Background
High-altitude illness may result from short-term exposures to altitudes in excess of 2000 m (6560 ft). This illness comprises a spectrum of clinical entities that are probably the manifestations of the same disease process. High-altitude pulmonary edema (HAPE) and high-altitude cerebral edema (HACE) are the most ominous of these symptoms, whereas acute mountain sickness (AMS), retinal hemorrhages, and peripheral edema are milder forms of the disease. The rate of ascent, the altitude attained, the amount of physical activity at high altitude, and individual susceptibility are contributing factors to the incidence and severity of high-altitude illness.
Despite the obvious dangers inherent in climbing and the altitude-related illness experienced by nearly all who spend significant time in the mountains, people continue to seek the remoteness and pleasures of high places. With the availability of easy transportation into the mountains, not just for climbing but also for skiing and other forms of recreation, thousands are exposed to high altitude each year. These individuals frequently experience acute illness soon after ascent.
The partial pressure of O2 (PaO2) in the atmosphere falls as blood pressure (BP) falls. Therefore, the change in BP at high altitude is the basic cause of decrease in the amount of O2 leading to hypobaric hypoxia (HH). A series of physiologic adjustments to compensate for this hypoxia, including increase in ventilation, hemodynamic and hematologic changes, and metabolic changes are usually termed acclimatization. [1]
Inadequate acclimatization may lead to altitude illness at 2438.4 m (8000 ft) or higher, and sometimes even at lower altitude. [2] Susceptibility and resistance to altitude illness are genetic traits, and no simple screening tests are available to predict risk. As noted earlier, risk is largely influenced by the altitude, rate of ascent, and exertion. Risk is not affected by training or physical fitness. Children are equally susceptible as adults; people older than 50 years have slightly lower risk. [3]
The magnitude of hypoxic stress depends on altitude, rate of ascent, and duration of exposure. Sleeping at high altitude produces the most hypoxemia; day trips to high altitude with return to low altitude are much less physiologically stressful. Adjustments to even moderate hypoxia require time. The process of acclimatization to high altitude takes 3–5 days; therefore, acclimatizing for a few days at 2438.4- 2743.2 m (8000–9000 ft) before proceeding to a higher altitude is recommended.
The Centers for Disease Control and Prevention (CDC) provides the following recommendations for travelers to high-altitudes [3, 4] :
-
Ascend gradually, if possible. Avoid going directly from low altitude to more than 2750 m (9000 ft) sleeping altitude in 1 day.
-
When above 2750 m (9000 ft) is reached, move the sleeping altitude no higher than 500 m (1600 ft) per day. Plan an extra day for acclimatization every 1000 m (3300 ft).
-
Consider taking acetazolamide to reach acclimatization more quickly, if abrupt ascent is unavoidable.
-
Avoid alcohol for the first 48 hours. If caffeine is regularly used, continue to do so.
-
Participate in only mild exercise for the first 48 hours.
-
Within 30 days before the trip, it is useful to have a high-altitude exposure at more than 2750 m (9000 ft) for at least 2 nights. However, it is better to have such exposure closer to the trip departure.
Consider prophylactic medications in addition to gradual ascent for adults and children at moderate to high risk. See the table below for a summary of medications used to prevent and treat altitude-related disorders.
Table. Major Treatment Modalities for High-Altitude Illnesses (Open Table in a new window)
Treatment |
Indication |
Dose |
Mechanisms of action and Comments |
Acetazolamide |
Prophylaxis and treatment of AMS and HACE |
Prophylaxis: 125-250 mg PO BID; 250 mg BID if >100 kg. Children: 2.5 mg/kg q12 hours
Treatment: 250 mg PO. Children Children: 2.5 mg/kg q 12 hours |
Carbonic anhydrase inhibitor: Causes bicarbonate diuresis and decreased production of CSF. Mechanism of action in AMS is unclear. |
Dexamethasone |
Prophylaxis of AMS and HAPE |
2 mg PO q6 hours or 4 mg q12 hours. Children: Do not use for prophylaxis |
Unknown |
Treatment of AMS and HACE |
AMS: 4 mg q6 hours PO, IV, or IM
HACE: 8 mg once PO, IV, or IM; then, 4 mg q6 hours. Children: 0.15 mg/kg/dose q6 hours up to 4 mg |
||
Nifedipine |
Prevention and treatment of HAPE |
Prophylaxis and treatment: 30 mg sustained-release version q12 hours PO |
Calcium channel blocker: Reduces pulmonary artery pressure. |
Sildenafil (and other phosphodiesterase 5 inhibitors) |
Prevention and treatment of HAPE |
Sildenafil: 50 mg PO q8 hours
Tadalafil: 10 mg PO BID |
Lowers pulmonary artery pressure. May worsen headache. |
Salmeterol |
Treatment and prophylaxis of HAPE |
125 mcg PO MDI q12h, used along with other prophylaxis/treatments |
Long-acting beta-agonist: Promotes ion channel mediated alveolar fluid clearance. |
Aspirin, ibuprofen, or other NSAIDs |
High-altitude headache |
|
May have some benefit in prevention/treatment of AMS. Descent advised if headache or AMS does not respond to these medications. |
Oxygen |
Treatment of AMS, HAPE, and HACE |
2-4 L/min by cannula or mask, titrated to keep SaO2 >90% |
Reduces hypoxic pulmonary vasoconstriction. |
Portable hyperbaric bag (Gamow bag) |
Treatment of HAPE and HACE |
|
Reduces effective altitude. |
Gingko biloba |
Prevention of HAPE |
100-120 mg PO BID |
Mixed results in studies; not recommended for AMS prevention |
AMS = acute mountain sickness; BID = twice daily; CSF = cerebrospinal fluid; HACE = high-altitude cerebral edema; HAPE = High-altitude pulmonary edema; IM = intramuscularly; IV = intravenously; MDI = metered dose inhaler; NSAIDS = nonsteroidal anti-inflammatory agents; PO = per os (by mouth); SaO2 = arterial oxygen saturation. |
|||
Related Medscape articles include Altitude Illness - Cerebral Syndromes and Altitude Illness - Pulmonary Syndromes.
Sleep at High Altitude
Most newcomers to altitude frequently report difficulty sleeping at night, even in the absence of other symptoms. Although some studies suggest an increase in arousals from sleep at high altitude, this is not a consistent finding. [5] Sleep disruption at altitude results from a combination of many factors, including the cold, windy environment and the often-crowded uncomfortable sleeping conditions, in addition to hypoxia. At high altitudes, reduced oxygen content of the blood induces breathing instability, with periods of deep and rapid breathing alternating with central apnea. This breathing pattern is called high-altitude periodic breathing. It occurs even in healthy persons at altitudes above 1828.8 m (6000 ft). It may lead to sleep disturbances with frequent awakenings and a feeling of lack of air. [1]
In a study of high-altitude sleep quality, healthy mountaineers ascending rapidly from 490 m (1607.6 ft) to 4559 m (14,957.4 ft), a reduction of total sleep time, sleep efficiency, and deep sleep (nonrapid eye movement [NREM] sleep stages 3 and 4) with a significant increase in arousals and pronounced periodic breathing were noted in the first night at high altitude. [6] However, sleep quality improved with acclimatization and improved oxygen saturation, whereas periodic breathing persisted. The researchers concluded that high-altitude sleep disturbances were related predominantly to hypoxemia rather than to periodic breathing. [6]
High-altitude periodic breathing may be an additional risk factor for high-altitude illness, and carbonic anhydrase inhibitors (eg, acetazolamide) decrease nocturnal periodic breathing, improve arterial oxygen saturation, and ameliorate daytime symptoms of AMS.
