 3) was associated with higher 90-day mortality, regardless of changes in serum creatinine levels. [4]
Not all cases of acute kidney injury are characterized by oliguria. Renal failure that results from nephrotoxic injury, interstitial nephritis, or neonatal asphyxia is frequently of the nonoliguric type, is related to a less severe renal injury, and has a better prognosis. In addition, the degree of oliguria depends on hydration and the concomitant use of diuretics.
In most clinical situations, acute oliguria is reversible and does not result in intrinsic renal failure. However, identification and timely treatment of reversible causes is crucial because the therapeutic window may be small. (See Prognosis, Presentation, Workup, Treatment, and Medication.)
Patient education
For patient education information from eMedicineHealth, see the Diabetes Center, as well as Acute Kidney Failure and Chronic Kidney Disease.
Etiology
Oliguria may result from prerenal, intrinsic renal, or postrenal processes.
Prerenal failure
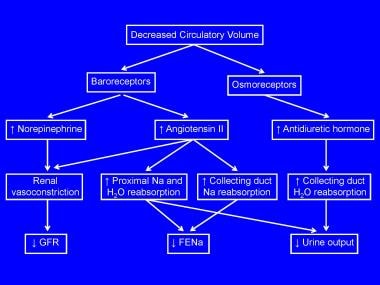
Prerenal insufficiency is a functional response of structurally normal kidneys to hypoperfusion. Globally, prerenal insufficiency accounts for approximately 70% of community-acquired cases of acute renal failure and as many as 60% of hospital-acquired cases. A decrease in circulatory volume evokes a systemic response aimed at normalizing intravascular volume at the expense of the glomerular filtration rate (GFR). Baroreceptor-mediated activation of the sympathetic nervous system and renin-angiotensin axis results in renal vasoconstriction and the resultant reduction in the GFR. The early phase also includes enhanced tubular reabsorption of salt and water (stimulated by the renin-angiotensin-aldosterone system and sympathetic nervous system), resulting in a decrease in fractional excretion of sodium (FENa) and decreased urine output. See the image below.
The early phase of renal compensation for reduced perfusion includes autoregulatory maintenance of the GFR via afferent arteriolar dilatation (induced by myogenic responses, tubuloglomerular feedback, and prostaglandins) and via efferent arteriolar constriction (mediated by angiotensin II). These changes are shown in the image below.
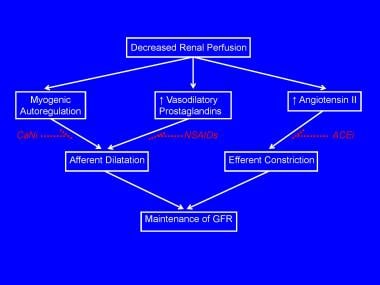 Compensatory mechanisms for preventing a fall in glomerular filtration rate (GFR) in the presence of prerenal failure
Compensatory mechanisms for preventing a fall in glomerular filtration rate (GFR) in the presence of prerenal failure
Iatrogenic interference with renal autoregulation by administration of vasoconstrictors (eg, cyclosporine, tacrolimus), inhibitors of prostaglandin synthesis (eg, nonsteroidal anti-inflammatory drugs), or angiotensin-converting enzyme (ACE) inhibitors can precipitate oliguric acute renal failure in individuals with reduced renal perfusion.
Rapid reversibility of oliguria following timely reestablishment of renal perfusion is an important characteristic and is the usual scenario in prerenal insufficiency. For example, oliguria in infants and children is most often secondary to dehydration and reverses without renal injury if the dehydration is corrected. However, prolonged renal hypoperfusion can result in a deleterious shift from compensation to decompensation.
This decompensation phase is characterized by excessive stimulation of the sympathetic and renin-angiotensin systems, with resultant profound renal vasoconstriction and ischemic renal injury.
Intrinsic renal failure
Intrinsic renal failure is associated with structural renal damage. This includes acute tubular necrosis (from prolonged ischemia, drugs, or toxins), primary glomerular diseases, or vascular lesions. Recent experimental studies indicate that prerenal and intrinsic renal failure are distinct molecular entities and hence different disease states, despite similar degrees of oliguria and similar increases in serum creatinine concentrations. Prerenal failure is characterized by induction of protective molecular mechanisms within the kidney, whereas intrinsic renal failure upregulates kidney genes and pathways of cell injury, cell death, and inflammation. [5]
Advancements in the care of critically ill neonates, infants with congenital heart disease, and children who undergo bone marrow and solid organ transplantation have led to a dramatic broadening of the etiology of pediatric acute kidney injury. Although multicenter etiologic data on pediatric acute renal failure are not available, single-center data and literature reviews from the 1980s and 1990s reported hemolytic uremic syndrome and other primary renal diseases as the most prevalent causes. [6, 7]
Subsequent single-center data have detailed the underlying causes of pediatric acute renal failure in large cohorts of children. In a study of 226 children with acute renal failure, Bunchman et al reported that congenital heart disease, acute tubular necrosis, sepsis, and bone marrow transplantation were the most common causes. [8]
A retrospective review of 248 patients with a diagnosis of acute renal failure upon discharge or death revealed acute tubular necrosis and nephrotoxins to be the most common causes of acute kidney injury. [9] Thus, the etiology of pediatric acute renal failure has evolved in industrialized countries from primary kidney diseases or prerenal failure to secondary effects of other systemic illnesses or their treatment.
