Practice Essentials
Background
Chloroquine and hydroxychloroquine belong to the quinolone family. Although their therapeutic and toxic doses differ, they are related drugs with similar clinical indications for use and similar manifestations of retinal toxicity. The image below depicts hydroxychloroquine retinopathy.
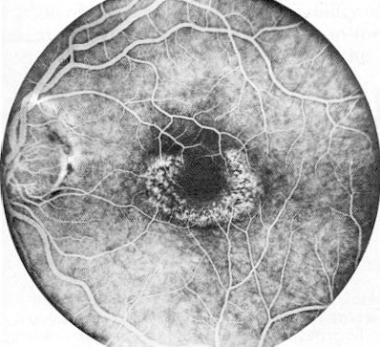 Fluorescein angiogram of left macula in patient with hydroxychloroquine retinopathy. Reprinted from American Journal of Ophthalmology, Vol 104, Johnson and Vine, Hydroxychloroquine therapy in massive total doses without retinal toxicity, pages 139-144, Copyright 1987, with permission from Elsevier Science.
Fluorescein angiogram of left macula in patient with hydroxychloroquine retinopathy. Reprinted from American Journal of Ophthalmology, Vol 104, Johnson and Vine, Hydroxychloroquine therapy in massive total doses without retinal toxicity, pages 139-144, Copyright 1987, with permission from Elsevier Science.
Coronavirus Disease 2019 (COVID-19) and Chloroquine/Hydroxychloroquine
Early in the coronavirus disease 2019 (COVID-19) pandemic, some studies reported that chloroquine and hydroxychloroquine displayed the ability to inhibit replication of multiple coronaviruses in vitro and that hydroxychloroquine could improve the clinical outcome of patients with COVID-19. [1, 2, 3] On March 28, 2020, the FDA granted emergency use authorization (EUA) for chloroquine phosphate and hydroxychloroquine sulfate for the treatment of COVID-19 in certain hospitalized patients when a clinical trial was not feasible.
However, the FDA revoked the EUA on June 15, 2020. Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that hydroxychloroquine is unlikely to be effective in treating COVID-19 for the authorized uses in the EUA. Additionally, in light of ongoing serious cardiac adverse events and other potential serious adverse effects, the known and potential benefits of hydroxychloroquine no longer outweigh the known and potential risks for the EUA. [4]
While additional clinical trials may continue to evaluate potential benefit, the FDA determined the EUA was no longer appropriate.
Etiology
Chloroquine/hydroxychloroquine retinopathy is influenced most by daily dose, length of use, and cumulative dose. The kinetics of chloroquine metabolism are complex, with the half-life increasing with increasing dosage.
Factors associated with hydroxychloroquine toxicity include the following:
-
Maintenance dose greater than 5 mg/kg/d based on real weight (most critical risk)
-
Daily dose greater than 400 mg
-
Cumulative dose greater than 1000 g
-
Age older than 60 years
Factors associated with chloroquine toxicity include the following:
-
Maintenance dose greater than 2.3 mg/kg/d
-
Obesity
Factors associated with toxicity in both drugs include the following:
-
Duration of treatment greater than 5 years (critical factor)
-
Tamoxifen use (5-fold risk)
-
Evidence of renal insufficiency
-
Underlying retinal disease or maculopathy (macular degeneration)
-
Evidence of liver disease
-
P450 polymorphisms leading to higher drug concentration in blood
Signs and symptoms
Retinopathy may be asymptomatic or may cause central or paracentral scotomas leading to difficulty reading or performing fine visual tasks (due to parafoveal metamorphopsia). Visual acuity usually remains intact until advanced disease.
Other reported visual symptoms include the following:
-
Dimness
-
Flickering or flashing lights of yellow
-
Green or red haloes
-
Cycloplegia
-
Amblyopia
-
Diplopia
-
Blindness
-
Photophobia
-
Oculogyric crisis
Systemic complaints include the following:
-
Nausea, abdominal pain, and vomiting
-
Occasionally, skin conditions, such as rashes, pruritus, and sensitivity to ultraviolet light
-
Rarely, neurologic symptoms, such as vertigo, tinnitus, irritability, cranial nerve palsies, and myasthenialike muscle weakness
Findings on eye examination include the following:
-
Corneal deposits
-
Posterior subcapsular lens opacity (chloroquine)
-
Irregularity in the macular pigmentation and blunting of the foveal reflex (early)
-
Bull’s eye maculopathy (classic finding in non-Asian patients)
-
Peripheral pigment irregularity and bone spicule formation, vascular attenuation, and optic disc pallor (end stage)
See Clinical Presentation for more detail.
