Practice Essentials
Hypercalcemia is not common among children; it is more frequently found in adults. The prognosis of pediatric hypercalcemia depends on the underlying disorder.
Signs and symptoms
Hypercalcemia can cause symptoms at levels as low as 12 mg/dL and consistently causes symptoms at 15 mg/dL. Hypercalcemia initially and predominantly affects the gastrointestinal (GI) and nervous systems. Symptoms may include the following:
-
Nervous system – Personality changes, malaise, headache, hallucinations, unsteady gait, proximal muscle weakness, central nervous system (CNS) depression, irritability, confusion
-
GI system – Abdominal cramping, constipation, anorexia, nausea, vomiting, epigastric pain
-
Renal effects – Kidney stones, polyuria, polydipsia, renal failure
-
Musculoskeletal system – Bone pain
See Presentation for more detail.
Diagnosis
Much of the laboratory workup should be guided by the history and physical examination. The differential diagnosis varies widely on the basis of the child's age.
See Workup for more detail.
Management
Treatment options for hypercalcemia include the following:
-
Hydration with isotonic sodium chloride solution
-
Loop diuretics
-
Bisphosphonates
-
Peritoneal dialysis or hemodialysis
-
Surgical correction of hyperparathyroidism
See Treatment and Medication for more detail.
Background
Calcium absorption and regulation involve a complex interplay between multiple organ systems and regulatory hormones. [1] The 3 predominant sources of calcium and targets for regulation are the bones, renal filtration and reabsorption, and intestinal absorption. The major regulators of calcium levels are parathyroid hormone (PTH) and vitamin D, which target the bones, intestine, and kidney to increase serum calcium. Calcitonin, a more minor player in regulation, decreases serum calcium by its effects on bone and kidney. Cyclically, high levels of calcium suppress PTH and thereby decrease levels of the active form of vitamin D by decreasing the activity of renal 1 α -hydroxylase.
The kidney serves as the rapid regulator of calcium fluxes but has limited capacity to handle large swings in the serum calcium levels. Sixty-five percent of the calcium filtered through the glomeruli is reabsorbed in the proximal tubule by a process linked to sodium reabsorption. Although dependent on concentration and voltage, this process is independent of PTH. Approximately 20-25% of filtered calcium is reabsorbed in the ascending limb of the loop of Henle, whereas the remaining 10% is reabsorbed under the influence of PTH and vitamin D in the distal tubule.
The bones serve as a reservoir, storing 99% of the body's calcium. Bony remodeling can engineer large, but slower, alterations in the serum calcium by a slow change in the balance between osteoblastic bone formation and osteoclastic bone resorption. However, deposition and release from hydroxyapatite can also provide slightly faster regulation. The intestine serves as a long-term homeostatic mechanism for calcium. Although the major source of calcium is dietary, seven eighths of dietary calcium is excreted unabsorbed in feces. Absorption occurs primarily in the ileum and jejunum by means of active transport and facilitated diffusion.
Pathophysiology
Half the plasma calcium is ionized and freely diffusible, whereas 10% is bound to citrate and phosphate but able to diffuse into cells. The remaining 40% is plasma protein bound and not diffusible into cells. In the setting of a calcium increase in a person with normal regulatory mechanisms, hypercalcemia suppresses the secretion of PTH. This plays a prominent role in calcium maintenance, however, only in the narrow range of serum calcium levels from 7.5-11.5 mg/dL. levels above or below this range are relatively ineffective at further stimulating or suppressing PTH and rely on direct exchange of calcium between bone and extracellular fluid.
Normally, PTH stimulates release of calcium from bone by direct osteolytic action and via osteoclast up-regulation. Therefore, a decline in serum PTH concentration decreases the flux of calcium from bone to extracellular fluid. PTH also acts to reabsorb calcium in the loop of Henle and distal tubule in the kidney and; when PTH is absent, much of the filtered calcium is excreted in the urine. Finally, PTH stimulates enzymatic conversion of 25-hydroxyvitamin D to the active metabolite 1,25-dihydroxyvitamin D.
Ultraviolet (UV) light converts 7-dehydrocholesterol in the skin to cholecalciferol (vitamin D-3). Alternatively, previtamin D is directly ingested and transported by proteins to the liver, where it is converted to 25-hydroxyvitamin D. In the kidney, 25-hydroxyvitamin D (calcidiol) is converted to the active metabolite 1,25-dihydroxyvitamin D by a PTH-stimulated process. 1,25-dihydroxyvitamin D (calcitriol) serves to promote intestinal absorption of calcium. When PTH is suppressed because of hypercalcemia, levels of 1,25-dihydroxyvitamin D decline, and thus intestinal calcium absorption declines.