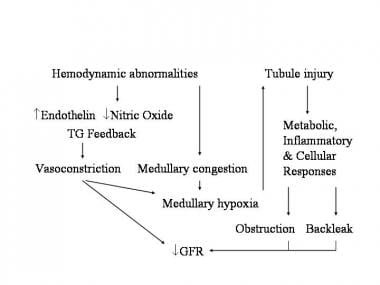
The pathophysiology of ischemic acute tubular necrosis is well studied. Ischemia leads to altered tubule cell metabolism (eg, depletion of adenosine triphosphate [ATP], release of reactive oxygen species) and cell death, with resultant cell desquamation, cast formation, intratubular obstruction, backleak of tubular fluid, and oliguria. (See the image below.)
In most clinical situations, the oliguria is reversible and associated with repair and regeneration of tubular epithelial cells.
Postrenal failure
Postrenal failure is a consequence of the mechanical or functional obstruction of the flow of urine. This form of oliguria and renal insufficiency usually responds to the release of the obstruction.
Principal causes of oliguric acute kidney injury in neonates
The etiology of oliguria varies with age, and the common causes in neonates and children are listed separately. Patients with acute kidney injury secondary to nephrotoxins, interstitial nephritis, and perinatal asphyxia frequently do not have oliguria.
Prerenal causes include the following:
-
Perinatal asphyxia
-
Respiratory distress syndrome
-
Hemorrhage - Eg, maternal antepartum, twin-twin transfusion, and intraventricular
-
Hemolysis
-
Polycythemia
-
Sepsis or shock
-
Congenital heart disease
-
Dehydration
-
Drugs - Eg, indomethacin, maternal nonsteroidal anti-inflammatory drugs (NSAIDs), and maternal ACE inhibitors
Intrinsic renal causes include the following:
-
Acute tubular necrosis
-
Exogenous toxins - Eg, aminoglycosides, amphotericin B, and contrast agents
-
Endogenous toxins - Eg, hemoglobin, myoglobin, and uric acid
-
Congenital kidney disease - Eg, agenesis, polycystic kidney, hypoplasia, and dysplasia
-
Vascular - Eg, renal vein thrombosis and renal artery thrombosis
-
Transient renal dysfunction of the newborn
Postrenal causes include the following:
-
Bladder outlet obstruction - Eg, posterior urethral valves and meatal stenosis
-
Neurogenic bladder
-
Ureteral obstruction, bilateral
Principal causes of oliguric acute kidney injury in children
Prerenal causes include the following:
-
Gastrointestinal (GI) losses - Eg, vomiting and diarrhea
-
Blood losses - Eg, hemorrhage
-
Renal losses - Eg, diabetes insipidus, diabetes mellitus, diuretics, and salt-wasting nephropathy
-
Cutaneous losses - Eg, burns
-
Third space losses - Eg, surgery, trauma, nephrotic syndrome, and capillary leak
-
Shock - Eg, septic, toxic, and anaphylactic
-
Impaired autoregulation - Eg, cyclosporine, tacrolimus, ACE inhibitors, and NSAIDs
-
Impaired cardiac output - Eg, congenital and acquired heart disease
Intrinsic renal causes include the following:
-
Acute tubular necrosis - Eg, prolonged prerenal failure
-
Glomerulonephritis
-
Interstitial nephritis, vascular - Eg, hemolytic-uremic syndrome and vasculitis
-
Exogenous toxins - Eg, aminoglycosides, amphotericin B, cyclosporine, chemotherapy, heavy metals, and contrast agents
-
Endogenous toxins - Eg, hemoglobin, myoglobin, and uric acid
-
Transplant rejection
Postrenal causes include the following:
-
Bladder outlet obstruction - Eg, posterior urethral valves, blocked catheter, and urethral trauma
-
Neurogenic bladder
-
Ureteral obstruction, bilateral
Epidemiology
Occurrence in North America
The frequency of oliguria widely varies depending on the clinical setting. In adults, the incidence is about 1% at admission, 2-5% during hospitalization, and 4-15% after cardiopulmonary bypass.
Oliguric acute kidney injury occurs in approximately 10% of newborn intensive care unit (ICU) patients. The incidence in children undergoing cardiac surgery is as high as 10-30%. Among critically ill children admitted to pediatric ICUs (PICUs), the incidence of acute kidney injury defined by doubling of serum creatinine is present in about 5-6%. This was illustrated by a prospective study from a Canadian PICU that identified 985 cases of acute kidney injury for an incidence rate of 4.5% of all PICU admissions. [10] In the largest study reported to date, 3396 admissions to a single PICU in the United States were retrospectively analyzed. [11] Using serum creatinine criteria, 6% of children had acute kidney injury on admission and 10% developed acute kidney injury during their PICU stay.