Diagnosis
Patients starting treatment with chloroquine/hydroxychloroquine should have a baseline examination by an ophthalmologist that includes the following:
-
History (including refraction)
-
Visual acuity (uncorrected visual acuity [UCVA] and best spectacle-corrected visual acuity [BSCVA])
-
Slit-lamp biomicroscopy
-
Direct and indirect ophthalmoscopy (this is not a screening tool, as it reveals relatively late toxic changes)
The examination should also include a Humphrey visual field central 10-2 white-on-white pattern (24-2 or 30-2 in Asian patients) and at least one of the following objective tests, if available (see Workup):
-
Spectral domain optical coherence tomography (SD-OCT)
-
Fundus autofluorescence (FAF) test
-
Multifocal electroretinogram (mfERG)
Ancillary tests used in the diagnosis of toxicity include the following:
-
Amsler grid
-
Color vision testing
-
Color fundus photography: documenting changes over time, especially in patients with preexisting retinal pathology
-
Full-field ERG or electro-oculogram
-
Fluorescein angiography: may assist in visualizing early subtle changes in the retinal pigment epithelium
See Workup for more detail.
Management
Withdrawal of the medication and shifting to another treatment is the standard of care. Coordination with the rheumatologist or the dermatologist may be warranted for comprehensive care of the patient. No diet or medical therapy has been proven effective to prevent, treat, or reduce risk of retinal toxicity.
See Treatment and Medication for more detail.
Background
Chloroquine and hydroxychloroquine belong to the quinolone family. Although their therapeutic and toxic doses differ, they are related drugs with similar clinical indications for use and similar manifestations of retinal toxicity.
Initially, chloroquine was given for malaria prophylaxis and treatment. Subsequently, it was used by rheumatologists for treating rheumatoid arthritis, systemic/discoid lupus erythematosus, and other connective tissue disorders. Dermatologists use these drugs for cutaneous lupus. Expanded use of these drugs for nonmalarial disease entities has resulted in prolonged duration of therapy and higher daily dosages than those used in antimalarial therapy.
Hydroxychloroquine has largely replaced chloroquine in the United States, except among patients who travel to areas endemic with malaria. Although the mechanisms of the two agents are presumed to be the same, many reports suggest that chloroquine is more toxic to the retina than hydroxychloroquine. The toxic dosage for chloroquine was derived from hydroxychloroquine retinal toxicity studies, although no definitive data have shown pharmacologic equivalence. [5]
The first reports of retinal toxicity attributed to chloroquine appeared during the late 1950s. In 1958, Cambiaggi first described the classic retinal pigment changes in a patient receiving chloroquine for systemic lupus erythematous (SLE). In 1959, Hobbs established a link between long-term use of chloroquine and subsequent development of retinal pathology. In 1962, J Lawton Smith coined the term bull's eye maculopathy, regarded as the classic finding of macular toxicity.
Physicians who prescribe chloroquine and hydroxychloroquine may not be fully aware of the potential ophthalmic implications. In the management of patients treated with these agents, the preferred practice is regular screening. Patients and referring physicians should understand that screening helps to identify toxicity early but cannot guarantee prevention of toxicity and vision loss.
Pathophysiology
The mechanism of chloroquine and hydroxychloroquine toxicity is not well understood. Chloroquine has an affinity for pigmented (melanin-containing) structures, which may explain its toxic properties in the eye. Melanin serves as a free-radical stabilizer and can bind toxins, including retinotoxic drugs. However, it is unclear whether this binding effect is beneficial or harmful.
Chloroquine and its principal metabolite accumulate in pigmented ocular structures at concentrations higher than other tissues and withdraws more slowly relative to other tissues upon withdrawal of therapy. [6, 7] Prolonged exposure can allow the drug to accumulate in the retina, where it remains in the pigmented structures long after its use is stopped. In patients with retinopathy, traces of chloroquine have been found in plasma, erythrocytes, and urine 5 years or more after discontinuation of the drug. [8] However, progression of retinopathy after discontinuation of therapy may not result from slow clearance but gradual decompensation of cells injured during drug therapy. [5]
Etiology
Chloroquine/hydroxychloroquine retinopathy is influenced most by daily dose, length of use, and cumulative dose. The kinetics of chloroquine metabolism are complex, with the half-life increasing with increasing dosage.
