Practice Essentials
The orthopedic entity known as a unicameral (or simple) bone cyst (UBC) is a common, benign, fluid-filled lesion found almost exclusively in children. [1, 2] It is not believed to be a new phenomenon. In 1942, Jaffe and Lichtenstein published their classic paper concerning solitary UBCs, [3] emphasizing the distinctiveness of UBC as follows:
Solitary unicameral bone cyst is a lesion sui generis. It bears no relation whatever to giant cell tumor of bone, and in particular it does not represent a cystic-healing phase of this tumor. Nor is it to be linked with enchondroma, fibroma or focus of fibrous dysplasia of bone that has undergone partial or extensive cystic degeneration. Further, it should not be regarded as representing cystic expression of osteitis fibrosa, since to throw it into this wastebasket category (one which to us is also meaningless) is to obliterate its distinctiveness. Correspondingly, solitary unicameral bone cyst ought no longer to be classed as an expression of localized fibrocystic disease of bone or localized fibrous osteodystrophy—likewise blanket designations dating from a more primitive era of bone pathology.
Much has been written about the diagnosis and management of UBCs, and evidence of a variety of successful treatment approaches can be found in the literature. Despite abundant clinical confidence in managing these lesions, many basic questions remain concerning their etiology and pathophysiology. [4] Among the questions that remain to be answered are the following:
-
What is the root cause of the UBC lesion?
-
Does a genetic basis to the problem exist?
-
Should pathologic fractures of the long bones (secondary to a UBC) be treated via immediate flexible intramedullary nailing?
Answering these and similar questions will require a far more coordinated research effort than has been made to date. A multicenter study (a combined effort of the Shriner's Hospital System and the Pediatric Orthopaedic Society of North America) holds some promise of refining the treatment approach to a UBC.
Most patients with a UBC present to the orthopedic surgeon after sustaining a pathologic fracture, most commonly involving either the proximal humerus or the proximal femur. Others may present to emergency department (ED) physicians, their primary care physicians, or orthopedic surgeons for other reasons, and radiographs obtained in the workup of other complaints may identify asymptomatic UBCs.
The decision to pursue surgical intervention in patients with UBCs is highly individualized. An asymptomatic lesion with satisfactory maintenance of cortical thickness may require only observation; a lesion with precarious cortical thinning (with or without insufficient pain) may demand surgery. The main contraindication for surgical treatment is a patient who otherwise meets indications for surgery but is unable to tolerate anesthesia. Another relative contraindication for surgery is a patient with a small asymptomatic latent cyst with a low likelihood of a pathologic fracture.
Anatomy
The anatomy that is relevant to UBCs is mainly that of the proximal humerus and proximal femur. Percutaneous approaches to the proximal humerus require the surgeon to avoid injury to the biceps tendon, as well as the axillary nerve, which innervates the deltoid musculature. The standard deltopectoral approach is the most common open surgical approach for proximal humeral lesions.
Key points of this approach include preservation of the cephalic vein, as well as careful medial retraction of the conjoined tendon (coracobrachialis and short head of the biceps) to avoid injuring the musculocutaneous nerve. Dissection in the region of the bicipital groove should be minimized; such dissection may injure the anterolateral ascending branch of the anterior humeral circumflex artery, which provides the bulk of the blood supply for the humeral head.
Pathophysiology
A UBC may appear in virtually any bone, but typically, it is found in either the proximal humerus or proximal femur. (See the images below.) A UBC often leads to thinning of adjacent areas of bone, such that fracture or pain from microfracture may occur.
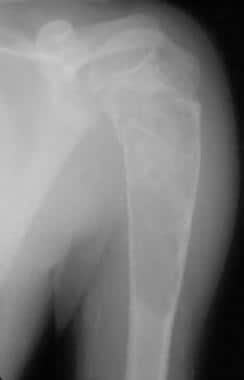 Large proximal humeral unicameral bone cyst demonstrates early cortical healing following pathologic fracture.
Large proximal humeral unicameral bone cyst demonstrates early cortical healing following pathologic fracture.
 Large unicameral bone cyst of pelvis. Pathologic fracture is depicted. Note extension of cyst into region of proximal femoral physis.
Large unicameral bone cyst of pelvis. Pathologic fracture is depicted. Note extension of cyst into region of proximal femoral physis.
When UBCs are immediately adjacent to a growth plate, they are referred to as active cysts, and when they have achieved some distance from the growth plate, they are considered to be latent cysts. This distinction has been used in the past; it was believed to have prognostic significance. A UBC usually presents as a unifocal (one-bone) problem, affecting patients who are skeletally immature.
Komiya and Inoue carried out a longitudinal study (with serial radiographs over 6 years) that documented the development of a UBC over time. [5] Initially, a small erosive lesion of the endosteal humeral metaphysis appeared, and over time, the lesion progressively enlarged into a typical UBC. The lesion analyzed by these authors was somewhat unusual in that it was located in the distal humerus. In addition, the lesion appeared following notation of a previous UBC in the proximal aspect of the same bone.