Several studies have evaluated the effect of hypnotic drugs on altitude-induced sleep disturbances, with conflicting results. In a randomized, double-blind study, 34 trekkers received either temazepam or acetazolamide on the first night sleeping at 3540 m (11,614.2 ft). Participants receiving temazepam reported a better subjective sleep quality and less frequent awakenings to urinate than those receiving acetazolamide. [7]
High-Altitude Retinal Hemorrhage
Retinal hemorrhages are relatively common at high altitude (HARHs), occurring in anywhere between 36% of climbers at 5334 m (17,500 ft) to as high as 79% in expedition climbers assessed at various altitudes up to and including the summit of Muztagh Ata (7545.9 m [24,757 ft]). [8, 9] HARHs may occur at altitudes of only 3352.8 m (11,000 ft), but there is a correlation showing more hemorrhages with increasing maximum altitude reached and duration at altitude.
Hypoxia, due to deficient oxygen at a high altitude, induces various compensatory mechanism in the retinal vasculature, which leads to retinal hemorrhages. The occurrence of hemorrhages is most often in the superficial retina than at a deeper level, and most go unnoticed unless these hemorrhages involve the macula. It is possible for retinal hemorrhages to result in residual scotomas or other visual changes, but most are self-limiting and without sequelae.
The precise relationship between HARHs and other altitude illnesses such as high-altitude cerebral edema (HACE) is unclear; thus, a history of HARHs should not necessarily preclude subsequent trips to high altitude. [10]
Acute Mountain Sickness and High Altitude Cerebral Edema
Case report
A 19-year-old student who lived at sea level drove to approximately 2438.4 m (8000 ft) in the Sierra Nevada Mountains to go skiing. After spending a restless night at altitude, he awoke the next morning with a severe headache. During the day, he felt tired, did not have much appetite, and vomited after attempting to eat lunch. By the next morning, however, he felt better and was able to ski with his friends.
Discussion
Acute mountain sickness (AMS) consists of nonspecific symptoms that occur at altitudes of ≥2438.4 m (8000 ft) in unacclimatized individuals with a usual delay of 4-12 hours after arrival at a new altitude. The symptoms are usually most pronounced after the first night spent at a new altitude and resolve spontaneously when appropriate measures are taken. A consensus conference held during the 1991 Hypoxia and Mountain Medicine Symposium at Lake Louise, Canada, defines AMS as the presence of headache in the setting of recent altitude gain and at least one of the following symptoms [11] :
-
Gastrointestinal disturbance (anorexia, nausea, or vomiting)
-
Fatigue or weakness
-
Dizziness or lightheadedness
-
Difficulty sleeping
The Lake Louise definition states that HACE "can be considered 'end stage' or severe AMS." High-altitude cerebral edema (HACE) is diagnosed in the setting of recent altitude gain and at least one of the following symptoms [11] :
-
Change in mental status and/or ataxia with AMS
-
Both mental status changes and ataxia without AMS
Without appropriate treatment, HACE usually leads to death, sometimes within 24 hours of onset. [12]
Many factors affect the incidence and severity of AMS, such as the rate of ascent, altitude attained (especially altitude of sleep), duration of exposure to altitude, and possibly the amount or intensity of exercise undertaken at altitude. The most important and least understood variable is the underlying physiologic susceptibility of the individual. Few people experience significant symptoms below 2133.6-2438.4 m (7000-8000 ft), whereas most unacclimatized persons ascending to 3048 m (10,000 ft) or higher experience at least a few symptoms.
The Wilderness Medical Society (WMS) classifies severity of AMS/HACE as follows [2] :
-
Mild AMS: All symptoms are of mild intensity
-
Moderate-severe AMS: All symptoms have moderate to severe intensity
-
HACE: Worsening of symptoms seen in moderate-severe AMS with ataxia, altered mental status, and encephalopathy
Prediction of AMS
A previous history of AMS suggests susceptibility to the syndrome and the likelihood of recurrence with reascent. Rapid ascent, especially with a final altitude above 3048-3962.4 m (10,000-13,000 ft) increases the likelihood of AMS; however, precise prediction of who will develop AMS is not currently possible. The Centers for Disease Control and Prevention (CDC) categorizes risk for AMS as follows [3] :
Low risk
-
No prior history of altitude illness and ascending to less than 2743.2 m (9000 ft)
-
Taking ≥2 days to arrive at 2499.4-2987 m (8200–9800 ft), with subsequent increases in sleeping elevation less than 487.7 m (1600 ft) per day, and an extra day for acclimatization every 1005.8 m (3300 ft)
Moderate risk
-
Prior history of AMS and ascending to 2499.4-2804.2 m (8200–9200 ft) or higher in 1 day
-
No history of AMS and ascending to more than 2773.7 m (9100 ft) in 1 day
-
Ascending more than 487.7 m (1600 ft) per day (increase in sleeping elevation) at altitudes above 3017.5 m (9900 ft), but with an extra day for acclimatization every 1005.8 m (3300 ft)
High Risk
-
History of AMS and ascending to more than 2804.2 m (9200 ft) in 1 day
-
Prior history of high-altitude pulmonary edema (HAPE) or HACE
-
Ascending to more than 3474.7 m (11,400 ft) in 1 day
-
Ascending more than 487.7 m (1600 ft) per day (increase in sleeping elevation) above 2987 m (9800 ft), without extra days for acclimatization
-
Very rapid ascents (such as < 7-day ascents of Mount Kilimanjaro)
Pathophysiology
Despite a great deal of study, the exact mechanism by which hypoxia causes AMS remains unknown. Hypoxia leads to increased cerebral blood flow, elevated hydrostatic capillary pressure, capillary leak, and, finally, edema. [13] Others have suggested that AMS develops in people who cannot compensate for brain swelling. [14, 15] People with a greater ratio of cerebrospinal fluid (CSF)-to-brain volume are less likely to develop AMS because the swelling brain is able to displace the CSF. Conversely, those with lesser CSF-to-brain volume ratio have limited space for compensation of brain swelling and are prone to AMS. The role of fluid retention in the pathogenesis of AMS remains uncertain. Secretion of antidiuretic hormone and atrial natriuretic factor is altered in AMS and may contribute to vasogenic edema. Hypoxia-induced alterations in oxidative stress and free radical metabolism have also been implicated in the pathophysiology of AMS. [16]
The pathophysiology of HACE shares many similarities with the pathophysiology of AMS. Despite similarities, the reason only a few persons with AMS develop HACE is unclear. Magnetic resonance imaging (MRI) in patients with HACE shows edema of the white matter, especially in the corpus callosum. [17] This MRI evidence also suggests that HACE is a vasogenic form of cerebral edema. [17]
Treatment and prevention of AMS/HACE
Slow, gradual ascent with adequate time for acclimatization provides the best protection from AMS and HACE. The ideal ascent rate varies based on individual susceptibility to AMS. Once symptoms of AMS occur, additional time for acclimatization before ascending further usually is the only treatment needed for mild AMS. If symptoms worsen despite additional time for acclimatization and taking of aspirin or other nonsteroidal anti-inflammatory (NSAIDs) medications, descent to a lower altitude (especially sleeping altitude) is needed. A descent of 304.8-914.4 m (1000-3000 ft) usually is sufficient to ameliorate symptoms. Supplemental oxygen, although rarely available in sufficient quantities, also effectively relieves symptoms of AMS. Maintenance of adequate fluid hydration is important because symptoms of dehydration may be similar to those of AMS—but excessive or “over”-hydration does not prevent AMS and should be avoided. [18]
HACE may be lethal if not recognized and promptly treated; thus, early recognition of HACE is crucial. A change in the level of consciousness or the onset of ataxia requires immediate descent. Supplemental oxygen, if available, should be administered along with dexamethasone. Diuretics, such as furosemide and mannitol, should not be administered because they may result in orthostatic hypotension from decreased intravascular volume, which makes descent difficult or impossible.
