Practice Essentials
Graft versus host disease (GVHD) is an immune-mediated condition resulting from a complex interaction between donor and recipient adaptive immunity. [1] Acute GVHD describes a distinctive syndrome of dermatitis (see the image below), hepatitis, and enteritis developing within 100 days after allogeneic hematopoietic cell transplantation (HCT). Chronic GVHD describes a more diverse syndrome developing after day 100. In addition to allogeneic HCT, procedures associated with high risk of GVHD include transplantation of solid organs containing lymphoid tissue and transfusion of unirradiated blood products.
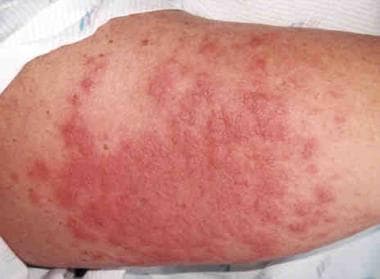 Autologous graft versus host disease (GVHD) involving the skin of a patient's arm appeared shortly after signs of engraftment appeared. The patient had undergone autologous peripheral blood stem-cell transplantation to treat ovarian cancer. Courtesy of Romeo A. Mandanas, MD, FACP.
Autologous graft versus host disease (GVHD) involving the skin of a patient's arm appeared shortly after signs of engraftment appeared. The patient had undergone autologous peripheral blood stem-cell transplantation to treat ovarian cancer. Courtesy of Romeo A. Mandanas, MD, FACP.
Signs and symptoms
Presentation in acute GVHD is as follows:
-
A pruritic or painful rash (median onset, day 19 posttransplantation; range, 5-47 days) [2]
-
Pruritus from initially asymptomatic liver involvement, anorexia, weight loss, followed rarely by hepatic coma
-
Diarrhea, intestinal bleeding, cramping abdominal pain, and ileus
Diarrhea in acute GHVD is green, mucoid, watery, and mixed with exfoliated cells forming fecal casts. Voluminous secretory diarrhea may persist despite cessation of oral intake.
Upper GI enteric GVHD occurs in approximately 13% of patients who receive HLA-identical transplants and manifests as anorexia and dyspepsia without diarrhea. It is most common in older patients.
Chronic GVHD may be an extension of acute GVHD, may occur de novo in patients who never have clinical evidence of acute GVHD, or may emerge after a quiescent interval after acute GVHD resolves. [3] Manifestations are as follows:
-
Ocular – Burning, irritation, photophobia, and pain from lack of tear secretion
-
Oral and GI – Mouth dryness, sensitivity to acidic or spicy foods, dysphagia, odynophagia, and insidious weight loss
-
Pulmonary – Obstructive lung disease, with symptoms of wheezing, dyspnea, and chronic cough that is usually nonresponsive to bronchodilator therapy
-
Neuromuscular – Generalized or focal weakness, neuropathic pain, vision loss, muscle cramps, myalgia
-
Joints – Arthralgia, arthritis
Physical examination
Skin findings are as follows:
-
Maculopapular exanthema – Red-to-violet lesions that typically first appear on the palms of the hands, soles of the feet, cheeks, neck, ears, and upper trunk, sometimes progressing to involve the whole body; in severe cases, bullae may be observed, and vesicles may form
-
Lichenoid skin lesions or sclerodermatous thickening of the skin, which sometimes causes contractures and limits joint mobility
-
Jaundice from hyperbilirubinemia; patients who subsequently develop pruritus may exhibit excoriations from scratching
Ocular findings may include the following:
-
Acute GVHD – Hemorrhagic conjunctivitis, pseudomembrane formation, and lagophthalmos
-
Chronic GVHD – Keratoconjunctivitis sicca, which may lead to punctate keratopathy
Additional findings are as follows:
-
Oral – Atrophy of the oral mucosa, erythema, and lichenoid lesions of the buccal and labial mucosae in chronic GVHD
-
Pulmonary – Prolonged expiratory breathing phase (wheezes) from bronchiolitis obliterans
-
GI – Diffuse abdominal tenderness with hyperactive bowel sounds may accompany secretory diarrhea of acute GVHD; in severe ileus, the abdomen is silent and appears distended
-
Neuromuscular – Findings of myasthenia gravis, polymyositis, optic neuritis, arthritis may occur in chronic GVHD
-
Vaginitis and vaginal strictures have been described in chronic GVHD
See Presentation for more detail.