The rarity of UBCs in adults supports a hypothesis of spontaneous resolution. In the absence of fracture through the cyst (or impending fracture), UBCs are asymptomatic. They are, at times, found serendipitously when radiographs are taken for other reasons. In the absence of symptoms and in the absence of mechanical compromise of the involved bone (eg, extensive cortical thinning), no treatment may be necessary other than observation.
However, treatment should be strongly considered for lesions that have resulted in a fracture or marked weakening of bone. Some evidence exists that spontaneous healing of a UBC may occur following fracture. Such healing occurs in only a minority of cases. Growth disturbance secondary to a UBC is also a concern. [6]
Case reports have been published in which a chondrosarcoma was found to arise within the same area as a previous histologically proven UBC. [7] In a separate case, an 8-year-old boy was reported to have sustained a pathologic fracture of the distal fibula that was believed to have resulted from a Ewing sarcoma infiltrating a UBC. [8]
The precise relation between such rare instances of apparent malignant transformation and the thousands (if not millions) of UBCs that have not demonstrated such behavior remains unclear. At any rate, a UBC is not considered to be a malignant or premalignant lesion; accordingly, routine biopsy or other treatment of asymptomatic and nonproblematic lesions based on a patient's or family's fear of cancer should not be undertaken.
Etiology
The specific etiology of a UBC has not been elucidated. [4] Many theories have been proposed. One commonly quoted theory was proposed in 1960 by Cohen, [9] who studied the cyst fluid from six children undergoing treatment for UBCs and found four to resemble plasma and two to resemble blood. Cohen proposed that the principal etiologic factor is blockage of the drainage of interstitial fluid in a rapidly growing and rapidly remodeling area of cancellous bone.
Chigira et al studied internal pressures in seven patients with UBCs and found them to be higher (by 2-7 mm Hg) than the contralateral normal bone-marrow pressures. [10] The arterial oxygen tension (PaO2) in the fluid from these same cysts was found to be impressively lower than that in venous or arterial samples taken at the same time. These authors suggested that venous obstruction within the bone appears to be a likely cause of such simple bone cysts.
Such vascular theories have been supported by other authors. [11] Mirra et al suggested that a UBC represents an area of a congenital rest of synovial tissue and was able to demonstrate both synovial type A (macrophagelike) and type B (fibroblastlike) cells in the lining of such cysts. [12] This description resembles that of an intraosseous synovial cyst. Yu et al also demonstrated how methylprednisolone influences the cellular physiology of synovial cells in culture, thus establishing a theoretic basis for steroid injection treatments for a UBC. [13]
Shindel et al reported increased prostaglandin E2 levels in the cyst fluid from seven of their patients and theorized that this may help explain the beneficial effect of steroid injection of such lesions. [14] Gerasimov led a group of Russian researchers who stressed that the fluid from UBCs possesses increased lysosomal enzyme activity regardless of the UBCs' status as active or latent. [15] These authors emphasized the role such enzymatic activity might play in permanent corrosion of the cyst cavity, as well as increasing osmotic pressure within the cyst.
High levels of cytotoxic oxygen free radicals have also been found in the fluid from UBCs. [16] Such free radicals are not only cytotoxic; they might be generated during the ischemic state following blockage of interstitial fluid drainage from UBCs. The Japanese researchers suggested that such oxygen scavengers may contribute to the bone destruction associated with UBCs. Reproduction of these results in other centers has not yet occurred.
A group of Brazilian researchers described specific genetic abnormalities in a pediatric patient with a UBC of the right distal femur. Vayego et al made their first report in 1996. [17] Cytogenetic analysis of the resected cyst initially demonstrated complex aberrations of chromosomes 4, 6, 8, 12, 16, and 21. Further study of the same patient (following bone cyst recurrence) later revealed specific mutations associated with amino acid substitutions (arginine for tryptophan, arginine for serine). [18]
More study in this area clearly is indicated, and there appears to be potential for future gene-based therapies.
Epidemiology
A UBC occurs most frequently in children aged 5-15 years (average, ~9 y). [19, 20] Many authors consider cysts that present in the first decade of life to be more aggressive. [19, 20] A UBC affects males approximately twice as often as females. These lesions constitute approximately 3% of all bone tumors. [4]
A UBC probably represents the third or fourth most common benign bone tumor that the orthopedic surgeon confronts (osteochondromas are commonly considered to be the most frequently encountered benign bone tumors in children, followed by fibromas and/or fibrous cortical defects). The lesion may occur in conjunction with other benign bone tumors, such as a nonossifying fibroma. [21]
By far the most common location for the lesion is the proximal humerus, followed by the proximal femur. The proximal humerus and femur together account for nearly 90% of all UBC sites. [19, 20] However, virtually any bone may be affected, with the calcaneus being one of these notable alternative locations. [22, 23, 24, 25, 26, 27]
Prognosis
The overall outcome and prognosis of a UBC are good. The lesion is believed to resolve spontaneously in most cases if given enough time.