The calcium sensing receptor (CaSR) is a regulator of calcium metabolism that has recently received significant attention. [2] Primarily expressed by the kidney and parathyroid gland, it controls parathyroid secretion and renal calcium reabsorption based on the extracellular calcium levels it senses. Inactivation of this receptor can cause hypercalcemia.
Regulators of calcium metabolism
The primary action of PTH is to increase serum calcium by the following mechanisms:
-
Directly causes rapid resorption of calcium from the bone into the plasma, elevating serum calcium both by directly stimulating the osteolytic calcium pump and by osteoclast up-regulation
-
Directly causes renal tubular reabsorption of calcium in the loop of Henle and distal tubule
-
Inhibits phosphate reabsorption, as well as that of sodium, water, and bicarbonate in the kidney
-
Promotes renal conversion of 25-hydroxyvitamin D to the more active form 1,25-dihydroxyvitamin D by stimulating renal 1 hydroxylase activity
-
Lowers serum phosphate
-
Is stimulated by increases in phosphate, decreases in calcium, adrenergic agents, magnesium, and certain vitamin D metabolites
-
Is suppressed by hypercalcemia and high levels of 1,25-dihyroxyvitamin D
Vitamin D in its active form of 1,25-dihydroxyvitamin D (also known as calcitriol [Rocaltrol]) increases serum calcium levels by the following mechanisms:
-
Increases calcium and phosphate absorption from the intestines
-
Increases mineralization of bone, possibly by increasing intracellular transport of calcium ions and by increasing circulating concentrations of calcium and phosphate
-
Increases calcium reabsorption in the distal tubule of the kidney
-
Is inhibited by phosphate and corticosteroids
Calcitonin causes an overall decrease in serum calcium by the following mechanisms:
-
Impairs osteoclast and bone osteolytic activity
-
Prevents osteoclast formation
-
Increases urinary excretion of calcium
Other factors altering serum calcium include the following:
-
Metabolic alkalosis, which causes an increase in tubular calcium reabsorption
-
Phosphate-induced decrease of serum calcium levels and increase of PTH
-
Stimulation of osteoclasts by cytokines, such as tumor necrosis factor, interleukin-1, and interleukin-6
-
Stimulation of osteoclasts by prostaglandins
-
Effect of glucocorticoids on bone formation and intestinal absorption of calcium
-
Inhibition of bone resorption by estrogens
-
CaSR
Etiology
Etiologies vary by age and other factors.
-
Neonates
Neonatal primary hyperparathyroidism can begin as soon as the parathyroid glands, functional in the first trimester of pregnancy, become hyperplastic.
Infants have malaise, constipation, and vomiting; serum calcium and parathyroid hormone (PTH) concentrations are elevated, and serum phosphate concentration is decreased. Aminoaciduria occurs. Rarification of bones leads to easier fracturing.
Rehydration with isotonic sodium chloride solution and forced diuresis with furosemide are urgently required, as well as administration of subcutaneous calcitonin. Common side effects of subcutaneous calcitonin include facial flushing, nausea, and vomiting.
Definitive treatment is performed by means of surgical resection, often with reimplantation of a small amount of tissue into a more accessible ectopic site (eg, forearm).
Neonatal primary hyperparathyroidism stems from a homozygous inactivating mutation in a calcium-sensing receptor.
Familial hypocalciuric hypercalcemia (FHH) is an autosomal dominant heterozygous mutation of the same calcium receptor-sensing gene (CASR) that is abnormal in the homozygous state in neonatal primary hyperparathyroidism.
If symptoms are observed (eg, chondrocalcinosis, pancreatitis, renal disease, neuropsychiatric disease), they generally begin in the neonatal period; however, patients are often asymptomatic. If the patient is asymptomatic, no treatment is required.
Because of the mutation, levels of calcium that are higher than usual are required to decrease secretion of PTH. The serum PTH concentration, although within the reference range, is inappropriately high for the degree of hypercalcemia.
Other laboratory findings include a decreased or normal serum phosphorus level, an increased magnesium level in 50% of babies, normal levels of alkaline phosphatase and serum 25-hydroxyvitamin D, and an appropriate level (to the PTH) of 1,25-dihydroxyvitamin D. Serum calcium levels rarely rise above 14 mg/dL. Urine calcium excretion is decreased to less than 200 mg/d, but the level of urine cyclic adenosine monophosphate (cAMP) is normal.
Radiographic findings are normal.
Excessive supplementation of calcium causes hypercalcemia.
Williams syndrome, which is associated with a deletion of elastin genes on chromosome 7, occurs as transient neonatal hypercalcemia, perhaps secondary to increased sensitivity to vitamin D. [3] The syndrome is associated with characteristic elfin facies, intellectual disability, and supravalvar aortic stenosis. Generally, hypercalcemia is symptomatic, with poor feeding and constipation, and spontaneously remits by age 9-18 months. Treatment is a dietary restriction of calcium to 100 mg/d and limited vitamin D intake. Hydrocortisone at 10-25 mg/kg/d or calcitonin is sometimes helpful.