Early use of a hyperbaric bag (ie, Gamow bag) may relieve symptoms and make descent easier, but it should not be considered a substitute for descent, especially because recovery often requires 10 or more days, even with treatment at low altitude.
Pharmacologic treatment of AMS
Acetazolamide (Diamox) is effective both for the prevention and for the treatment of AMS. [19, 20, 21, 22, 23, 24, 25, 26] For AMS prevention, acetazolamide 125 mg twice daily usually is effective, whereas 250 mg twice daily is recommended for treatment of established AMS. [2] Smaller doses may be effective in some people. Starting acetazolamide 1 day before ascent and continuing for a couple of days while at altitude is recommended. Acetazolamide also decreases hypoxemia during sleep by reducing the amount of nocturnal periodic breathing. [25, 26]
Dexamethasone, 2 mg every 6 hours or 4 mg every 12 hours, is effective in preventing AMS. [27, 28, 29, 30, 31, 32] For treatment of AMS, 4 mg every 6 hours is recommended. [2]
The over-the-counter herbal supplement Ginkgo biloba has been of interest in AMS prophylaxis, primarily due to its low adverse effect profile. Although early studies were promising, later ones do not support the use of Ginkgo biloba. In a couple of studies, Ginkgo biloba was no better than placebo in prophylaxis of AMS. [20, 33] As such, the mainstay of pharmacologic treatment remains acetazolamide and dexamethasone.
Portable hyperbaric bags (eg, Gamow bag) simulate descent to a lower altitude. These bags are effective for treating AMS, although they are rarely needed unless AMS is complicated with HACE or HAPE (see High-Altitude Pulmonary Edema).
High-Altitude Pulmonary Edema
Case report
A 25-year-old student and two companions drove from sea level to nearly 2438.4 m (8000 ft) in the Sierra Nevada Mountains of California. They then hiked to 2743.2 m (9000 ft), where they spent their first night. The next day, they continued to 3352.8 m (11,000 ft), and on the third day, after considerable exertion digging a snow cave, they camped at 3779.5 m (2400 ft).
That night, the student developed a mild cough but was otherwise asymptomatic. On the morning of the fourth day, approximately 60 hours after leaving sea level, the group attempted an ice-climbing route. During the climb, the student noted considerable fatigue and shortness of breath, and he was unable to keep up with his climbing partners. By early afternoon, they abandoned the climb and began their descent. The student was, by then, extremely fatigued and reported a slight headache. His cough increased, and shortly thereafter, he began coughing up thin straw-colored fluid. He continued the descent unaided but with some difficulty. Finally, after approximately 12 hours of descent, the party arrived at their car. After driving to 1219.2 m (4000 ft), the student felt markedly improved but exhausted.
A chest radiograph shown below, obtained approximately 18 hours after his descent, revealed marked patchy opacities, particularly on the right side. A follow-up chest film taken 3 days later showed considerable improvement.
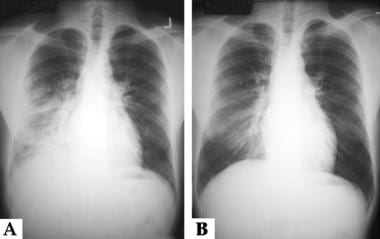 Altitude-Related Disorders. These are the chest radiographs of a young male patient who developed high-altitude pulmonary edema while climbing in the Sierra Nevada Mountains in California. Panel A is a radiograph taken a few hours after returning to near sea level; it shows patchy opacities predominantly involving the right chest. Panel B is a radiograph taken after 3 days of recovery; it shows minimal residual changes in the right lung.
Altitude-Related Disorders. These are the chest radiographs of a young male patient who developed high-altitude pulmonary edema while climbing in the Sierra Nevada Mountains in California. Panel A is a radiograph taken a few hours after returning to near sea level; it shows patchy opacities predominantly involving the right chest. Panel B is a radiograph taken after 3 days of recovery; it shows minimal residual changes in the right lung.
Signs and symptoms
High-altitude pulmonary edema (HAPE) generally occurs 1-4 days after rapid ascent to altitudes in excess of 2438.4 m (8000 ft). Young people and previously acclimatized people reascending to a high altitude following a short stay at low altitude appear to be more predisposed to HAPE. Cold weather and physical exertion at high altitude are other predisposing factors.
Signs and symptoms of HAPE include the following:
-
Decreased exercise tolerance and slow recovery from exercise
-
Extreme fatigue/weakness
-
Dyspnea on exertion
-
Tachycardia and tachypnea at rest
-
Nonproductive cough, frothy sputum
-
Rales
-
All symptoms are worse at night
HAPE may be fatal within a few hours if left untreated. In general, people who recover from HAPE have rapid clearing of edema fluid and do not develop long-term complications.
Diagnosis
The diagnostic criteria for HAPE are at least two symptoms and two signs in the setting of a recent gain in altitude.
Symptoms include the following:
-
Dyspnea at rest
-
Cough
-
Weakness or decreased exercise performance
-
Chest tightness or congestion
Signs include the following:
-
Rales or wheezing in at least one lung field
-
Central cyanosis
-
Tachypnea
-
Tachycardia
A chest radiograph, if facilities are available, and a measurement of arterial oxygen saturation may contribute to making the diagnosis and excluding other disorders. Marked hypoxemia is an important and common finding in HAPE.
Radiographic features
Chest radiographs are useful to confirm the diagnosis of HAPE and may show abnormalities, even 24-48 hours after descent to sea level. With HAPE, homogeneous or patchy opacities appear in the mid lung areas and involve one or both sides of the chest. Opacities are more likely to be present in the right lung than in the left lung. Unilateral involvement of only the left lung is rare and should raise the suspicion of a congenital absence or hypoplasia of the right pulmonary artery. The pulmonary arteries are frequently enlarged; however, the cardiac silhouette is usually normal. Kerley lines may or may not be present.
Pathophysiology
The exact pathophysiology of HAPE is hampered by the lack of a good animal model. Any model must account for several factors, as follows: (1) elevated pulmonary artery pressures with wedge and left atrial pressures within the reference range, (2) no evidence of left ventricular failure, (3) capillary and arterial thromboses (in many fatal cases of HAPE), and (4) intense exercise (which makes HAPE more likely, whereas bedrest is beneficial). A summary of the pathogenesis of HAPE is shown below.
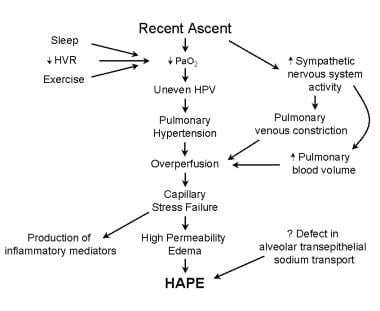 Altitude-Related Disorders. This image shows the pathophysiology of high-altitude pulmonary edema (HAPE) following ascent to high altitude. Factors leading to a low partial pressure of oxygen (PO2), such as exercise, sleep, or a low ventilatory response to hypoxia, increase the likelihood of developing HAPE. Alterations in the sympathetic nervous system are also believed to contribute to the development of HAPE. Evidence exists to suggest that a defect in sodium transport across the alveolar epithelium may be important in susceptible individuals. HPV = hypoxic pulmonary vasoconstriction; HVR = hypoxic ventilatory response; and PaO2 = partial arterial pressure of oxygen.
