Background
Glucose intolerance is an umbrella term for a group of metabolic conditions that result in higher than normal blood glucose levels. Both the World Health Organization (WHO) and the American Diabetes Association (ADA) have released classification systems and diagnostic criteria for diabetes mellitus (DM) and allied categories of glucose intolerance. [1, 2, 3, 4] Although similiar, there are a number of variances in recommendations which may result in differences in an individual’s classification.
The major categories of the disorders of glycemia or glucose tolerance are as follows:
-
Type 1 diabetes mellitus (DM) (due to autoimmune B-cell destruction, usually leading to absolute insulin deficiency)
-
Type 2 diabetes mellitus (due to a progressive loss of B-cell insulin secretion frequently on the background of insulin resistance)
-
Gestational diabetes mellitus (GDM) (diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation) [5]
-
Specific types of diabetes due to other causes (such as neonatal diabetes and maturity-onset diabetes of the young, diseases of the exocrine pancreas, and drug- or chemical-induced diabetes)
-
Impaired glucose tolerance (IGT: prediabetes/intermediate hyperglycemia)
-
Impaired fasting glucose (IFG: prediabetes/intermediate hyperglycemia)
Conditions secondarily associated with glucose intolerance also occur. Etiologic types and stages of the major disorders of glucose intolerance are shown in the image below.
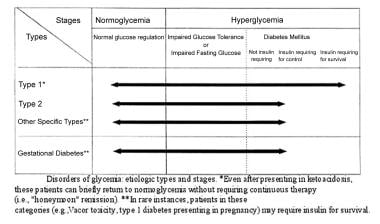 Glucose intolerance. Etiologic types and stages of the major disorders of glucose tolerance are displayed.
Glucose intolerance. Etiologic types and stages of the major disorders of glucose tolerance are displayed.
In most cases, the diagnosis of a type of diabetes or glucose intolerance is based on the patient’s condition at the time, but not all patients have a set of symptoms that fit readily into a particular class (see Presentation).
When hyperglycemia is present, its severity may change in time, depending on the underlying process. Choosing an appropriate management approach to any disorders of glucose intolerance necessitates a strong understanding of the mechanisms involved in the disease process. [6, 7] (See Treatment and Medication.)
Pathophysiology
Heterogeneity occurs in most glucose intolerance disorders, including diabetes mellitus syndromes.
Type 1 diabetes mellitus
Type 1 diabetes mellitus is characterized by absolute insulin deficiency. In type 1A, a cellular-mediated autoimmune destruction of beta cells of the pancreas occurs. The disease process is initiated by an environmental factor—that is, an infectious or noninfectious agent in genetically susceptible individuals.
Some genes in the histocompatibility leukocyte antigen (HLA) system are thought to be crucial. A stress-induced epinephrine release, which inhibits insulin release (with resultant hyperglycemia), sometimes occurs and may be followed by a transient asymptomatic period known as "the honeymoon." Lasting weeks to months, the honeymoon precedes the onset of overt, permanent diabetes.
Amylin, a beta-cell hormone that is normally cosecreted with insulin in response to meals, is also completely deficient in persons with type 1 diabetes mellitus. Amylin exhibits several glucoregulatory effects that complement those of insulin in postprandial glucose regulation. Idiopathic forms of type 1 diabetes also occur, without evidence of autoimmunity or HLA association; this subset is termed type 1B diabetes.
