Practice Essentials
Worldwide, coal remains one of the largest sources for energy, contributing to approximately one fourth of the global energy supply and over one third of the fuel that is used to produce electricity. [1] It has been projected that coal will be used to produce approximately 40% of the electricity worldwide and 34% in the United States until 2040. [2]
Coal dust particles are created during the process of coal production. Excessive exposures to coal dust can overwhelm the lungs’ mechanism to clear these particulates, causing them to accumulate over time. [3] Because of modern technological advancements, significant volumes of coal can be mined per shift. [4] Through constant exposure and inhalation of coal dust particles, coal miners are at an increased risk for developing respiratory illnesses categorized as coal mine dust lung disease (CMDLD). These pulmonary conditions can range from airflow limitation or obstruction to causing interstitial lung diseases.
When these particles are introduced into the respiratory tract, they can cause a reactive process in the lung tissue known as pneumoconiosis. [5] Coal workers’ pneumoconiosis (CWP) is also known as “black lung disease,” one of the most common conditions that belong in the category of CMDLD, along with silicosis, mixed-dust pneumoconiosis with coexistent silica exposure, chronic bronchitis, emphysema, and dust-related diffuse fibrosis. [1, 4]
CWP is divided into two categories: simple CWP (SCWP) and complicated CWP (CCWP), or progressive massive fibrosis (PMF), [3, 4] depending on the extent and severity of the disease. Also see Silicosis and Coal Worker Pneumoconiosis. Note the images below.
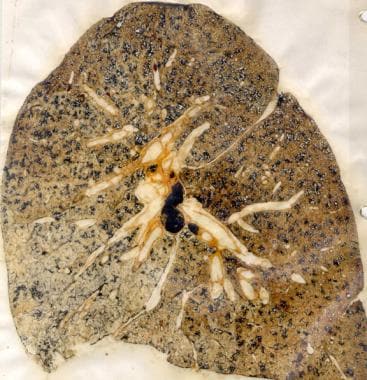 Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating simple coal worker's pneumoconiosis.
Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating simple coal worker's pneumoconiosis.
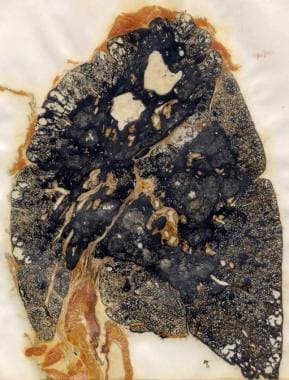 Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating progressive massive fibrosis in a coal miner.
Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating progressive massive fibrosis in a coal miner.
No cure for CWP currently exists. Treatment for both SCWP and CCWP is symptomatic, and prevention is the key to management of this condition. Lung transplantation has been used in the setting of end-stage pulmonary disease.
Pathophysiology
Anthracosis has previously been used synonymously for coal workers’ pneumoconiosis (CWP) (black lung disease) or for describing the process of detecting a substantial amount of pulmonary carbon deposits on autopsies secondary to recurrent exposure to several factors, such as air pollution, smoke inhalation, or coal dust fragments. [6, 7] Dust particles as small as five microns can enter the lungs and infiltrate the peripheral bronchioles and alveoli. The presence of these particles can obstruct the airways and lead to primary lesions composed of coal dust, macrophages, and fibroblasts. [5] Accumulation of these particulate matter in the lung tissues can be the stimulus to the development of several pathologic conditions described as harmless pulmonary anthracosis, emphysema, and/or lung fibrosis. [4]
The exact mechanism for how pneumoconiosis develops is still unclear; however, several theories have been proposed to help explain its pathogenesis, including oxidative stress theory, immunology, cytokine network theory, and single nucleotide polymorphisms. [8] Mossman described that lung tissue as a common organ affected by inhaled pollutants such as metals, mineral dust, particulates, and reactive gases. [9] Vanhee and coworkers reported three phenomena that occur after the pulmonary tissues are exposed to coal dust: (1) Inflammatory cells accumulate and activate inflammatory cells in the lower respiratory tract; (2) fibroblast proliferation; and (3) augmented formation of extracellular matrix components. [10] It has also been suggested that macrophages play a significant role in the development of pneumoconiosis. [8, 9, 10, 11, 12, 13] Zhang and colleagues indicated that the presence of dust causes the alveolar macrophages to induce an immune response that may ultimately lead to fibrosis. [8]