Altitude-Related Disorders. This image shows the pathophysiology of high-altitude pulmonary edema (HAPE) following ascent to high altitude. Factors leading to a low partial pressure of oxygen (PO2), such as exercise, sleep, or a low ventilatory response to hypoxia, increase the likelihood of developing HAPE. Alterations in the sympathetic nervous system are also believed to contribute to the development of HAPE. Evidence exists to suggest that a defect in sodium transport across the alveolar epithelium may be important in susceptible individuals. HPV = hypoxic pulmonary vasoconstriction; HVR = hypoxic ventilatory response; and PaO2 = partial arterial pressure of oxygen.
Alveolar hypoxia leads to hypoxic pulmonary vasoconstriction following ascent to high altitude. The extent of vasoconstriction is highly variable among individuals, probably due to different genetic characteristics. Individuals susceptible to HAPE have more severe pulmonary arterial hypertension than normal at altitude; however, not everyone with exaggerated hypoxic pulmonary vasoconstriction develops HAPE.
Decades ago, Hultgren proposed the overperfusion concept for the development of HAPE, which postulates that uneven hypoxic pulmonary vasoconstriction results in lung areas with decreased blood flow while other areas receive excessive flow. [34, 35] Leakage of edema fluid occurs in these overperfused lung regions. Magnetic resonance imaging (MRI) studies confirm the increased blood flow heterogeneity in individuals susceptible to HAPE. [36]
Bronchoalveolar lavage studies show that the edema fluid in HAPE has a high protein concentration, along with various inflammatory markers, such as complement C5a and leukotriene B4. These inflammatory markers are felt to be an epiphenomenon rather than a direct cause of the pulmonary capillary leakage in HAPE. [37] More recently, investigators have reported upregulation of novel inflammation-related genes (eg, high-mobility group box 1 [HMGB1], Toll-like receptor 4 [TLR4], LY96, FASLG, SMAD7), as well as enriching nuclear factor-κB and TLR signaling pathways, in the first 3 days of high-altitude exposure, which may play a role in immune system sensitization. [38] These findings have the potential to contribute to understanding how hypoxia-induced inflammation may be factors in high-altitude illnesses and exacerbate inflammatory responses in hypoxemia-related critical conditions. [38]
The nonhomogeneous vasoconstriction allows high pulmonary artery pressures to be transmitted to pulmonary capillaries in overperfused areas of the lung. These overperfused pulmonary capillaries are subjected to high wall stresses from the high capillary pressure and may rupture in a process known as capillary stress-failure. [39, 40, 41]
Clearance of fluid from the alveoli and interstitial space is important in the prevention and resolution of pulmonary edema. [42] The epithelial sodium channel (ENaC) appears to be the most important regulator of this process. Both beta agonists and steroids upregulate the ENaC ion channels within the alveolar epithelial cells, a concept used to show that inhaled salmeterol is useful in preventing HAPE. [43] Ruh et al described a 60% reduction in the mRNA for the epithelial sodium channel in humans following acute exposure to high altitude. [44]
Treatment and prevention of HAPE
The treatment of HAPE includes rest, administration of oxygen, and descent to a lower altitude. If diagnosed early, recovery is rapid with a descent of only 500-1000 m (1640.4-3280.8 ft). Immediate use of a portable hyperbaric chamber or administration of supplemental oxygen increases oxygen saturation and reduces pulmonary artery pressure, heart rate, respiratory rate, and symptoms. In situations where descent is difficult, these treatments can be lifesaving. [2, 3]
Portable hyperbaric chambers (Gamow, CERTEC) are in wide use by trekkers. A physiologic (simulated) descent of approximately 2000 m (6561.7 ft) may be achieved in a few minutes. Patients are typically treated in 1-hour increments; these individuals should be closely observed for rebound symptoms after hyperbaric treatments.
Prophylaxis is indicated for persons who have been identified (from past experience) as being susceptible to developing high-altitude illness or who must ascend rapidly to a high altitude. Because acetazolamide hastens acclimatization, it should be effective at preventing all forms of acute altitude illness. This drug has been shown to blunt hypoxic pulmonary vasoconstriction, but there are no data specifically supporting a role in HAPE prevention. Clinical observations suggest acetazolamide may prevent reentry HAPE, a disorder seen in individuals who reside at high altitude, travel to lower elevation, and then develop HAPE upon rapid return to their homes. [2]
Based on a single randomized, placebo-controlled study and extensive clinical experience, the Wilderness Medical Society recommends nifedipine for HAPE prevention in high risk individuals. [2]
Other preventive measures include the following:
-
Eating a high-carbohydrate diet
-
Avoiding heavy exertion at high altitude
-
Slow ascent
-
Avoiding abrupt ascent to sleeping elevations higher than 3000 m (9842.5 ft): If possible, spend two nights at altitudes of 2500-3000 m (8202.1-9842.5 ft) before further ascent
-
Avoiding alcohol and sedatives
Special Populations at High Altitude
Before undertaking high-altitude travel, persons with medical conditions, such as heart failure, myocardial ischemia (angina), sickle cell disease, any form of pulmonary insufficiency or preexisting hypoxemia, or obstructive sleep apnea (OSA) should consult a physician familiar with high-altitude medical issues. [3, 45]
Coronary artery disease
Unacclimatized persons with coronary artery disease may develop increased anginal symptoms following ascent to altitude because of an increase in cardiac work, as well as possible vasoconstriction of the coronary arteries. Cardiac arrhythmia, including atrial fibrillation or flutter, may worsen after rapid ascent to altitude, even without underlying coronary artery disease. During exercise testing at 3093.7 m (10,150 ft), cardiac patients developed angina or ST-segment depression at the same double product (ie, heart rate times systolic blood pressure) as they did at 1609.3 m (5280 ft). Thus, ascent to altitudes of about 3048 m (10,000 ft) has little direct effect on myocardial ischemia but may produce symptoms by increasing heart rate and blood pressure during submaximal exercise.
Despite the increase in cardiac symptoms following rapid ascent to high altitude, the increased risk for cardiac death is low. In a large survey of trekkers in Nepal, no deaths from cardiac disease were reported, although several individuals required evacuation for cardiac problems. Other studies conducted at moderate altitudes in the Colorado Rocky Mountains among unacclimatized elderly individuals suggest a relatively low risk. In a review of the effects of altitude on patients with cardiovascular disease, Hultgren suggested an approach for the evaluation of a patient with heart disease prior to trekking at high altitude (including when to perform a pre-ascent exercise test). [46]
With sufficient time for acclimatization, patients with coronary heart disease are likely to experience decreased symptoms because of a lower blood pressure. With long-term exposure to altitude, coronary artery disease mortality in these individuals actually are lower than that observed at sea level.
Pulmonary disease
Low-altitude predictors (symptoms, functional class, exercise capacity, exertional oxygen desaturation) for adverse health effects at high altitude are clinical risk factors for pulmonary vascular disease. [45] Clinicians should educate patients about the challenges of predicting altitude-related adverse health effects and instruct them that in the event of worsening symptoms to immediately descend to lower altitude (accompanied by others) and receive oxygen therapy.
Chronic obstructive pulmonary disease
Shortness of breath occurs in everyone, including those without heart or lung disease, after ascent to altitude. Even at sea level, patients with chronic obstructive pulmonary disease (COPD) are frequently limited by impaired lung mechanics and dyspnea.