Diagnosis
Laboratory study results in GVHD are as follows:
-
CBC – Autoimmune cytopenias (eg, thrombocytopenia, anemia, leukopenia) may be observed with chronic GVHD
-
Liver function tests – Elevation of the alkaline phosphatase level, an early sign of liver involvement by GVHD; hypoalbuminemia is typically due to GVHD-associated intestinal protein leakage and a negative nitrogen balance
-
Serum electrolytes and chemistries (eg, potassium, magnesium, bicarbonate levels) may be altered; massive diarrhea and diminished oral intake can lead to serious electrolyte abnormalities
Other tests
-
Schirmer test – To measure the degree of tear formation by the lacrimal glands in chronic GVHD
-
Pulmonary function tests and arterial blood gas analysis – To identify obstructive pulmonary disease (eg, obliterative bronchiolitis) in chronic GVHD
-
Manometric studies of the esophagus
Imaging studies
-
Hepatic and Doppler sonography
-
Barium swallow study
Procedures
-
Skin punch biopsy
-
Upper GI endoscopy and biopsy in patients with persistent anorexia and vomiting
-
Flexible sigmoidoscopy or colonoscopy with biopsy of sigmoid or colonic lesions in patients with diarrhea
-
Liver biopsy (rarely done, usually only in patients with isolated hepatic findings)
See Workup for more detail.
Management
The criterion standard for primary prophylaxis of acute GVHD is cyclosporine for 6 months and short-course methotrexate in T-cell–replete allogeneic HCT (criterion standard); currently, tacrolimus is often substituted for cyclosporine because of its more potent immunosuppressant capacity and lower risk of nephrotoxicity. Antithymocyte globulin (ATG) is given before HCT in unrelated-donor transplants.
Primary therapy for acute GVHD is as follows:
-
For skin GVHD of stage I or II, observation or a trial of topical corticosteroids (eg, triamcinolone 0.1%) may be used
-
For grade II-IV acute GVHD, continuing the original immunosuppressive prophylaxis and adding methylprednisolone (commonly starting at 2 mg/kg/day in 2 divided doses); in patients who respond to initial therapy, the steroid will be tapered weekly thereafter until off
-
Other therapies are ATG, cyclosporine, sirolimus [4] , mycophenolate mofetil (MMF), daclizumab, anti–interleukin-2 (IL-2) receptor, alone or in combination
Secondary therapy for acute GVHD is as follows:
-
ATG or multiple pulses of methylprednisolone (at doses higher than those used in initial therapy)
-
Tacrolimus, for GVHD with cyclosporine resistance or neurotoxicity or nephrotoxicity
-
MMF at 2 g daily, added to the steroid regimen [5]
-
Psoralen and ultraviolet A irradiation (PUVA), for cutaneous lesions
-
Ruxolitinib
-
Muromomab-CD3 (Orthoclone OKT3)
-
Humanized anti-Tac antibody to the IL-2 receptor
Primary therapy for chronic GVHD is as follows:
-
Prednisone, 1 mg/kg every day
-
Tacrolimus
-
Cyclosporine, 6 mg given every 12 hours every other day in patients at high risk for GVHD with thrombocytopenia
-
Sirolimus
-
Thalidomide
Secondary therapy for chronic GVHD is as follows:
-
MMF, added to standard tacrolimus, cyclosporine, sirolimus, and/or prednisone, for steroid-refractory chronic GVHD
-
Azathioprine, alternating cyclosporine/prednisone, or thalidomide for steroid-refractory chronic GVHD
-
Low-dose (100-cGy) total lymphoid irradiation to thoracoabdominal areas
-
Imatinib [10]
-
Pentostatin
-
Belumosudil, for adults and children aged ≥12 years in whom 2 prior systemic therapies for chronic GVHD have failed
-
Ruxolitinib, for adults and children aged ≥12 years in whom 1 or 2 prior systemic therapies for chronic GVHD have failed
Treatment of cutaneous, musculoskeletal, or oral lesions of chronic GVHD includes the following:
-
Clofazimine, for treating cutaneous and oral lesions of chronic GVHD and as a steroid-sparing agent
-
PUVA therapy, for refractory cutaneous chronic GVHD
-
Rituximab, mainly for musculoskeletal and cutaneous chronic GVHD [10]
See Treatment and Medication for more detail.
Background
Barnes and Loutit first described (in mice) what is now known as graft versus host disease (GVHD) as a syndrome called secondary disease to differentiate it from primary disease of radiation sickness. [13] Mice that were given allogeneic spleen cells after irradiation developed fatal secondary disease (skin abnormalities and diarrhea), which was a result of introducing immunologically competent cells into an immunoincompetent host. Human GVHD has features similar to those observed in animal studies.
