Practice Essentials
Patients presenting with orbital tumor most often will have some type of proptosis wherein the eye is displaced in the opposite direction from the tumors present; for example, a superolateral tumor will displace the most often inferomedial. The orbital anatomy can affect the direction and degree of proptosis. Many tumors are benign and can take decades to grow. Other tumors that are not invasive can have a rapid onset and can arise from adjacent structures such as a sinus within the orbit directly or be metastatic from elsewhere. [1] Bony changes and pain are often associated with a more unfavorable diagnosis. Both CT and MRIs are required for further evaluation. Specialized studies such as angiography and orbital ultrasound may be required. Biopsy versus removal of the tumor is decided in the context of the potential for spread as well as the risk to the orbital structures including the eye, extraocular muscles, neurovascular structures, and surrounding tissues.The potential risk for hemorrhage with resultant visual impairment and extraocular motility dysfunction must be taken into account.
Background
The orbit is an anatomically complex structure containing the globe; extraocular muscles; fat; and vascular, nerve, glandular, and connective tissues.
The orbit in the broadest sense describes the cavity containing structures essential for ocular function and the bony architecture that encases them. This complex anatomic area was first described by Whitnall to resemble a pear, with its widest aperture anterior and narrowing posteriorly.
The bony orbit has its cellular ancestry from the mesenchymal cells surrounding the optic vesicle. Composed of 7 bones, a normal adult orbit holds a volume of 30 mL with a medial length of 45 mm, a width of 40 mm, and a height of 35 mm at its most anterior point.
Since the orbit is a relatively small anatomic area with little wasted space, space-occupying lesions that increase orbital volume may result in proptosis of the globe and may adversely affect visual and extraocular muscle function. However, Lin and colleagues reported that proptosis of less than 4 mm might go undetected, obscuring occult pathology. [2]
History of the Procedure
In 1888, Krönlein first described the lateral orbitotomy approach. The Berke-Reese modification of this approach used an extended canthotomy. In place of the curved incision used by Krönlein, Stallard altered the approach taking it into the upper lateral brow area. Avoiding the lateral canthal region was at the core of the Wright modification of the Stallard approach. A hemicoronal (or bicoronal) approach, also referred to as the coronal approach, has been used by Kennerdal and others. [3] Goldberg et al have popularized the transconjunctival approach and the transcaruncular approach. [4]
Endoscopic approaches have become more common, especially to treat medial orbital tumors. [5] Image guidance also has become more frequently used in orbital surgeries performed via both nonendonasal and endonasal approaches. [6, 7, 8]
Problem
Orbital tumors have protean manifestations. Rootman et al reported that the major presenting symptom was proptosis, resulting from the mass effect. [9] This occurred in 269 of the 601 patients evaluated or 44.8% of these patients.
Epidemiology
Frequency
The top 3 pediatric orbital tumors are dermoid cysts, capillary hemangiomas, and rhabdomyosarcoma. Retinoblastoma can spread from the globe to the orbit. Neuroblastoma can involve the orbit via metastases and is the most common metastatic tumor to the orbit in children. [10, 11]
The top 3 adult orbital tumors are lymphoid tumors, cavernous hemangiomas, and meningiomas. Other tumors include those of the lacrimal gland, tumors form the surrounding sinuses, metastatic tumors such as breast cancer in women, and neural-based tumors. [12]
Etiology
Primary orbital tumefaction, although quite rare, encompasses a lexicon of benign and malignant neoplasia. All anatomic structures of the orbit can give rise to neoplasia. Direct extension from contiguous anatomic structures, lymphoproliferative disorders, and hematogenous metastasis results in secondary orbital invasion.
Capillary hemangiomas are the most common orbital tumors found in children. Lined by vascular endothelium and pericytes, these histologic benign lesions manifest at birth or within the first 3 months of life, enlarge rapidly, and begin to commence contracting around age 1 year. Other benign orbital lesions include dermoids, lymphangiomas, and histiocytic tumors.
Rhabdomyosarcoma, a mesenchymal tumor, is the most common primary malignant orbital tumefaction in children. These devastating lesions usually occur in children younger than 2 years or older than 6 years, and they have a predilection for the superior nasal orbit.