Because of the increased ventilatory requirements of exercise at altitude, patients with COPD may experience a worsening of their symptoms during exposure to altitude. Patients with COPD without evidence of cor pulmonale exposed to 1920.2 m (6300 ft) altitude developed few altitude-related symptoms except fatigue (and headache in one individual), despite a decrease in resting arterial partial pressure of oxygen (PO2) from 66 to 52 mm Hg. [47] In these patients, the authors attributed the lack of symptoms of acute mountain sickness (AMS) to partial acclimatization resulting from hypoxemia. They concluded that patients with mild or moderate COPD without cor pulmonale tolerate altitude exposure quite well. [47]
Patients with COPD living at altitude, as opposed to sojourners, develop cor pulmonale and have an increased mortality when compared to similar patients living at low altitude. Although the cause for this increased mortality is unknown, it probably is related to the higher pulmonary artery pressure observed in these residents.
Pulmonary hypertension
Hypoxic pulmonary vasoconstriction raises pulmonary artery pressure in sojourners to high altitude. With idiopathic pulmonary hypertension, ascent to altitude results in even higher pulmonary artery pressures. These patients are likely to experience additional symptoms, such as fatigue, dyspnea, or even syncope. An increase in supplemental oxygen or the use of pulmonary vasodilators may be helpful to ameliorate altitude symptoms. Prior to traveling to high altitude, persons with idiopathic pulmonary hypertension should consult a physician familiar with altitude problems who can evaluate the potential risks. Individuals chronically living at high altitude who develop significant pulmonary hypertension should be encouraged to consider moving to lower altitude.
Asthma
Asthma is a common disorder affecting many young, active individuals; therefore, a significant number of altitude sojourners have asthma or reactive airways. The dry, cold air often encountered at high altitude may cause bronchoconstriction; however, this climate also contains fewer allergens. As a result, many people with asthma report doing as well or even better at high altitude than at lower elevations. The reduced barometric pressure results in decreased air density. Thus, even though the ventilatory demands of activity at high altitude are greater, the reduced air density at least partially compensates. Patients with asthma who want to travel to high altitude should be encouraged to do so, but they should bring an adequate supply of their medications and pay attention to their respiratory symptoms.
Diabetes
Individuals with diabetes can safely and successfully participate in high-altitude climbing, although significant challenges must be overcome. Although individuals with diabetes adapt to the hypoxia of high altitude, elevated counter-regulatory hormones can impair glycemic control, particularly if mountain sickness occurs. In addition, high-altitude–induced anorexia and increased energy expenditure can predispose individuals to dysglycemia unless careful adjustments in medication are performed. Frequent blood glucose monitoring is imperative, but not all glucose meters read accurately at high altitudes—thus, results must be interpreted with caution. [48]
The Centers for Disease Control and Prevention (CDC) advises that individuals with diabetes should be accustomed to exercise and carefully monitor their blood glucose when traveling to high altitudes. Diabetic ketoacidosis may be triggered by altitude illness and may be more difficult to treat if acetazolamide has been taken. [3]
Sickle cell disease
Travel to high altitudes is contraindicated in individuals with sickle cell anemia, because exposure to the hypoxia at high altitude may precipitate a sickle cell crisis among these patients. Although rare, the modest hypoxemia associated with airline travel has been reported to trigger splenic syndrome in susceptible individuals. [49]
WMS and CDC Guidelines
The Wilderness Medical Society (WMS) [2, 50] and Centers for Disease Control and Prevention (CDC) [3, 4] have available guidelines for the prevention and treatment of acute altitude illness.
The coronavirus disease 2019 (COVID-19) pandemic has raised concerns over whether affected patients with respiratory distress have presentations more like high-altitude pulmonary edema (HAPE) than that of acute respiratory distress syndrome (ARDS). [51, 52] Therefore, this Guidelines section also contains the following COVID-19-related guidance:
Prevention
A gradual ascent is the primary recommendation for the prevention of AMS, HACE and HAPE. In planning the rate of ascent, the sleeping altitude is considered more important than the altitude reached during waking hours. Above an altitude of 3000 m (9842 ft), individuals should not increase the sleeping elevation by more than 500 m per day (1640 ft/day) and should include a rest day every 3-4 days. In the event that logistical factors prevent strict adherence to 500 m per day (1640 ft/day) sleeping elevation, additional acclimatization days should be included in the itinerary before or after larger gains and elsewhere as needed to ensure that the overall ascent rate averaged over the entire trip falls below the 500 m/day (1640 ft/day) threshold. [2, 50]
Additional recommendations for the prevention of AMS/HACE include the following [2, 50] :
-
Prophylactic medications should be considered in addition to gradual ascent for moderate-to-high risk adults and children.
-
Acetazolamide is the preferred agent for both adults and children
-
Dexamethasone may be used as an alternative in adults with prior history of intolerance of or allergic reaction to acetazolamide, but is contraindicated in children.
-
Dexamethasone in very high doses may be considered in very high-risk situations (ie, search and rescue personnel being airlifted to altitudes greater than 3500 m (11,500 ft) with immediate performance of physical activity) but should not be used outside these limited circumstances.
Additional recommendations for the prevention of HAPE include the following [2, 50] :
-
Drug prophylaxis should only be considered for individuals with a prior history of HAPE, especially multiple episodes.
-
Nifedipine is the preferred agent; initiate the day before ascent and continue nifedipine either until descent begins or the individual has spent 4 days at the highest elevation, perhaps up to 7 days if the rate of ascent was faster than recommended. (Note: These durations are longer than use of acetazolamide for prevention of acute mountain sickness.)
-
Stop prophylactic medications when beginning descent for individuals who ascend to a high point and then descend toward the trailhead.
-
Further research is needed before tadalafil or dexamethasone can be recommended over nifedipine for prophylaxis.
-
In general, acetazolamide facilitates acclimatization, but this agent should not be relied on as the sole preventive agent in individuals with known HAPE susceptibility.
The CDC strongly recommends acetazolamide prophylaxis in all individuals with a prior history of HAPE or HACE, as well as with the following [3, 4] :
-
History of acute mountain sickness and ascending more than 2800 m (9200 ft) in 1 day
-
All people ascending to more than 3500 m (11,500 ft) in 1 day
-
All people ascending more than 500 m (1640 ft) per day (increase in sleeping elevation) above 3000 m (9850 ft), without extra days for acclimatization
-
Very rapid ascents
The CDC recommends the following pharmacologic agents and regimens for HAPE prophylaxis [3, 4] :
-
Oral nifedipine (generally reserved for HAPE-susceptible individuals): 30 mg sustained-release formulation every 12 hours (same regimen for HAPE treatment)
-
Oral tadalafil: 10 mg twice daily
-
Oral sildenafil: 50 mg every 8 hours
In addition the CDC suggests travelers are educated with three rules for prevention of death or serious consequences from altitude illness, as follows [3, 4] :
-
Know the early symptoms of altitude illness, and be willing to acknowledge when they are present.
-
Never ascend to sleep at a higher elevation when experiencing symptoms of altitude illness, no matter how minor they seem.
-
Descend if the symptoms become worse while resting at the same elevation.