Severe autosomal recessive hypophosphatasia is a disease of bone mineralization due to a deficiency in tissue nonspecific alkaline phosphatase (TNSALP). Associations vary from rachitic changes to fetal death. These children require a low-calcium high-phosphate diet.
Secondary hyperparathyroidism is a neonatal response to maternal hypocalcemia with similar symptoms to primary hyperparathyroidism, except that the child undergoes a progression from hypocalcemia to normocalcemia to hypercalcemia quickly after birth. PTH is generally elevated. During the first few months, the parathyroid glands and skeletal lesions normalize; therefore, only symptomatic nonsurgical treatment is required.
Idiopathic infantile hypercalcemia (IIH) is a poorly understood disorder possibly related to non–malignancy-associated PTH-related protein (PTHrP), which spontaneously resolves by age 12 months. In a study of 20 children with mild IIH who were followed up for a median of 21 months, dietary calcium and vitamin D restriction reduced serum and urinary calcium levels; however, serum concentrations of 1,25 dihydroxyvitamin D remained elevated. In addition, renal calcification worsened in 2 of the children in the study. [4]
Blue diaper syndrome is a selective defect in the intestinal transport of tryptophan. The diagnosis is confirmed by analyzing urine indoles.
Jansen metaphyseal chondrodysplasia is a rare disease of endochondral bone formation characterized by short stature, leg bowing, short-limbed dwarfism, and a waddling gait. Neonatally, these children appear normal but have radiographic and laboratory abnormalities. In early childhood, the external changes become more obvious. The condition arises from an activating mutation in the PTH/PTHrP receptor. Radiographic findings reveal cupped and ragged metaphyses and osteitis fibrosa cystica, and laboratory findings reveal a serum calcium level of 13-15 mg/dL, a low phosphate level, a high level of 1,25-dihydroxyvitamin D, a high alkaline phosphatase level, and urine hydroxyproline.
-
Infants: Subcutaneous fat necrosis, which manifests in neonate as violaceous plaques or nodules overlying fatty areas, can lead to life-threatening hypercalcemia at age 1-6 months. It is likely mediated by prostaglandin E (PGE) or due to macrophage production of 1,25-dihydroxyvitamin D. Treatment includes corticosteroids and symptomatic support of patient.
-
School-aged children
Hyperparathyroidism secondary to parathyroid adenoma or autosomal dominant hereditary hyperparathyroidism is a rare problem in older children. Children may be asymptomatic or symptomatic with constipation and personality changes. Levels of urine and serum calcium are high, whereas the serum phosphorus level is low and urine phosphorus level is high. Unlike in most forms of hypercalcemia, which are associated with systemic alkalosis, patients with hyperparathyroidism tend to have acidosis. This acidosis is due to a loss of bicarbonate in the urine, giving a picture consistent with renal tubular acidosis. Radiographic findings of osteitis fibrosa cystica may be present. Treatment is surgical, and corticosteroids have no role. [5]
Multiple endocrine neoplasia (MEN) type 1 (ie, Wermer syndrome) is a rare autosomal dominant constellation of hyperparathyroidism, pancreatic tumors, and pituitary tumors treated by subtotal parathyroidectomy. Molecular diagnosis is now available for MEN types 1 and 2.
-
General factors
Malignancies produce hypercalcemia much less frequently in the pediatric patients than in adults. However, pediatric malignancies that can elevate calcium include the following:
Langerhans cell histiocytosis [6]
Rhabdomyosarcoma with metastases to breast or bone marrow in adolescents
Ovarian small cell carcinoma in adolescents
Renal tumors with rhabdoid histology in infants
Three different mechanisms are responsible, and resultant laboratory abnormalities slightly differ.
Primarily in leukemia, PTHrP increases osteoclast resorption of bone, renal reabsorption of calcium, and renal loss of phosphorous, leading to decreased serum phosphate levels, increased urinary cAMP, and detectable PTHrP.
Burkitt lymphoma and multiple myeloma, as well as bony tumors or sarcomas with bony metastases, can cause cytokine-mediated bone resorption.
Hodgkin and non-Hodgkin lymphoma may cause increased intestinal absorption of calcium via production of 1,25-dihydroxyvitamin D by macrophages, which contain 1-alpha-hydroxylase activity, and may maintain a normal serum phosphorus level.
Generally, patients with malignancy-induced hypercalcemia have decreased chloride levels, alkalosis, increased BUN levels, increased uric acid levels, urine calcium levels higher than 400 mg/dL, and increased urine cAMP levels. Serum alkaline phosphatase levels may be elevated, and serum PTH levels are decreased, except in the uncommon setting of direct stimulation of PTH production by the tumor. Serum calcium levels greater than 14 require treatment, primarily with hydration and steroids at a dose of 1.5-2 mg prednisone equivalent/kg/d for several days.