Factors associated with hydroxychloroquine toxicity include the following:
-
Maintenance dose greater than 5 mg/kg/d based on real weight (most critical risk)
-
Daily dose greater than 400 mg
-
Cumulative dose greater than 1000 g
-
Age older than 60 years
Factors associated with chloroquine toxicity include the following:
-
Maintenance dose greater than 2.3 mg/kg/d
-
Obesity
Factors associated with toxicity in both drugs include the following:
-
Duration of treatment greater than 5 years (critical factor)
-
Tamoxifen use (5-fold risk)
-
Evidence of renal insufficiency
-
Underlying retinal disease or maculopathy (macular degeneration)
-
Evidence of liver disease
-
P450 polymorphisms leading to higher drug concentration in blood
A study of chloroquine/hydroxychloroquine retinopathy in a Turkish cohort found no significant difference between affected and unaffected patients with respect to several risk factors. Rather, the cumulative dose of hydroxychloroquine was significantly higher in the unaffected patients. These findings suggest that the currently widely accepted risk factors may not be applicable to all patients and that there may be risk factors previously not reported that may play a role in the development of toxicity. [9]
Epidemiology
Despite variability of statistics in published reports, a consistent trend found in the literature is that the incidence of retinopathy from chloroquine/hydroxychloroquine increases with both the daily dose and the duration of treatment. The risk of developing retinal toxicity is less than 2% in patients who use dosages below the recommended threshold for up to 10 years. The risk increases significantly after 20 years of therapy and/or daily dose above the recommended threshold. [10]
Older patients are believed to be at a higher risk because of the higher rate of retinal comorbidities.
Prognosis
If the maximum daily dosage recommendations are followed, the likelihood of toxicity from chloroquine or hydroxychloroquine is less than 1% the first five years of treatment. [5] Corneal epithelial changes are usually reversible, but retinopathy caused by these agents are not. If diagnosed early, before RPE damage, there is mild and limited progression of the disease upon discontinuation of the agents. Once the appearance of a bull's eye maculopathy is noted, which indicates advanced stage of toxicity, disease progression can continue for years after discontinuation of the agents. Risk to vision and disease progression are a function of disease severity at the time of detection. [11, 12]
Patient Education
When starting patients on chloroquine or hydroxychloroquine, clinicians should counsel patients about the benefits and limitations of screening. Patients should be informed that screening can detect toxicity at early stages and limit progression of vision loss but cannot necessarily prevent all toxicity and associated vision risk.
Advise patients to consult their physician and an ophthalmologist if changes in visual acuity or if blurred vision occurs while on treatment, as risk of vision loss may warrant discontinuation of the medication.
-
A 53-year-old female with a complaint of something "funny" with her vision. The possibility of hydroxychloroquine toxicity was entertained, although clinical evidence was not found. Color vision testing and funduscopic examination were normal. A full field electroretinogram was normal, but foveal cone electroretinograms were reduced bilaterally. These findings prompted the question of possible early hydroxychloroquine retinopathy.
-
Fluorescein angiogram of left macula in patient with hydroxychloroquine retinopathy. Reprinted from American Journal of Ophthalmology, Vol 104, Johnson and Vine, Hydroxychloroquine therapy in massive total doses without retinal toxicity, pages 139-144, Copyright 1987, with permission from Elsevier Science.
-
Membranous cytoplasmic bodies in ganglion cell of retina. (N=nucleus) (X12,500.) Reprinted from American Journal of Ophthalmology, Vol 67, Gleiser CA, Dukes TW, Lawwill T, Read WK, Bay WW, Brown RS. Ocular changes in swine associated with chloroquine toxicity, pages 399-405, Copyright 1969, with permission from Elsevier Science.
-
Swollen ganglion cells with foamy cytoplasm (Hematoxylin-eosin, X500). Reprinted from American Journal of Ophthalmology, Vol 67, Gleiser CA, Dukes TW, Lawwill T, Read WK, Bay WW, Brown RS. Ocular changes in swine associated with chloroquine toxicity, pages 399-405, Copyright 1969, with permission from Elsevier Science.
-
The same patient as described in the image above (other eye, left eye). The patient (with foveal cone electroretinogram reduction) had abnormal computerized acuity mapping of the macula results.
-
An Amsler grid is used to assess the central portion of the macula. This simple test is helpful for patients to monitor their vision at home.