The oxidative stress theory is one of the possible mechanisms to help describe the pathogenesis of pneumoconiosis. This theory suggests that exposure to dust particulates in the lung tissues provokes an inflammatory response, which then leads to the formation of reactive oxygen and nitrogen species (ROS/RNS) by alveolar and interstitial macrophages, along with polymorphonuclear cells. [9] Mossman describes alveolar and interstitial macrophages as playing a role in oxidative stress through an oxidative burst response that produces ROS/RNS. However, other cells, such as epithelial cells, can also generate a similar reaction. [9] Therefore, the reactive process by macrophages and epithelial cells may be the stimulus for an immune response and functional changes that occur in surrounding cells, including fibroblasts, which can lead to the development of fibrosis. In addition, Pinho and coworkers described the production of ROS leading to other changes in the lungs, such as inactivation of antiproteases, disturbances in the basal membrane, and injury to the alveolar cells. [11]
Another possible mechanism to explain the occurrence of pneumoconiosis is the production of cytokines. Besides the production of ROS, [10, 12, 13, 14] macrophages are also thought to release mediators (eicosanoid metabolites, destructive proteolytic enzymes, and inflammatory growth and differentiation factors), [10] and cytokines have been implicated in the development of pneumoconiosis. These cytokines have been described as signaling proteins that influence inflammatory and fibrotic reactions. Several proinflammatory cytokines have been identified with the development of this condition including interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and IL-1β. [14]
An investigative study by Ulker and colleagues evaluated the serum and bronchoalveolar lavage (BAL) inflammatory cytokine levels in a total of 85 subjects with simple pneumoconiosis (SP) and progressive massive fibrosis (PMF). [12] Active and retired miners served as control subjects. The study results demonstrated that subjects with SP and PMF had higher levels of IL-1β, IL-6, and TNF-α in serum and BAL. [12]
Single nucleotide polymorphisms (SNPs) are another suggested mechanism for the pathogenesis of CWP, entailing the notion that certain individuals may be at increased risk for developing CWP because of their genetics. There have been discussions regarding the concept of allelic variations with SNPs being more frequently identified, and that there are certain functional variants that may be conducive to potentiating an increased risk for certain disease states. [15, 16] Previous studies investigating an association between gene polymorphisms involving TNFα and CWP in Belgian and Japanese coal miners demonstrated that those with CWP had a higher rate of TNFα-308 variant compared to those without the lung disease. [16] In a case control study, Wang and coworkers evaluated the association between three functional SNPs in the SELE gene (T1880C/rs5335, T1559C/rs5368, and A16089G/rs4786) with the risk for developing CWP in the Han Chinese population. [15] Their results demonstrated that there may be an association between SELE rs5368 and an increased risk for CWP. [15]
Etiology
The distinguishing feature of coal workers’ pneumoconiosis (CWP) (black lung disease) is the primary lesion, coal macule, characterized as a small (≤ 4 mm) pigmented lesion “...of dust particles, collagen, fibrin, and dust laden macrophages” [17] located around the respiratory bronchioles. [18] Reticulin fibers develop in the vicinity of the coal macule, leading to airspace dilatation and giving the appearance of focal emphysema, considered a form of centriacinar emphysema. With these focal emphysema, bullae do not project from the lung surface and are thought to be due to a mechanical disturbance within the secondary lobules of the lung. [19]
In contrast, coal nodules are lesions that contain dust larger than those found in macules ( >4 mm in size), with increased fibrosis and collagen arranged in a random arrangement. Unlike coal macules, these nodules are not restricted to the bronchioles but may be found throughout the lung parenchyma, including subpleural sites in the interlobular septa. [20] Coal nodules have a tendency to develop when there has been exposure to coal dust containing silica. [18]
Once the process of fibrosis has developed with pneumoconiosis, it is deemed irreversible even if exposure to the offending agent has ceased. The causes of these fibrotic changes remain unknown; however, one hypothesis is that persistent damage to the alveolar epithelial cells along with increased extracellular matrix are linked to the development of fibrosis. An investigative study by Zhang and coworkers on rats demonstrated that DNA methylation (defined as epigenetic modification of DNA) affecting gene expression may be a factor in pulmonary fibrosis. [8] They also observed that treatment with a DNA methylation inhibitor, 5-aza-dC, decreases the fibrotic changes.