The underlying pathophysiology of beta cell demise or dysfunction is currently more understood in type 1 diabetes than in type 2 diabetes. The rate of progression in type 1 diabetes is dependent on the age at first detection of antibody, number of antibodies, antibody specificity, and antibody titer. [1, 8] Three distinct stages of type 1 diabetes have been recognized. [1, 8] Both stages 1 and 2 are characterized by autoimmunity and a presymptomatic status; although there is still normoglycemia in stage 1, dysglycemia (impaired fasting glucose [IFG] and/or impaired glucose tolerance [IGT]) is present in stage 2. Stage 3 is characterized by new-onset symptomatic hyperglycemia. [1, 8]
Type 2 diabetes mellitus
In a state of health, normoglycemia is maintained by fine hormonal regulation of peripheral glucose uptake and hepatic production. Type 2 diabetes mellitus results from a defect in insulin secretion and an impairment of insulin action in hepatic and peripheral tissues, especially muscle tissue and adipocytes. [9] A postreceptor defect is also present, causing resistance to the stimulatory effect of insulin on glucose use. As a result, a relative insulin deficiency develops, unlike the absolute deficiency found in patients with type 1 diabetes. The specific etiologic factors are not known, but genetic input is much stronger in type 2 diabetes than in the type 1 form. [10]
Impaired glucose tolerance (IGT) is a transitional state from normoglycemia to frank diabetes, but patients with impaired glucose tolerance exhibit considerable heterogeneity. Type 2 diabetes, or glucose intolerance, is part of a dysmetabolic syndrome (syndrome X) that includes insulin resistance, hyperinsulinemia, obesity, hypertension, and dyslipidemia. Current knowledge suggests that the development of glucose intolerance or diabetes is initiated by insulin resistance and worsened by the compensatory hyperinsulinemia. Insulin resistance is not only predictive for type 2 diabetes and associated with myriad metabolic derangements in fasting conditions, but nondiabetic insulin-resistant individuals are subjected to a similar adverse postprandial metabolic setting and cardiometabolic risk as those with type 2 diabetes. [11] In addition, the prevalence of hypertension rises with exacerbation of stages of impaired glucose metabolism; however, only in the early stages of impaired insulin metabolism do hyperglycemia and hyperinsulinemia appear to be significant contributors to the presence of hypertension. [12]
The paths to beta-cell dysfunction or demise are less well defined in type 1 diabetes. The progression to type 2 diabetes is influenced by genetics and environmental or acquired factors, such as a sedentary lifestyle and dietary habits that promote obesity. Most patients with type 2 diabetes are obese, and obesity is associated with insulin resistance. Insulin resistance is not only predictive for type 2 diabetes and associated with myriad metabolic derangements in fasting conditions, but nondiabetic insulin-resistant individuals are subjected to a similar adverse postprandial metabolic setting and cardiometabolic risk as those with type 2 diabetes. [11] Central adiposity is more important than increased generalized fat distribution. In patients with frank diabetes, glucose toxicity and lipotoxicity may further impair insulin secretion by the beta cells. [13] [14] [15] [16] Moreover, "in obesity, inflammation, with increased accumulation and inflammatory polarization of immune cells, takes place in various tissues, including adipose tissue, skeletal muscle, liver, gut, pancreatic islet, and brain, and may contribute to obesity-linked metabolic dysfunctions, leading to insulin resistance and type 2 diabetes." [17]
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM) was previously described as any degree of glucose intolerance in which onset or first recognition occurs during pregnancy. [5] The definition was limited by imprecision. Women diagnosed with diabetes in the first trimester are now classified as having type diabetes. GDM is diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes. Insulin requirements are increased during pregnancy because of the presence of insulin antagonists, such as human placental lactogen or chorionic somatomammotropin, and cortisol; these promote lipolysis and decrease glucose use.
Another factor in increased insulin requirements during pregnancy is the production of insulinase by the placenta. Various genetic defects of the beta cell, insulin action, diseases of the exocrine pancreas, endocrinopathies, drugs, chemical agents, infections, immune disorders, and genetic syndromes can cause variable degrees of glucose intolerance, including diabetes.
To see complete information on Diabetes Mellitus and Pregnancy, please go to the main article by clicking here.
Other specific types of diabetes mellitus
These are specific types of diabetes due to other causes, which include monogenic diabetes syndromes, diseases of the exocrine pancreas, and drug- or chemical induced diabetes. Various genetic defects of the beta cell, insulin action, diseases of the exocrine pancreas, endocrinopathies, drugs, chemical agents, infections, immune disorders, and genetic syndromes can cause variable degrees of glucose intolerance, including diabetes.
Varying forms of glucose intolerance
Glucose intolerance may be present in many patients with cirrhosis due to decreased hepatic glucose uptake and glycogen synthesis. Other underlying mechanisms include hepatic and peripheral resistance to insulin and serum hormonal abnormalities. Abnormal glucose homeostasis may also occur in uremia, as a result of increased peripheral resistance to the action of insulin.
The gastrointestinal tract plays a significant role in glucose tolerance. [18] With food ingestion, incretin hormones glucagonlike peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are synthesized and secreted by specialized gut cells. Oral glucose administration results in a higher insulin secretory response than does intravenous glucose administration; this difference is due in part to incretin hormones.
The significance of incretin hormones has been noted as a result of efforts to develop agents that may improve glycemic control in patients with type 2 diabetes through new mechanisms. [19] These strategies include inhibition of dipeptidyl peptidase IV (DPP-4), the major enzyme responsible for degrading incretin hormones in vivo, and the use of GLP-1 agonists. [20] Incretin hormones also significantly affect the differentiation, mitogenesis, and survival of beta cells.