For travel to remote high-altitude areas, where descent to a lower altitude could be problematic, a pressurization bag (such as the Gamow bag) can be beneficial. A foot pump produces an increased pressure of 2 lb/in2, mimicking a descent of 1500-1800 m (5000-6000 ft) depending on the starting elevation. [3, 4]
AMS/HACE treatment
According to the WMS guidelines, disorders whose symptoms and signs may resemble those seen in AMS and HACE, such as dehydration, exhaustion, hypoglycemia, hypothermia, or hyponatremia must be excluded prior to initiating treatment. Ascension should stop if symptoms of altitude illness of any severity are present. Descent should be considered based on clinical circumstances and severity of illness. [2, 50]
Additional key recommendations for treatment of AMS include the following [2, 50] :
-
AMS can be treated at the current altitude with use nonopiate analgesics for headache and antiemetics for gastrointestinal symptom relief. that may be all that is required.
-
For mild illness, acetazolamide facilitates acclimatization through increased ventilation and diuresis.
-
For moderate-to-severe disease, dexamethasone;
-
Decent should be initiated if symptoms are not responding to treatment with acetazolamide or dexamethasone.
-
Individuals with AMS may resume their ascent once symptoms resolve, but further ascent or reascent to a previously attained altitude should never be undertaken in the face of ongoing symptoms.
-
After resolution of AMS, reascent with acetazolamide
HACE is differentiated from severe AMS by neurological signs including ataxia, confusion, or altered mental status and may follow AMS or occur concurrently with HAPE.
If HACE is suspected, treatment recommendations include the following [2, 50] :
-
In populated areas with access to hospitals or specialized clinics, supplemental oxygen and dexamethasone
-
In remote areas away from medical resources, dexamethasone should be given and descent initiated
-
If descent is not feasible, supplemental oxygen or a portable hyperbaric chamber should be considered in addition to dexamethasone
-
No further ascent should be attempted until patient is asymptomatic and no longer taking dexamethasone
HAPE treatment
The WMS guidelines caution that before treatment is initiated, other causes of high-altitude respiratory distress should be considered including pneumonia, viral upper respiratory tract infection, mucus plugging, bronchospasm, or myocardial infarction. Once HAPE is diagnosed, descent is the treatment priority.
If logistics prohibit descent, supplemental oxygen or a portable hyperbaric chamber should be used. [2, 50] When available, use supplemental oxygen sufficient to achieve an SpO2 above 90% or to relieve symptoms while waiting to initiate descent when descent is infeasible and during descent in severely ill patients.
Nifedipine should be used for HAPE treatment when descent is impossible or delayed and reliable access to supplemental oxygen or portable hyperbaric therapy is unavailable. [2, 50] Tadalafil or sildenafil can be used for HAPE treatmenct when descent is impossible or delayed, access to supplemental oxygen or portable hyperbaric therapy is impossible, and nifedipine is unavailable. ontinuous positive airway pressure (CPAP) or expiratory positive airway pressure (EPAP) may be considered for treatment of HAPE when supplemental oxygen or pulmonary vasodilators are not available or as adjunctive therapy in patients not responding to supplemental oxygen alone.
Diuretics or acetazolamide should not be used for treatment of HAPE. [2, 50] Diuretics or acetazolamide should not be used for treatment of HAPE.
Additional treatment recommendations for HAPE include the following [2, 50] :
-
Patients with access to oxygen in a hospital or high altitude medical clinic can be treated with oxygen at the current elevation.
-
In the field setting, nifedipine can be used as an adjunct to descent, oxygen, or portable hyperbaric therapy; only use as primary therapy if other measures are unavailable.
-
A phosphodiesterase inhibitor may be used if nifedipine is not available, but concurrent use of multiple pulmonary vasodilators is not recommended.
-
In the hospital setting, CPAP can be considered as an adjunct to supplemental oxygen, and nifedipine can be added if patients fail to respond to oxygen therapy alone.
-
There is no established role for acetazolamide, beta-agonists, or diuretics in the treatment of HAPE.
After treatment for HAPE, patients may further ascend or reascend under the following conditions:
-
Symptoms of their disease have resolved
-
Stable oxygenation is maintained at rest and with mild exercise while off supplemental oxygen or vasodilator therapy
-
Consideration may be given to using nifedipine or another pulmonary vasodilator on resuming ascent
Consideration may be given to using nifedipine or another pulmonary vasodilator on resuming ascent.
Concurrent HAPE and HACE treatment
Some patients with HAPE may have neurologic dysfunction caused by hypoxic encephalopathy rather than true HACE, but differentiating between the diagnoses in the field can be difficult. The WMS guidelines recommend adding dexamethasone to the treatment regimen for patients with HAPE and neurologic dysfunction that does not resolve rapidly with administration of supplemental oxygen and improvement in the patient’s oxygen saturation. [2, 50] If supplemental oxygen is not available, initiate dexamethasone in addition to medications for HAPE in those with mental status changes and/or suspected concurrent HACE.
Other key recommendations include the following [2, 50] :
-
Dexamethasone should be administered at recommended doses for the treat HACE
-
Nifedipine or other pulmonary vasodilators may be used to treat HAPE, but avoid lowering mean arterial pressure, as this may decrease cerebral perfusion pressure and thereby increase the risk for cerebral ischemia.
FDA Policy for Face Masks, Face Shields, and Respirators in COVID-19 (2021)
The guidelines on policy for face masks and respirators during the coronavirus disease 2019 (COVID-19) public health emergency were released on March 25, 2020, by the US Food and Drug Administration (FDA) and revised in April 2020 and September 2021. [53]
Face Masks, Face Shields, and N95 Respirators Not Intended for a Medical Purpose
Face masks, face shields, and respirators are devices when they are intended for a medical purpose, such as prevention of infectious disease transmission (including uses related to COVID-19). Face masks, face shields, and respirators are not devices when they are intended for a nonmedical purpose, such as for use in construction.
When considering whether face masks, face shields, and respirators are intended for a medical purpose, among other considerations, FDA will assess the following:
-
Whether they are labeled or otherwise intended for use by a healthcare professional
-
Whether they are labeled or otherwise for use in a healthcare facility or environment
-
Whether they include any drugs, biologics, or antimicrobial/antiviral agents
Face Masks and Barrier Face Coverings Intended for a Medical Purpose That Are Not Intended to Provide Liquid Barrier Protection
In general, FDA recommends that healthcare providers follow current Centers for Disease Control and Prevention (CDC) guidance regarding personal protective equipment (PPE) that should be used during the COVID-19 outbreak.
For the duration of the public health emergency, FDA does not intend to object to the distribution and use of face masks and barrier face coverings, with or without a face shield (not including respirators), that are intended for a medical purpose (whether used by medical personnel or by the general public), without compliance with prior submission of a premarket notification where the face mask does not create an undue risk in light of the public health emergency.
FDA currently believes that such devices would not create an undue risk in the following cases:
-
The product's labeling accurately describes the product as a face mask (as opposed to a barrier face covering, surgical mask, or filtering facepiece respirator [FFR]) and includes a list of the body-contacting materials.
-
For FFRs not approved by the National Institutes of Occupational Safety and Health (NIOSH) to be used as face masks, the non-NIOSH-approved FFRs should be segregated from NIOSH-approved FFRs, and they must be clearly identified as a face mask for use as source control only.
-
The product does not include any drugs, biologics, or nanoparticles.
-
The product's labeling makes recommendations that would sufficiently reduce the risk of use—for example, recommendations against use in any surgical setting or a setting where significant exposure to liquid, bodily fluids, or other hazardous fluids, may be expected; use in a clinical setting with a high risk of infection through inhalation exposure; and use in the presence of a high-intensity heat source or flammable gas.
-
The product is not intended for any use that would create an undue risk in light of the public health emergency—for example, the labeling does not include uses for antimicrobial/antiviral protection or related uses or uses for infection prevention/reduction or related uses, and does not include particulate filtration claims in the labeling.