As mentioned previously, CWP is divided into two categories: simple CWP (SCWP) and complicated CWP (CCWP), or progressive massive fibrosis (PMF). [3, 4] With SCWP, the coal macules are surrounded by fibrosis smaller than 10 mm, often located in the upper lung regions, with a latency period of at least 10 years. [1, 4, 5, 14] In addition, SCWP tends to affect older miners with fewer, less severe symptoms, such as dyspnea or cough; individuals with SCWP may not have any symptoms at all.
The early development of SCWP is the most important risk factor for the development of complicated pneumoconiosis. which is closely linked to the intensity and duration of respirable dust exposure. PMF is distinguished from CWP by the presence of scars in the lungs larger than 10 mm. Lesions with PMF are often located in the “posterior segments of the upper lobes or apical segments of the lobes” and frequently bilateral. [18] Younger miners have been observed to have increased diagnoses of PMF in relatively recent years. [3] Symptoms are usually more severe than those of SCWP such as black sputum, chronic cough, and frequent pneumonia. [5]
Several factors have been identified that raise an individual’s risk for developing CWP, such as the concentration of respirable coal dust, the size and composition of the dust particles, quartz or free silica content, as well as the duration of exposure, individual’s age, the work environment, and work practices. [21] However, the composition of respirable dust, coal rank, and duration of exposure are deemed the most significant factors. Respirable dust has been defined as “the fraction of airborne particulates that can be deposited anywhere in the lung gas-exchange regions.” [22] Coal rank is dependent on the age and carbon content of coal, and there appears to be a direct relationship between an increased risk for CWP and higher carbon content in the coal. [21]
Huang and colleagues found a correlation between bioavailable iron (BAI), pyrite concentration, and the regional progression of lung disease. [23] BAI is iron that dissolves in 10 mmol/L of phosphate solution at a pH 4.5, which mimics the interior of lysosomes. They observed an increased prevalence of CWP and PMF at Pennsylvania mines, where BAI values are higher, compared to Utah mines, where BAI levels are lower. [23] The investigators also demonstrated that pyrite-containing coal contributed to the higher prevalence of progression to CWP and PMF in Pennsylvania. In addition, McCunney and coworkers have suggested that iron, not quartz, is the active agent in coal responsible for CWP. [24]
When mixed with water, pyrite produces hydrogen peroxide [25, 26] and hydroxyl radicals. [27, 28] These reactive agents have been shown to degrade yeast RNA, ribosomal RNA, and DNA. [26] Cohn and colleagues demonstrated that these pyrite-induced reactive oxygen species can be implicated as the cause of the cellular damage and chronic inflammation that lead to chronic disease in the lungs of coal miners. [29] In order to proceed to RNA degradation, the concentration of sulfur (pyrite) in the coal necessary to produce hydrogen peroxide and hydroxyl radicals must exceed 1%. [29] These findings suggest that personnel at individual mines can measure the amount of sulfur in the coal and implement proper measures to ensure that miners in these high-risk areas either have improved protective gear or decreased long-term exposure to coal with increased BAI.
Exposure to coal mine dust is determined by the mining methods used as well as a miner’s job duties. It has been widely accepted that miners who work underground have higher exposure levels compared to surface coal miners. In addition, transportation of coal out of the mines can cause further dispersal of dust. For surface miners, considerations must take into account whether or not miners are exposed to dust in open or enclosed cabs, as well as the amount of time spent outside cutting, drilling or blasting rock. [2, 4]
Epidemiology
In 2017, the three top coal-producing countries were China, the United States, and Australia. [5] The prevalence of coal workers’ pneumoconiosis (CWP) (black lung disease) was higher in China compared to the other two countries because of China's earlier focus on occupational health. There was an average of 21,719 new cases of occupational diseases reported each year in the 14-year period between 2003 and 2016, with 44.83% of these cases being lung diseases due to coal dust in China. In the United States, there were 37,965 cases of CWP reported from 1968 to 2015; approximately 75,000 deaths of coal miners were reported to either be the underlying or contributing cause. Australia has the lowest prevalence of CWP among these three countries, with over 1000-related deaths in Australia between 1979 to 2002. [5]
In the United States, most coal is mined in eastern Pennsylvania, western Maryland, West Virginia, Virginia, and Kentucky. Disease prevalence varies in different areas of the country and from mine to mine because coal content varies by region. Following decades of decline, the prevalence of CWP among active US underground coal miners has been increasing since the late 1990s. [30, 31, 32] In addition, an increase in the progression to CWP and progressive massive fibrosis (PMF) has been seen in Kentucky and Virginia. [33, 34]
In a retrospective (2000-2009) chart review of 138 West Virginian coal miners, Wade and colleagues found an increased number of cases of rapidly progressive pneumoconiosis and PMF in young coal miners after 2001, resulting in increased morbidity and mortality. [3] These coal miners developed PMF at a mean age of 52.6 years, after an average of 30 years of exposure and after an average of 12 years from the last normal chest radiographic finding. This report asserts the need for close surveillance for this disease and a need for improvements in preventive measures. [3, 14]
The changing epidemiologic patterns of CWP in the Appalachian region of the United States has led to an increased exposure to respirable silica, as suggested by radiographic abnormalities consistent with silicosis. [3, 35]
The onset of CWP does not occur at any specific age but depends on the length and severity of exposure to coal dust and, thus,s on when the coal miner began working in the mines and the specific nature of their exposure.