Pathologic defects observed in type 2 diabetes mellitus and sometimes in impaired glucose tolerance include postprandial hyperglucagonemia, dysregulation of gastric emptying, and loss of incretin effect.
Postprandial hyperglycemia in diabetes and impaired glucose tolerance (IGT) is related to a lower rate of glucose disposal, whereas insulin secretion and action, as well as postprandial turnover, are essentially normal in individuals with isolated IGT. [21]
Etiology
Genetic defects of beta-cell function include the following:
-
Mutation on chromosome 12, the hepatocyte nuclear factor (HNF-1) alpha -MODY3
-
Mutation on chromosome 7p, the glucokinase gene -MODY2
-
Mutation on chromosome 20, HNF-4 alpha -MODY1
-
Point mutations in mitochondrial DNA
Defects in insulin action include the following:
-
Structure and function of insulin receptor: postreceptor signal transduction pathways
-
Type A insulin resistance
-
Leprechaunism
-
Rabson-Mendenhall syndrome
-
Lipoatrophic diabetes
Diseases of the exocrine pancreas include the following:
-
Trauma
-
Infection
-
Pancreatectomy
-
Fibrocalculous pancreatopathy
(Note that the malnutrition-related diabetes has been eliminated from the above list, as evidence is lacking on protein deficiency as a direct cause of diabetes, and fibrocalculous pancreatopathy has been reclassified as a disease of the exocrine pancreas.)
Endocrine diseases associated with excess production of insulin antagonists include the following:
-
Acromegaly
-
Glucagonoma
-
Pheochromocytoma
-
Somatostatinoma
-
Aldosteronoma
Drugs or chemical agents with adverse effects on glucose tolerance include the following:
-
Thiazides
-
Diazoxide
-
Glucocorticoids
-
Calcineurin inhibitors, such as cyclosporine and tacrolimus
-
Oral contraceptives
-
Beta-adrenergic agonists
-
Nicotinic acid
-
Thyroid hormone
-
Pentamidine
-
Alpha interferon
-
Atypical antipsychotics, especially clozapine and olanzapine
-
Antiretroviral drugs
-
Vacor
Infections associated with beta-cell destruction include the following:
-
Rubella
-
Coxsackievirus B
-
Mumps
-
Cytomegalovirus
-
Adenovirus
Genetic syndromes that predispose an individual to impaired glucose tolerance include the following:
-
Klinefelter syndrome
-
Turner syndrome
-
Wolfram syndrome
-
Friedreich ataxia
Pregnancy can be associated with gestational diabetes mellitus, and the risk of diabetes increases with parity.
Obesity is a powerful determinant of glucose intolerance in the general population and develops through the interaction of genetics and acquired factors such as physical inactivity and dietary habits.
Immune-mediated causes of impaired glucose tolerance include stiff person syndrome and anti-insulin receptor abnormalities. Other causes of glucose intolerance are liver disease (as in cirrhosis) and renal failure.
Epidemiology
United States statistics
Approximately 29 million people in the United States (9.3%) have diabetes, with 1.7 million new cases diagnosed in adults each year. The CDC estimates 25% of individuals with diabestes are undiagnosed. Eighty-six million adults aged 20 years and older have prediabetes. [22]
Type 1 diabetes, which usually occurs in children and adolescents, accounts for 5-10% of diabetes cases. Approximately 1 out of every 400-500 children and adolescents in the United States has type 1 diabetes.
Type 2 diabetes, which most commonly occurs in middle age, is the predominant form of clinical disease, constituting 90-95% of cases. This type of diabetes is reaching epidemic proportions. Minority populations, especially American Indians, Hispanic persons, African Americans, and Asian Americans are at particularly high risk.
Gestational diabetes develops in approximately 4% of pregnancies in the United States. The prevalence is 1-14%, depending on the population studied and the diagnostic criteria.