Face Shields Intended for a Medical Purpose
In general, FDA recommends that healthcare providers follow current CDC guidance regarding PPE that should be used during the COVID-19 outbreak.
For the duration of the public health emergency, FDA does not intend to object to the distribution and use of face shields that are intended for a medical purpose (whether used by medical personnel or the general public), without compliance with the following regulatory requirements where the face shield does not create an undue risk in light of the public health emergency: Registration and Listing requirements in 21 CFR Part 807, Quality System Regulation requirements in 21 CFR Part 820, reports or corrections and removals in 21 CFR Part 806, and Unique Device Identification requirements in 21 CFR Part 830 and 21 CFR 801.20.
FDA currently believes that such devices would not create an undue risk in the following cases:
-
The product's labeling accurately describes the product as a face shield and includes a list of the body-contacting materials (which does not include any drugs or biologics).
-
The face shield does not contain any materials that will cause flammability, or the product meets class I or class II flammability requirement per 16 CFR Part 1610 (unless labeled with a recommendation against use in the presence of a high-intensity heat source or flammable gas).
-
The product is not intended for any use that would create an undue risk in light of the public health emergency—for example, the labeling does not include uses for antimicrobial/antiviral protection or related uses or uses for infection prevention/reduction or related uses, or for radiation protection.
Surgical Masks Intended to Provide Liquid Barrier Protection
Surgical masks are class II devices that cover the user’s nose and mouth and provide a physical barrier to fluids and particulate materials and are tested for flammability and biocompatibility.
For the duration of the declared public health emergency, FDA does not intend to object to the distribution and use of surgical masks without prior submission of a premarket notification in instances where the surgical masks do not create an undue risk in light of the public health emergency.
FDA currently believes that such devices would not create an undue risk in the following cases:
-
The product meets fluid resistance testing (liquid barrier performance) requirements in a manner consistent with standard methods.
-
The product meets standard class I or class II flammability requirements (unless labeled with a recommendation against use in the presence of high-intensity heat sources or flammable gas).
-
The product's labeling accurately describes the product as a surgical mask and includes a list of the body-contacting materials (which does not include any drugs or biologics).
-
The product is not intended for any use that would create an undue risk in light of the public health emergency—for example, the labeling does not include uses for antimicrobial/antiviral protection or related uses or uses for infection prevention/reduction or related uses and does not include particulate filtration claims.
Emergency Use Authorizations (EUAs) for Face Masks Intended for a Medical Purpose, Surgical Masks, Face Shields, and and Respirators
Wherever possible, healthcare facilities should continue to use FDA-cleared surgical masks and NIOSH-approved air-purifying respirators and/or NIOSH-approved and FDA-cleared respirators. In response to the COVID-19 pandemic, FDA issued an EUA that authorizes NIOSH-approved airpurifying respirators for use in healthcare settings by HCPs. In addition, EUAs have also been issued for face masks for use by the general public and HCPs as source control, and surgical masks, and face shields for use by HCPs in healthcare settings. These EUAs have helped increase availability of these devices to HCPs and the general public, as applicable, during the public health emergency.
For any face mask or FFR (including N95 respirators) issued an EUA, FDA will include appropriate conditions of authorization in accordance with section 564 of the FD&C Act on a case-by-case basis. The following conditions will likely be included:
-
Appropriate conditions designed to ensure that healthcare professionals administering the device, and individuals being administered the device, are informed of FDA EUA of the device; and of the significant known potential benefits/risks of the emergency use of the device, and of the extent to which such benefit/risks are unknown.
-
Appropriate conditions designed to ensure healthcare professionals administering the device are informed of the available alternatives to the device, and of their benefits/risks
-
Appropriate conditions designed to ensure individuals being administered the device are informed of the option to accept/refuse administration of the device, of the consequence, if any, of refusing administration of the device, and of the available alternatives to the device and of their benefits/risks
-
Appropriate conditions for the monitoring and reporting of adverse events associated with the emergency use of the device
-
For device manufacturers, appropriate conditions concerning recordkeeping and reporting, including records access by FDA, with respect to emergency use of the device
Alternatives When FDA-Cleared or NIOSH-Approved N95 Respirators Are Not Available
See the CDC-published Strategies for optimizing the Supply of N95 Respirators: Crisis/Alternate Strategies, which, as part of a set of crisis management recommendations, identifies alternatives to FDA-cleared or NIOSH-approved N95 respirators approved under standards used in other countries, some of which were evaluated under methods that are similar to NIOSH-approved N95 respirators.
As of September 2021, current CDC and FDA recommendations are that healthcare facilities should not be using crisis capacity strategies at the time of issuance of this guidance. During the public health emergency, FDA generally does not intend to object to stockpiled, non-NIOSH-approved disposable FFRs being further distributed and used as face masks for source control (as opposed to use as FFRs for respiratory protection) by the general public and HCP where such use does not create an undue risk in light of the public health emergency.
COVID-19–Related Airway Management Clinical Practice Guidelines (SIAARTI/EAMS, 2020)
In March 2020, the Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI) Airway Research Group and the European Airway Management Society released coronavirus disease 2019 (COVID-19) recommendations that included guidance on airway management and tracheal intubation. [54]
Perform airway management procedures electively rather than as an emergency, employing any means required to maximize first-pass success.
Carry out procedures in a negative pressure chamber (if available) or an isolation area that is equipped with a replenished, complete, and checked emergency airway trolley.
Strict monitoring of entry and departure of staff from the immediate clinical area is necessary, with restriction of personnel to whoever is required.
Through thorough airway evaluation, clinicians should determine whether it is safe to employ asleep tracheal intubation, rather than awake tracheal intubation (ATI).
The use of ATI requires careful consideration owing to the fact that it is potentially a highly aerosol-generating procedure.
Tracheal Intubation
Effective pre-oxygenation is mandatory in patients with COVID-19 owing to their risk of rapid arterial oxygen desaturation.
Following preemptive optimization and correction of hemodynamic disturbances, perform pre-oxygenation with a fraction of inspired oxygen of 1.0 for at least 3 minutes at tidal volume breathing or eight vital capacity breaths.
Rapid sequence intubation, indicated for all cases to minimize the apnea time, can result in significant aerosolization with facemask ventilation. Therefore, facemask ventilation should only be performed gently should critical arterial oxygen desaturation occur.
Unless otherwise indicated, cricoid force should not be performed, so that first-pass success can be maximized and optimal ventilation (if needed) is not compromised.
Apneic oxygenation is recommended to prevent desaturation, with low-flow nasal oxygenation ideally used during tracheal intubation attempts.
Because it is an aerosol-generating technique, high-flow nasal oxygen should be avoided.
It is recommended that general anesthetic agents be administered, cautiously, to minimize hemodynamic instability, and that rocuronium 1.2 mg/kg or suxamethonium 1 mg/kg be provided to ensure rapid onset of neuromuscular blockade, maximize first-pass success, and prevent coughing and associated aerosolization.
It is advisable to perform neuromuscular monitoring.
Employment of a videolaryngoscope, ideally disposable but with a separate screen to minimize patient contact, is strongly recommended.
Should tracheal intubation fail, gentle manual ventilation may be used, with a maximum of two attempts at tracheal intubation subsequently employed (with consideration of position change, device, and technique between attempts).
Should tracheal intubation fail twice, or if a rescue airway is needed, it is strongly advised that a second-generation supraglottic device, preferably one that permits flexible bronchoscopic intubation, be used.