Thyrotoxicosis can cause sufficient bone resorption to increase serum calcium in 20% of cases. In these patients, thyrotoxicosis can also decrease serum PTH and increase urine excretion of cAMP and calcium. Although hypercalcemia is rarely subjectively symptomatic to the patient, it can lead to nephrocalcinosis and renal failure. This condition is rare in childhood, but it is possible in neonates of mothers with Graves disease or in older children who develop Graves disease.
Granulomatous disease, including sarcoidosis, tuberculosis (TB), Wegener disease, berylliosis, and Pneumocystis carinii pneumonia, may cause hypercalcemia via overproduction of 1,25-dihydroxyvitamin D by macrophages and increased extrarenal alpha1-hydroxylase activity.
Adrenal insufficiency can decrease the renal clearance of calcium.
Hypercalcemia may appear in the oliguric phase of acute renal failure due to the PTH increase stimulated by hyperphosphatemia. Also, children with renal failure treated with calcitriol for secondary hyperparathyroidism can develop a mild hypercalcemia. [7]
Immobilization can cause hypercalcemia.
-
Medication and iatrogenic causes
Total parenteral nutrition may cause hypercalcemia.
Vitamin D intoxication due to ingestion of more than 50 mcg/d in infants or more than 500 mcg/d in adults can cause hypercalcemia, even in the absence of a markedly elevated 1,25-dihydroxyvitamin D. Symptoms of hypercalcemia, including hypertension, aortic valvular sclerosis, retinopathy, renal damage, and bony abnormalities, can also occur 1-3 months after a large overdose of vitamin D. Serum PTH is decreased. Levels of water-soluble preparations can drop quickly, but hypercalcemia from an excess intake of fat-soluble preparations may persist for months.
Vitamin A in high doses, such as those found in retinoid therapy for acne, can directly stimulate bone resorption by functioning as a transcription factor in osteoclast stimulation. Trans -retinoic acid, used for treatment of some leukemias, can elevate calcium with this mechanism, particularly when coupled with voriconazole. [8]
Thiazide diuretics (eg, Diuril) may cause hypercalcemia because of their action on the distal tubule.
Lithium causes a mild increase in serum calcium, which can occasionally increase further in the few months after cessation of the drug secondary to parathyroid hyperplasia or adenoma.
Tamoxifen and oral contraceptives can exacerbate existing hypercalcemia.
Milk-alkali syndrome (ie, Burnett syndrome) from exogenous ingestion of calcium-containing antacids leads to renal insufficiency and metastatic calcinosis with increased phosphorus levels, increased levels of 1,25-dihyroxyvitamin D, decreased PTH levels, normal levels of serum alkaline phosphatase, normal urine calcium levels, and decreased urine phosphate levels. [9] If continued over time, this may lead to osteomalacia. This condition is particularly sensitive to the development of hypocalcemia following treatment with bisphosphonates.
Theophylline can cause increases in calcium via beta-adrenergic stimulation. This may be treated with propranolol.
Oral dietary phosphate deficiency may cause hypercalcemia.
Vitamin-D receptor modulators (eg, paricalcitol) are newer medications used to treat malignancy and hyperparathyroidism, which can increase serum calcium levels.
Epidemiology
United States statistics
Hypercalcemia is not a common pediatric problem; the actual incidence in children is unknown, although it is less common than in adults. In adults, hypercalcemia is the primary malignancy-associated endocrine/electrolyte disorder; it is present in 5% of all malignancies, or in 15 per 100,000 total patients. Cancer-related hypercalcemia in adults most often occurs in later-stage malignancies. [10]
Prognosis
Hypercalcemia is frequently noted during laboratory testing while the patient is asymptomatic or mildly symptomatic. Prognosis depends on the underlying disorder.
Morbidity/mortality
Mortality from hypercalcemia itself is rare, although cardiovascular collapse and neonatal seizures are reported. The survival rate is more than 80%, even with malignancy-associated hypercalcemia in adults requiring ICU admission. Clearly, in certain disorders associated with hypercalcemia (eg, Williams syndrome, cancer), the underlying disorder may prove fatal or provide significant morbidity.
Complications
Primarily ectopic calcifications may occur.
-
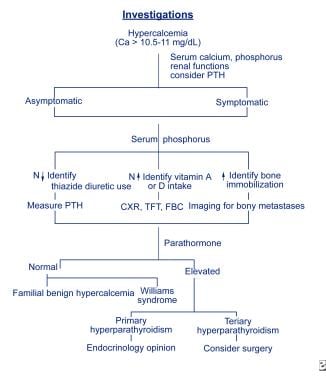
Investigations flowchart.