Neuroblastomas, Ewing sarcoma, Wilms tumor, and leukemias are the more common metastatic orbital lesions afflicting children.
Other malignant lesions include Burkitt lymphoma and granulocytic sarcoma.
In adults, cavernous hemangiomas are the most common de novo orbital tumefaction. CT scan reveals a round, encapsulated, well-defined orbital lesion. Histologically, large blood-filled, endothelial-lined spaces with fibrous interstitial tissue and smooth muscle are discerned. These lesions usually are well tolerated by the patient and managed by conservative therapy and reassurance, unless visual acuity or field loss is found.
Pathophysiology
Orbital tumefactions increase intraocular volume and cause a mass affect. Although a mass may be histologically benign, it can encroach on intraorbital or adjacent orbital structures and be considered anatomically or positionally malignant. Visual acuity or field compromise, diplopia, extraocular motility disturbances, or pupillary abnormalities can result from invasion or compression of intraorbital contents secondary to solid tumor or hemorrhage. Lid dysfunction and lagophthalmos or lacrimal gland dysfunction can result in exposure keratopathy, keratitis, and thinning of the cornea.
Presentation
Evaluation of the patient with a presumed orbital mass begins with a thorough ophthalmic and medical history. When concomitant sinus disease or an intranasal source is suspected, a speculum or endoscopic intranasal examination is warranted. Special emphasis on the duration and rate of progression of the patient's signs and symptoms is essential. Pain, diplopia, pulsation, change in effect or size with position or Valsalva maneuver, and disturbance of visual acuity are symptoms that should be explored. Past trauma and family history also may aid in the diagnosis. [13]
A complete ophthalmic examination is warranted. Periorbital changes can be noted easily on gross examination in a well-illuminated examination room. Hypertelorism, exorbitism, eyeball protrusion (proptosis), eyelid lesions or edema, chemosis, and engorged conjunctival vessels are several periorbital signs. Blepharoptosis, lagophthalmos (incomplete lid closure), and interpalpebral fissure distance are additional signs to be considered during the examination.
Protrusion of the eye is an important clinical manifestation of orbital disease. This ocular prominence is referred to as proptosis or exophthalmos. Henderson has suggested reserving exophthalmos to describe orbital manifestations of endocrinopathies. As recommended by Henderson, proptosis will be used to describe the change in anteroposterior axis of the eye as a result of orbital masses. In addition to proptosis, one should note the displacement of the eye in planes other than the anteroposterior dimension (eg, downward and lateral). Hertel exophthalmometry is a well-accepted tool to quantitate proptosis. Its use requires intact lateral orbital rims. If the rim is not intact, a Luedde exophthalmometer can be used. Relative protrusion can be observed by simply standing behind a seated patient and gazing downward toward the chin from the forehead to assess the displacement of one globe compared to the contralateral side.
Palpation of the anterior orbit can assess the level of tenderness, texture, and mobility of the mass. Tenderness may denote an inflammatory process or neural invasion by a neoplasm, such as adenoid cystic carcinoma of the lacrimal gland. Attention also should be paid to regional lymph nodes. Tactile inspection of the globe may reveal pulsations secondary to arteriovenous communications or physiological intracranial pulsations transmitted through a bony defect of the orbit, such as an encephalocele.
Auscultation of the orbit may detect a high flow state in the orbit or intracranially. The bell is useful for this examination. If a high-flow lesion is suspected (eg, carotid cavernous fistula), arteriography should be sought to further qualify these lesions. It is important to have the contralateral eye remain fixated on a target while auscultating the orbit.
Decreased visual acuity, change of refraction, and pupillary abnormalities should be noted. Extraocular motility dysfunction and diplopia should be carefully assessed and documented. Forced duction testing may qualify the dysfunction as restrictive or neurogenic in nature. Intraocular pressure may be elevated, and slit lamp examination can discern chemosis, engorged, or sentinel vessels. Dilated funduscopic examination may reveal optic disc edema or pallor, retinal detachment, choroidal folds, vascular engorgement or shunt vessels, or indentation of the posterior pole.