Prognosis
Prognosis is poor once the patient with coal workers’ pneumoconiosis (CWP) (black lung disease) has been determined to have progressive massive fibrosis (PMF). Treatment is mainly palliative.
Mortality and morbidity are strictly related to the type of coal dust and the length of exposure. Disease severity increases as coal rank increases and with greater exposure to respirable dust. Over the past two decades, there has been a rapidly progressive form of pneumoconiosis observed in some US coal miners, which is associated with significant respiratory compromise and premature death. [20] This severe lung disease is seen in younger miners. [20]
In a 41-month retrospective study (1998-2002), Shen and coworkers described a prognostic relationship between CWP and the first episode of respiratory failure requiring mechanical ventilation. [36] The investigators found that radiographic evidence of PMF was not associated with increased intensive care unit (ICU) mortality. No mortality difference was delineated between patients with simple CWP (SCWP) and those with complicated CWP (CCWP). The following three independent variables predicted outcomes [36] :
-
Hypercapnia (partial pressure of carbon dioxide [PaCO2] >45 mmHg) at the time of intubation exhibited a protective effect, suggesting a less severe acute illness as the cause of the respiratory failure relative to normocapnic individuals.
-
An Acute Physiology and Chronic Health Evaluation II (APACHE II) score greater than 25 at the time of intubation was associated with a worse mortality.
-
A ratio of partial pressure of oxygen (PaO2) to the fraction of inspired oxygen in the air (FiO2) of less than 200 mmHg at the time of intubation was associated with an increased mortality.
There was a 40% ICU mortality for patients with CWP with their first episode of respiratory failure requiring mechanical ventilation, and a 43% in-hospital mortality. [36]
Complications
Complications of CWP include the following:
-
Airflow obstruction
-
Chronic bronchitis
-
Respiratory tract infections
-
Respiratory failure
-
Hypoxemia
-
Cor pulmonale
-
Arrhythmias
-
Pneumothorax
Complications such as airflow obstruction, respiratory tract infection, respiratory failure/ hypoxemia, cor pulmonale, arrhythmias, and pneumothorax may occur. If there has been significant crystalline silica exposure, Mycobacterial infection is a possible complication. Diffuse interstitial fibrosis is a potent accelerator of peripheral squamous cell carcinoma (SCC). [37]
First described in 1953 in Welsh miners, Caplan syndrome is a condition with combined features of rheumatoid arthritis (RA) and pneumoconiosis. There are several well-defined, rounded pulmonary nodules often located in the lung periphery of patients with RA and concurrent inorganic dust exposure. These nodules may eventually cavitate or calcify. [1] The pathogenesis and a causal relationship between pneumoconiosis, RA, and pulmonary changes in Caplan syndrome are still unclear. Questions remain regarding whether coal dust exposure or the presence of an autoimmune disease predispose the other condition to occur or develop. For the majority of patients, the nodules with Caplan syndrome are asymptomatic do not cause any significant changes to pulmonary function testing. Approximately 70% of affected patients will have a positive rheumatoid factor (RF). [38]
-
Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating simple coal worker's pneumoconiosis.
-
Coal workers' pneumoconiosis (black lung disease). Gross specimen demonstrating progressive massive fibrosis in a coal miner.