Pathophysiology
Several criteria, as first described by Billingham in 1966, [14] are traditionally required to diagnose GVHD, including the following:
-
The graft must contain immunologically competent cells.
-
The host must possess important transplantation alloantigens that are lacking in the donor graft so that the host appears foreign to the graft and can therefore stimulate it antigenically.
-
The host itself must be incapable of mounting an effective immunologic reaction against the graft, or it must at least allow for sufficient time for the latter to manifest its immunologic capabilities (ie, it must have the security of tenure).
Certain patient groups are at risk for GVHD, as outlined below in Table 1.
Table 1. Procedures Associated with a High Risk of GVHD* (Open Table in a new window)
Procedure |
Groups at High Risk |
Allogeneic HCT |
Patients receiving no GVHD prophylaxis Older patients Recipients of HLA-nonidentical stem cells Recipients of grafts from allosensitized donors Recipients of grafts from unrelated donors |
Solid-organ transplantation (organs containing lymphoid tissue) |
Recipients of small-bowel transplants |
Transfusion of unirradiated blood products |
Neonates and fetuses Patients with congenital immunodeficiency syndromes Patients receiving immunosuppressive chemoradiotherapy Patients receiving directed blood donations from partially HLA-identical, HLA-homologous donors |
*Modified from Ferrara and Deeg, 1991. [15] HLA = Human leukocyte antigen. |
|
Current understanding of the biology of GVHD includes the occurrence of autologous GVHD and transfusion-associated GVHD. The former suggests that inappropriate recognition of host self-antigens may occur, and the latter is an example of GVHD in an individual who is immunocompetent (see image below).
 Autologous graft versus host disease (GVHD) involving the skin of a patient's arm appeared shortly after signs of engraftment appeared. The patient had undergone autologous peripheral blood stem-cell transplantation to treat ovarian cancer. Courtesy of Romeo A. Mandanas, MD, FACP.
Autologous graft versus host disease (GVHD) involving the skin of a patient's arm appeared shortly after signs of engraftment appeared. The patient had undergone autologous peripheral blood stem-cell transplantation to treat ovarian cancer. Courtesy of Romeo A. Mandanas, MD, FACP.
GVHD is an immune-mediated disease resulting from a complex interaction between donor and recipient adaptive immunity. [1] The main effectors are donor T cells, which are activated in the presence of co-stimulatory molecules by a storm of proinflammatory cytokines [16] (see image below). The successful use of B-cell–targeted therapy such as rituximab in chronic GVHD has sparked an interest in defining the role of B cells in the pathophysiology of GVHD. [17]
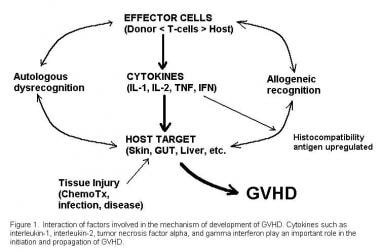 Interactive factors involved in the pathogenesis of graft versus host disease (GVHD.) Courtesy of Romeo A. Mandanas, MD, FACP.
Interactive factors involved in the pathogenesis of graft versus host disease (GVHD.) Courtesy of Romeo A. Mandanas, MD, FACP.
Chronic GVHD is a syndrome that mimics the autoimmune diseases. Donor T cells play an important role in its development, but humoral immunity is also implicated. The targets of attack may include host non-HLA antigens such as minor histocompatibility antigens. In some studies, host dendritic cells may also be at play. A close relationship exists between the development of chronic GVHD and a helpful graft-versus-tumor/leukemia effect. [3]
Etiology
Important factors in determining occurrence and severity of GVHD include the following:
-
Donor-host factors
-
Stem cell source
-
Immune modulation
-
High-dose chemotherapy and radiation therapy
Donor-host factors are as follows:
-
The incidence of GVHD increases with unrelated matched donor transplants compared with related matched transplants.
-
With increasing HLA disparity, the incidence and severity of GVHD increases.
-
Sex mismatching and increasing age of both donor and recipient increase the frequency of GVHD.
Stem-cell source factors are as follows:
-
Cryopreservation of marrow before its infusion apparently reduces the rate of GVHD.
-
Use of umbilical-cord blood rather than marrow may also lower the incidence of GVHD.