Cases that present to the orthopedic surgeon typically involve patients who demonstrate a combination of a cyst that has caused cortical thinning and the right stressful event (eg, being tackled while playing football). In general, treatment may be summarized as doing nothing more than trying to promote natural healing. Flexible intramedullary nailing may do nothing more than mechanically support the bone while the natural healing process occurs.
Few comparative studies have been conducted regarding the various treatment alternatives for individuals with UBCs. [28, 29, 30, 31] Some of the more important ones are summarized below.
In a retrospective study of 36 patients with UBCs who were surgically treated over a period of 45 years, [28] Farber et al found that curettage and bone grafting (with some patients receiving allograft and others receiving autograft) yielded a 53% (10/19) healing rate, whereas aspiration and injection with steroid yielded a 70% (12/17) success rate. In 25% (3/12) of the patients, only one injection was required. Although this difference in overall healing rates might appear clinically significant, it was not statistically significant.
Farber et al also did not explicitly define their criteria for success. [28] Because of the similar healing rates and the lower morbidity of the steroid injection, the authors concluded that they favored the percutaneous approach over traditional open curettage and bone grafting.
In a retrospective review, Canavese et al compared the outcomes of percutaneous curettage, intralesional injection of methlyprednisolone, and intralesional injection of bone marrow. [32] The three treatment groups included 46 patients with radiologically confirmed UBCs and at least 2 years of follow-up. Results showed that the rate of satisfactory healing was 70% in the percutaneous curettage group, 21% in the bone-marrow injection group, and 41% in the methylprednisolone group.
Oppenheim et al evaluated 37 patients treated via open surgical techniques (35 with curettage and bone grafting and two with subperiosteal total or subtotal resection) against 20 patients treated via steroid injection. [33] They found a 40% recurrence rate and a 15% major complication rate in their open group, whereas the steroid injection group had only a 5% recurrence rate and a 5% major complication rate. Although not calculated by the authors, this difference in recurrence rates was statistically significant. [33] These authors used reconstitution of cortical thickness as their endpoint of healing rather than cyst obliteration.
Glaser et al published a comparative multicenter study focused on calcaneal UBCs. [23] The calcaneus is a somewhat uncommon (ie, the third to sixth most common) site for UBC. [22, 23, 27, 34] The study by Glaser et al suggested that percutaneous steroid injection procedures were less effective in the calcaneal lesions and that curettage and bone grafting may be a more predictable and successful procedure for simple bone cysts in this location. [23]
Flexible intramedullary nailing of UBCs in long bones, though not a new concept by any means, has been reported to yield good results. [35, 36] Roposch et al documented a 94% (30/32) good response rate to flexible nailing of UBCs of the long bones. [36] Complete cyst healing or healing with minor residual lucent areas occurred at an average of 36 months. Thus, this technique appears to support the compromised bone while the UBC follows its natural history and spontaneously resolves.
Some authors have stated that they believe such flexible nails allow continuous decompression of the UBC, with a resulting decrease in intralesional pressure. [36, 37, 38] However, 28% of patients in a study by Roposch et al required at least one further operation because of inadequate nail length in the face of continued bone growth. [36]
In a systematic review and meta-analysis (62 studies; N = 3211; 3217 UBCs) by Kadhim et al, active treatment of these lesions (eg, curettage, grafting, injection of steroid or bone marrow, flexible intramedullary nailing, and continuous decompression with cannulated screws) was associated with variable healing rates, and the outcomes were favorable in comparison with conservative treatment. [39]
In a smaller study (N = 68; 54 boys, 14 girls) focused specifically on humeral UBCs in children, Kadhim et al found that whereas complete healing was a challenge with any treatment modality, better healing was generally achieved when these lesions were treated surgically. [40]
Gundle et al evaluated the outcomes of injecting bone-marrow aspirate (BMA) and demineralized bone matrix (DBM) for the treatment of UBCs of the proximal humerus in 51 patients. [41] Of the 51, 11 (22%) underwent only one injection, 19 (37%) completed treatment after two injections, four (8%) healed after three injections (8%), and one (2%) healed after four injections. The cumulative success rate was 22% (11/51) after one injection, 58% (30/51) after two, 67% (34/51) after three, and 69% (35/51) after four. Open curettage and bone grafting was ultimately performed in 11 patients (22%) and injection of calcium phosphate bone substitute in five (10%).
-
Large proximal humeral unicameral bone cyst demonstrates early cortical healing following pathologic fracture.
-
Large unicameral bone cyst of pelvis. Pathologic fracture is depicted. Note extension of cyst into region of proximal femoral physis.
-
Typical appearance of cyst fluid is depicted. Initial aspiration often yields thin, clear, yellow fluid that rapidly becomes blood-tinged.
-
Double-cannula technique demonstrates intraoperative use of contrast material for evaluation of cyst's interior. In this case, large partial septum remains along inferior portion of cyst.