Consider an early emergency front-of-neck airway (surgical or percutaneous cricothyroidotomy) before a “cannot intubate, cannot oxygenate” scenario independently of critical arterial oxygen desaturation.
An experienced operator should perform an indicated ATI; employment of intravenous sedation may minimize coughing.
Minimize aerosol or vaporized local anesthesia delivery, and consider using mucosal atomizers, swabs, and tampons, as well as (if clinical expertise permits) nerve blocks.
To reduce the risk of cross-contamination, employ single-use flexible bronchoscopes; a separate screen is strongly advised.
Because it is faster than flexible bronchoscopy, ATI with videolaryngoscopy can be considered.
Despite the potential for aerosolization, tracheostomy with local anaesthesia must be considered in the event of a failed ATI.
In the event of a “cannot intubate, cannot oxygenate” scenario, carry out an emergency front-of-neck airway.
If emergency tracheal intubation is required for a COVID-19 patient, personal protective equipment (PPE) must be donned by team members prior to airway management. Gentle facemask ventilation may be required in a hypoxic patient to give more time to the patient and clinicians.
Place high-efficiency particulate air filters between the primary airway device and the breathing circuit, including the expiratory limb of the circuit once the patient is connected to the ventilator.
Unnecessary respiratory circuit disconnections should be avoided, in order to prevent viral dispersion. If disconnection is required, optimize patient sedation to prevent coughing, turn the ventilator to stand-by mode, and clamp the tracheal tube.
COVID-19 Ventilation Clinical Practice Guidelines (ESICM, 2020)
Ventilation clinical practice guidelines in adults with coronavirus disease 2019 (COVID-19) were released by the European Society of Intensive Care Medicine and the Society of Critical Care Medicine. [55]
Ventilation-Related Recommendations and Suggestions for Adults With COVID-19
It is suggested to start supplemental oxygen if the peripheral oxygen saturation (SPO2) is less than 92%. It is recommended to start supplemental oxygen if the SPO2 is less than 90%.
In the event of acute hypoxemic respiratory failure on oxygen, it is recommended that the SPO2 be maintained at no higher than 96%.
In patients with acute hypoxemic respiratory failure despite conventional oxygen therapy, it is suggested that a high-flow nasal cannula be used rather than conventional oxygen therapy.
In patients with acute hypoxemic respiratory failure, it is also suggested that a high-flow nasal cannula be used over noninvasive positive-pressure ventilation.
In these patients with acute hypoxemic respiratory failure, in the event a high-flow nasal cannula is not available and the patient has no urgent indication for endotracheal intubation, it is suggested that a trial of noninvasive positive-pressure ventilation be conducted, with close monitoring and short-interval assessment for worsening of respiratory failure.
While considered an option, no recommendation was made regarding helmet noninvasive positive-pressure ventilation versus mask noninvasive positive-pressure ventilation.
In patients receiving either noninvasive positive-pressure ventilation or high-flow nasal cannula, it is recommended they be closely monitored for worsening respiratory status; early intubation in a controlled setting is recommended if worsening occurs.
In patients with acute respiratory distress syndrome (ARDS) who are on mechanical ventilation, it is recommended to use low-tidal-volume ventilation (4-8 mL/kg of predicted body weight) versus higher tidal volumes (>8 mL/kg).
In patients with ARDS who are on mechanical ventilation, it is recommended to target plateau pressures at less than 30 cm water.
In patients with moderate-to-severe ARDS who are on mechanical ventilation, it is suggested to use a higher positive end-expiratory pressure (PEEP) strategy versus a lower PEEP strategy. When using a higher PEEP strategy (ie, PEEP >10 cm water), monitor patients for barotrauma.
In patients with ARDS who are on mechanical ventilation, it is suggested to use a conservative fluid strategy versus a liberal fluid strategy.
In patients with moderate-to-severe ARDS who are on mechanical ventilation, it is suggested to use prone ventilation for 12-16 hours versus no prone ventilation.
In patients with moderate-to-severe ARDS who are on mechanical ventilation, it is suggested to use, as needed, intermittent boluses of neuromuscular blocking agents versus a continuous infusion, to facilitate protective lung ventilation.
Use of a continuous infusion of neuromuscular blocking agents is suggested in the event of persistent ventilator dyssynchrony, a need for ongoing deep sedation, prone ventilation, or persistently high plateau pressures.
In patients with ARDS who are on mechanical ventilation, routine use of inhaled nitric oxide is not recommended.
In mechanically ventilated patients with severe ARDS and hypoxemia despite optimization of ventilation and other rescue strategies, a trial of inhaled pulmonary vasodilator is suggested as rescue therapy; if rapid improvement in oxygenation is not observed, taper off treatment.
In mechanically ventilated patients with severe ARDS and hypoxemia despite optimization of ventilation, use of recruitment maneuvers is suggested over not using recruitment maneuvers. If recruitment maneuvers are used, staircase (incremental PEEP) recruitment maneuvers are not recommended.
In those patients on mechanical ventilation who have refractory hypoxemia despite optimization of ventilation and who have undergone rescue therapies and proning, it is suggested to use venovenous extracorporeal membrane oxygenation (EMCO) if available; alternatively, refer the patient to center that has ECMO. However, because EMCO is resource-intensive and it requires experienced centers/healthcare workers and infrastructure, it should only be considered in carefully selected patients with severe ARDS.
-
Altitude-Related Disorders. This graph shows the periodic breathing during sleep at simulated high altitude in a decompression chamber during the Operation Everest II studies. The top portion of the graph shows arterial oxygen saturation, which shows fluctuations. During the last minute of the tracing, saturation increases when supplemental oxygen is administered to the person, causing elimination of the apneic episodes (not shown). The bottom portion of the figure shows tidal volume. The breathing pattern consists of 3-5 breaths followed by cessation of breathing for several seconds. This cyclical pattern is characteristic of the breathing pattern during sleep of most people not acclimatized to high altitude.
-
Altitude-Related Disorders. These are the chest radiographs of a young male patient who developed high-altitude pulmonary edema while climbing in the Sierra Nevada Mountains in California. Panel A is a radiograph taken a few hours after returning to near sea level; it shows patchy opacities predominantly involving the right chest. Panel B is a radiograph taken after 3 days of recovery; it shows minimal residual changes in the right lung.
-
Altitude-Related Disorders. This image shows the pathophysiology of high-altitude pulmonary edema (HAPE) following ascent to high altitude. Factors leading to a low partial pressure of oxygen (PO2), such as exercise, sleep, or a low ventilatory response to hypoxia, increase the likelihood of developing HAPE. Alterations in the sympathetic nervous system are also believed to contribute to the development of HAPE. Evidence exists to suggest that a defect in sodium transport across the alveolar epithelium may be important in susceptible individuals. HPV = hypoxic pulmonary vasoconstriction; HVR = hypoxic ventilatory response; and PaO2 = partial arterial pressure of oxygen.
Tables
What would you like to print?
- Background
- Sleep at High Altitude
- High-Altitude Retinal Hemorrhage
- Acute Mountain Sickness and High Altitude Cerebral Edema
- High-Altitude Pulmonary Edema
- Special Populations at High Altitude
- WMS and CDC Guidelines
- FDA Policy for Face Masks, Face Shields, and Respirators in COVID-19 (2021)
- COVID-19–Related Airway Management Clinical Practice Guidelines (SIAARTI/EAMS, 2020)
- COVID-19 Ventilation Clinical Practice Guidelines (ESICM, 2020)
- Show All
- Media Gallery
- Tables
- References