-
Allogeneic peripheral blood stem cells (PBSC) may increase the incidence of chronic GVHD and prolong follow-up. [20]
Immune modulation factors are as follows:
-
The efficacy of posttransplantational immunosuppressive prophylaxis affects the development of GVHD. [21]
-
Triple therapy with cyclosporine, short-course methotrexate (MTX), and prednisone lowers the incidence of GVHD compared with double therapy with cyclosporine and MTX alone. The addition of sirolimus to tacrolimus and MTX also reduces GVHD incidence compared with double therapy alone. [22]
-
Anti–T-cell globulin, when added to standard immunosuppressive prophylaxis, can decrease the incidence of acute and chronic GVHD in recipients of matched unrelated donor transplants. [23]
-
Statins have been found, in the preclinical setting, to affect or inhibit human antigen-presenting cell (APC) function and to reduce the expression of co-stimulatory molecules and major histocompatibility complex (MHC) class II. [24, 25] In the clinical setting, a retrospective analysis of 567 patients who received allogeneic transplantation from HLA-identical sibling donors for various hematologic malignancies noted that statin use by the donor (but not by the recipient) was associated with a decreased risk of grade 3-4 acute GVHD. [26]
High-dose chemotherapy and radiation therapy have the following effects:
-
After high-dose chemotherapy, levels of circulating cytokines increase; this is known as a cytokine storm. These cytokines are thought to increase the ability of graft immune cells to recognize host antigens. [16]
-
High-dose chemotherapy can also lead to localized tissue damage, exposing cryptic antigens in certain organs (eg, skin, liver, gut).
-
Conditioning regimens that include total-body irradiation are associated with an increased incidence and severity of GVHD compared with chemotherapy alone. [27]
-
Administration of nonmyeloablative but immunosuppressive chemotherapy followed by allogeneic transplants (ie, minidose transplantations, or "transplant-light") decreases the original cytokine storm and tissue damage. This strategy lowers the incidence of GVHD and is aimed at maintaining a graft-versus-tumor effect. [27, 28]
Epidemiology
Frequency
United States
Autologous GVHD occasionally occurs after autologous or syngeneic HCT (7-10%). Tissue damage caused by high-dose chemotherapy or secondary cytokine production may expose cryptic self-antigens, which the immune system may newly recognize only after HCT. Mild and usually self-limited episodes of dermal GVHD or even hepatic and GI abnormalities have been described. GVHD-like symptoms and findings can also be induced in autologous recipients after the administration (and withdrawal) of cyclosporine and interleukin (IL)-2. [29]
Transfusion-associated GVHD occurs 4-30 days after transfusion and resembles hyperacute GVHD after allogeneic HCT. Marrow aplasia is a frequent and often fatal complication. This serious complication of transfusion can be prevented by irradiating blood products with at least 2500 cGy before transfusion in individuals at risk. In Japan (where inbred populations share common haplotypes), marrow aplasia is estimated to occur in 1 in 500 open-heart operations in individuals who are immunocompetent.
The occurrence of acute GVHD in patients who receive marrow from HLA-identical siblings varies widely depending on several recognized risk factors. About 19-66% of recipients are affected, depending on their age, on donor-recipient sex matching, and on donor parity. The incidence of GVHD increases with HLA-nonidentical marrow donors who are related or in HLA-matched unrelated donors, with rates of 70-90%. [30]
Chronic GVHD is observed in 33% of HLA-identical sibling transplantations, in 49% of HLA-identical related transplantations, in 64% of matched unrelated donor transplantations. The rate could be as high as 80% in 1-antigen HLA-nonidentical unrelated transplantations. [3]
The source of donor graft affects the incidence of GVHD. Although acute GVHD does not differ significantly among recipients of HLA-identical sibling bone marrow (BM) versus peripheral blood stem cells (PBSC), the cumulative incidence of chronic GVHD (and extensive GVHD) is higher in those who received PBSC (73% vs 55%). [31, 32] The cumulative incidence of grades III-IV acute and extensive chronic GVHD is much lesser in unrelated cord blood recipients than in recipients of either HLA-identical sibling BM or PBSC transplants. [33]
Prognosis
The overall grade of acute GVHD is predictive of the patient's outcome, with the highest rates of mortality in those with grade IV, or severe, GVHD. The response to treatment is also predictive of outcomes in GVHD of grades II-IV. Patients with no response or with progression have a mortality rate as high as 75%, compared with 20-25% in those with a complete response. [30] Factors associated with impaired survival are HLA-nonidentical marrow donors, liver abnormalities in addition to GVHD, and early time to onset and treatment of GVHD.