International statistics
The global prevalence of diabetes has nearly doubled since 1980, rising from 4.7% to 8.5% in the adult population, and results in an estimated 1.5 million deaths each year. Higher-than-optimal blood glucose levels caused an additional 2.2 million deaths, by increasing the risks of cardiovascular and other diseases. Forty-three percent of these 3.7 million deaths occur before the age of 70 years. [23]
Race characteristics
In the United States, African Americans, Hispanic persons, and Native Americans are about twice as likely to have diagnosed diabetes as non-Hispanic white adults. The percentage of US adults with prediabetes is similar for whites (35%), African Americans (39%), and Hispanics (38%). [22]
Whites have the highest rates of type 1 diabetes, especially those of northern European descent. Type 2 diabetes is more prevalent in ethnic minorities. The disease is unknown or rare among certain ethnic groups (eg, Japanese, Chinese, African). Type 1B diabetes is more common in patients of Asian or African origin.
Sex characteristics
In the World Health Organization’s global data, the prevalence ratio of diabetes for men and women varies markedly, with no consistent trend; however, impaired glucose tolerance is more common in women than in men. The relative difference in frequency between the sexes is probably related to the presence of underlying factors such as pregnancy and obesity, rather than to a sex-specific genetic tendency. [24]
Age characteristics
Type 1 diabetes occurs most commonly in children and adolescents but may occur in individuals of any age. Type 2 diabetes typically begins in middle life or later, usually after age 30 years; its prevalence rises with age. Maturity-onset diabetes of youth can be expressed in childhood or in early adolescence. In the United States, 208,000 people younger than 20 years have either type 1 or type 2 diabetes. [22]
Prognosis
Several studies have demonstrated a relationship between high plasma glucose distributions and the risk for cardiovascular disease and increased mortality, even within the normoglycemic range. [25, 26, 27, 28, 29, 30]
Diabetes is the sixth leading cause of death by disease worldwide and the seventh leading cause of death in the United States. Those with impaired glucose tolerance have a propensity for acute metabolic complications. IGT is a leading cause of end-stage renal disease and of blindness. Individuals with this condition also are at higher risk for neuropathy and gangrene.
Gestational diabetes
Gestational diabetes mellitus brings an increased risk for fetal and neonatal morbidity and mortality, as well as obstetric complications. There is an associated increased risk for obesity in offspring, as well as for glucose intolerance and type 2 diabetes. [29, 30, 31]
For gestational diabetes mellitus, reclassification is performed at 6-12 weeks postpartum. In most patients with gestational DM, glucose tolerance becomes normal after delivery. The lifetime risk for IGT and diabetes is increased substantially in these women, however.
Impaired glucose tolerance
IGT is a major risk factor for diabetes, with 20-50% of affected persons progressing to diabetes within 10 years. Approximately one third revert to normal glucose tolerance, while others persistently demonstrate IGT, as determined by using the oral glucose tolerance test. [32, 33, 34, 35]
Baseline plasma glucose is the most consistent predictor of progression to diabetes. Individuals who progress to diabetes tend to have rates of cardiovascular risk factors that are intermediate between persons with normal glucose tolerance and those with diabetes. They are at an increased risk of macrovascular complications (eg, coronary disease, gangrene, stroke). Progression to diabetes is not clearly associated with microvascular complications (eg, nephropathy, retinopathy, neuropathy). However, microvascular complications have been found in certain individuals with IGT. [36, 37]
Impaired fasting glucose is not associated with the same risk level as IGT, and the risk of cardiovascular disease is much lower in those with impaired fasting glucose.
Patient Education
It is important to educate patients on the disease, including treatment, monitoring, complications, and primary and secondary preventive measures. In addition, family members should be educated on various related issues, including the management of hypoglycemia.
For patient education resources, see Diabetes Center, as well as Diabetes (Type 1 and Type 2), How Is Glucose Tolerance Testing Used to Diagnose Diabetes?, Hypoglycemia (Low Blood Sugar), Diabetic Ketoacidosis, and Diabetic Eye Disease. See also the Medscape Drugs and Diseases articles Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus.
-
Glucose intolerance. Etiologic types and stages of the major disorders of glucose tolerance are displayed.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Guidelines
- Medication
- Medication Summary
- Sulfonylureas
- Meglitinide derivatives
- Biguanides
- Thiazolidinediones
- Alpha-glucosidase inhibitors
- Rapid-acting insulins
- Short-acting insulins
- Intermediate-acting insulins
- Long-acting insulins
- Premixed Insulins
- Glucagon-like Peptide-1 (glp-1) Receptor Agonists
- Amylinomimetic agents
- Dipeptidyl Peptidase-IV Inhibitors
- Sodium-glucose Cotransporter 2 Inhibitors
- Dopamine Agonists
- Antidiabetics, Glucagon-like Peptide-1 Agonists
- Show All
- Questions & Answers
- References