Indications
Initiation of surgical intervention occurs when confirmatory biopsy is needed or when the lesion is directly or indirectly adversely affecting the globe or the vision. In a patient with a salmon-patch colored lesion, confirmatory biopsies are needed to aid in the diagnosis and subtyping of the presumed lymphomatous lesion. Other lesions exert their destructive effects through their bulk, and diminishing these lesions is essential in restoring orbital integrity. In other situations, compression of the optic nerve requires decompression of the orbital contents.
Relevant Anatomy
The anatomic localization of orbital neoplasia is referenced with regard to the bony walls comprising it and vital structures, which travel within or in close proximity to these walls.
From an osteologic standpoint, the orbit is composed of 4 bony walls that are the sum total of contributions from 7 bones.
The adult orbital floor has contributions from the maxillary, zygomatic, and palatine bones. It is the shortest of all the walls, not reaching the orbital apex, measuring 35-40 mm, and terminating at the posterior edge of the maxillary sinus. The infraorbital groove, canal, and foramen are contiguous, tunneling through the maxilla, and entombing the maxillary branch of the trigeminal nerve. The maxillary branch of cranial nerve V (V2) exits as the infraorbital nerve provides sensory innervations to the floor, mid face, and posterior upper gingiva in an ipsilateral fashion.
The lamina papyracea of the ethmoid bone, lacrimal bone, maxillary bone, and greater wing of sphenoid bone conjoin to form the adult medial orbital wall. The medial wall ending at the optic foramen serves as a barrier between the ethmoid air cell complex and the orbit. The most anterior bone, the lacrimal bone, forms the posterior one half of the lacrimal sac fossa. The anterior and posterior ethmoid foramina found in the superomedial orbit along the frontoethmoid suture line envelop branches of the nasociliary nerve and ophthalmic artery en route to the nose and ethmoid air cells. The former usually is identified 20-25 mm posterior to anterior lacrimal crest, while the latter is 30-35 mm behind the anterior lacrimal crest. If one identifies the posterior foramen, keep in mind that the optic canal is not much farther posterior and that the cribriform plate is looming about superiorly and must not be insulted.
Projecting in a posterior and inferior fashion, the orbital roof comes to an end at the superior orbital fissure and optic canal. Comprised of the frontal bone and lesser wing of the sphenoid bone, the orbital roof isolates the orbit from the frontal sinus and the anterior cranial fossa. The supraorbital notch, occasionally found to be a foramen, serves as a conduit for the supratrochlear nerve, which is an end branch of the frontal nerve. The ophthalmic division of the trigeminal nerve (V1) gives rise to frontal, nasociliary, and lacrimal nerves when dividing in the cavernous sinus. Orbital lesions or surgical intervention in the superomedial region of the orbit can cause lesions of this neural network. In addition, the lacrimal gland and trochlear fossae are found within the roof.
Within the confines of the superior and inferior orbital fissure is the lateral orbital wall. This prominent orbital structure receives bony contributions from the greater wing of the sphenoid bone and the zygoma. The lateral orbital tubercle of Whitnall is a bony protuberance serving as an anchoring site for the lateral canthal tendon. The zygomatic artery and nerve, an offshoot of the lacrimal artery and nerve, respectively, exit the zygomatic foramen.
The lacrimal gland is situated in the superolateral, anterior orbit. It is comprised of the palpebral and orbital lobes consisting of lacrimal, lymphoid, and epithelial tissues. One half of neoplasia that afflict this gland are malignant. Of these malignant lesions, 50% are of epithelial ancestry and 50% are lymphomatoid in origin.
Contraindications
Patients with complex medical histories should undergo thorough preoperative assessment by a medical specialist. Anticoagulative medications must be discontinued at an appropriate time prior to the surgical procedure to prevent excessive or uncontrolled intraoperative hemorrhage. If these medications cannot be stopped, a risk-benefit analysis must be contemplated.
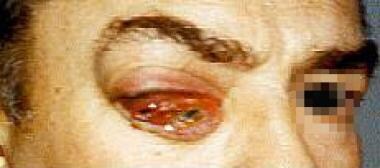
-
Patient with orbital tumor on the right. Note chemosis, ptosis, and proptosis. Patient is also lifting the right brow in an effort to elevate the ptotic lid.
-
Axial CT scan revealing lateral orbital neoplasm.
-
Coronal MRI showing left orbital cavernous hemangioma.
-
Left lateral lid crease incision to access the superolateral orbit.