A recent landmark international study of 4683 critically ill children utilized the KDIGO definition of AKI to demonstrate that the incidence of AKI was 27% when both serum creatinine and urine output criteria were used. Notably, more than two-thirds of the patients with oliguria did not meet the serum creatinine criteria for AKI – and low urine output alone conferred a significantly increased risk of death compared to elevated serum creatinine alone. [12]
Age-related demographics
Oliguria affects people of all ages. It is more common in neonatal and older age groups because of comorbid conditions and is more common in early childhood because of the high incidence of illnesses that lead to dehydration.
Prognosis
Mortality rates in oliguric acute kidney injury widely vary according to the underlying cause and associated medical condition. It ranges from 5% for patients with community-acquired kidney injury failure to 80% among patients with multiorgan failure in the ICU.
In general, severe acute kidney injury can have serious short- and long-term consequences. The outcome depends upon the etiology, age of the child, and comorbidities. In terms of mortality, severe acute kidney injury requiring renal replacement therapy in children is still associated with a mortality rate of about 30-50%, and this has not changed appreciably over the past 3 decades. Infants younger than 1 year have the highest mortality rate.
In a PICU cohort, patients who presented with acute kidney injury on admission had a 32% mortality rate, and those who developed acute kidney injury at any time during the PICU stay had a 30% mortality rate. [11] Additionally, those with any degree of acute kidney injury at the time of PICU admission had higher PICU mortality than those with normal kidney function. Moreover, patients who developed any degree of acute kidney injury during PICU stay had higher ICU mortality than those without acute kidney injury during PICU stay. Multivariate logistic regression modeling controlling for age, sex, weight, race, and pediatric index of mortality score confirmed that acute kidney injury on admission to the PICU was associated with an increased risk of mortality (adjusted odds ratio, 5.4; 95% CI, 3.5-8.4). Development of acute kidney injury during the PICU stay was associated with an even greater risk of mortality (adjusted odds ratio, 8.7; 95% CI, 6.0-12.6) and a 4-fold increase in length of hospital stay.
In a retrospective analysis of 344 patients from the Prospective Pediatric Continuous Renal Replacement Therapy (ppCRRT) Registry, the overall mortality rate was 42%. [13] Survival was lowest in liver disease/transplantation (31%), pulmonary disease/transplantation (45%), and bone marrow transplantation (45%). Overall survival was better for children who weighed more than 10 kg (63% vs 43%; P = .001) and for those who were older than 1 year (62% vs 44%; P = .007).
Thus, it is now clear that patients die of acute kidney injury and its complications, and not simply with acute kidney injury. [14] The patient succumbs largely because of involvement of multiple other systems during the period of severe oliguric renal insufficiency. The most common causes of death are sepsis and cardiovascular or pulmonary dysfunction.
Information regarding the long-term outcome of children after an episode of severe acute kidney injury is scant but is beginning to accumulate. [15]
In a multicenter pooled analysis of 3476 children with hemolytic uremic syndrome followed for a mean of 4.4 years, [16] the combined average death and end-stage renal disease (ESRD) rate was 12% (95% CI, 10-15%) and the combined average renal sequelae rate (chronic kidney disease, proteinuria, hypertension) was 25% (95% CI, 20-30%). Thus, long-term follow-up appears to be warranted after an acute episode of hemolytic uremic syndrome.
In a retrospective study of 176 children who developed acute kidney injury in a single center, 34% had either reduced kidney function or were dialysis dependent at hospital discharge. [9] Upon 3-5 years of follow up of the same cohort, the mortality rate was 20%. [17] Approximately 60% developed evidence for chronic kidney disease (proteinuria, decreased glomerular filtration rate, hypertension) and 9% developed ESRD.
Collectively, these data strongly suggest that long-term follow-up is warranted for children who survive an episode of acute kidney injury.
In contrast to the above, the prognosis from prerenal causes of acute kidney injury or from acute tubular necrosis in the absence of significant comorbid conditions is usually quite good if appropriate therapy is instituted in a timely fashion.
Complications
Infections develop in 30-70% of patients and affect the respiratory system, urinary tract, and indwelling catheters. Impaired defenses due to uremia and the inappropriate use of broad-spectrum antibiotics may contribute to the high rate of infectious complications.
Cardiovascular complications are a result of fluid and sodium retention. They include hypertension, congestive heart failure, and pulmonary edema. Hyperkalemia results in electrocardiographic abnormalities and arrhythmias.
Other complications include the following:
-
GI - Anorexia, nausea, vomiting, ileus, and bleeding
-
Hematologic - Anemia and platelet dysfunction
-
Neurologic - Confusion, asterixis, somnolence, and seizures
-
Other electrolyte/acid-base disorders - Metabolic acidosis, hyponatremia, hypocalcemia, and hyperphosphatemia
-
Pathogenesis of prerenal failure
-
Compensatory mechanisms for preventing a fall in glomerular filtration rate (GFR) in the presence of prerenal failure
-
Mechanisms of intrinsic acute renal failure.