Late GI symptoms (more than 100 days posttransplant) were reported in 71 allogeneic stem cell transplant patients. Following an endoscopy, 45 (63%) were diagnosed with GI-GVHD. Of these 45 patients, 39 (87%) had late acute GVHD. The median survival time from the first endoscopy was 8.5 months. The incidence of nonrelapse mortality at 6 months was 31% in patients with GI-GVHD compared to 19% in patients without GI-GVHD (P = 0.42). All patients with GI-GVHD were on steroid therapy, and close to one-third of these patients needed total parenteral nutrition. [34]
In chronic GVHD, mortality rates are increased in patients with extensive disease, progressive onset, thrombocytopenia, and HLA-nonidentical marrow donors. The overall survival rate is 42%, but patients with progressive onset of chronic GVHD have a survival rate of 10%. [35] Other factors linked to high mortality rates are as follows:
-
Extensive disease
-
Thrombocytopenia
-
HLA-nonidentical marrow donors
-
Elevated bilirubin value at 2 mg/dL or greater
-
Lichenoid histology on skin biopsy
-
Failure to taper prednisone treatment for acute GVHD before the onset of progressive chronic GVHD
Multiple immune defects are observed in patients with chronic GVHD, such as impaired mucosal defense, chemotactic defects, functional asplenia, T-cell alloreactivity, and qualitative and quantitative B-cell abnormalities. Bacteremia and sinopulmonary infections due to Streptococcus pneumoniae and Haemophilus influenzae can occur. [36] The incidence of pulmonary infections after day 100 is 50% in patients with chronic GVHD versus 21% in those without GVHD.
In patients who undergo unrelated donor transplantation, the risk of bacteremia and septicemia due to chronic GVHD and HLA-nonidentity is increased, and hypogammaglobulinemia occurs frequently.
Treatment of patients with chronic GVHD with azathioprine is associated with an increased risk of secondary neoplasms, such as squamous cell carcinomas of the skin and buccal mucosa.
Sclerodermatous lesions can lead to joint contractures, impairing mobility and the patient's ability to perform certain routine body movements.
Patient Education
To decrease the incidence of sunburn, which can exacerbate GVHD reactions, patients should avoid excessive exposure to the sun by using sunblock lotion, sun-blocking headgear, and appropriate garments.
Patients should pay attention to good skin care, using moisturizing lotions or creams to prevent skin breakdown.
While receiving corticosteroid therapy, patients should be encouraged to preserve muscle tone and mass by avoiding sedentary activity and by exercising regularly.
Patients should avoid unnecessary exposure to potentially hazardous infections while they are receiving highly immunosuppressive treatment for GVHD. Examples of unnecessary exposure are the inhalation of fungal spores from the soil while gardening, working on farms, and working with animal excreta.
-
Autologous graft versus host disease (GVHD) involving the skin of a patient's arm appeared shortly after signs of engraftment appeared. The patient had undergone autologous peripheral blood stem-cell transplantation to treat ovarian cancer. Courtesy of Romeo A. Mandanas, MD, FACP.
-
Acute graft versus host disease (GVHD) involving desquamating skin lesions in a patient after allogeneic bone marrow transplantation for myelodysplasia. Courtesy of Romeo A. Mandanas, MD, FACP.
-
Oral mucosal changes in a patient with chronic graft versus host disease (GVHD). Note the skin discoloration (vitiligo), which can be a result of GVHD. Courtesy of Romeo A. Mandanas, MD, FACP.
-
Interactive factors involved in the pathogenesis of graft versus host disease (GVHD.) Courtesy of Romeo A. Mandanas, MD, FACP.
-
This boy developed stage III skin involvement with acute graft versus host disease (GVHD) despite of receiving prophylaxis with cyclosporin A. The donor was his HLA-matched sister; the sex disparity increased the risk for acute GVHD. Courtesy of Mustafa S. Suterwala, MD.
-
Same boy as in previous image progressed to grade IV graft versus host disease (GVHD). High-dose cyclosporin A and methylprednisolone had been administered intravenously. He later died from chronic pulmonary disease due to chronic GVHD. Courtesy of Mustafa S. Suterwala, MD.
-
Acute graft versus host disease (GVHD). Hematoxylin and eosin–stained tissue shows dyskeratosis of individual keratinocytes and patchy vacuolization of the basement membrane. Moderate superficial dermal and perivascular lymphocytic infiltrate are also observed. Courtesy of Melanie K. Kuechler, MD.








