Background
Nodular disease of the thyroid gland is prevalent in the United States. The lifetime risk for developing a palpable thyroid nodule is estimated to be 5-10%, however, high resolution ultrasound has revealed thyroid nodules in 19-68% of randomly selected individuals [1] ; the condition affects more women than men.
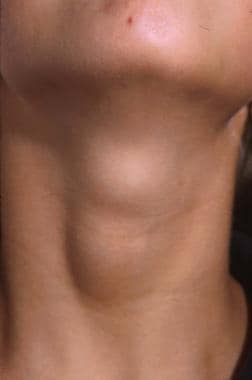 A 12-year-old patient with an asymptomatic, palpable thyroid nodule, which was noticed upon routine physical examination.
A 12-year-old patient with an asymptomatic, palpable thyroid nodule, which was noticed upon routine physical examination.
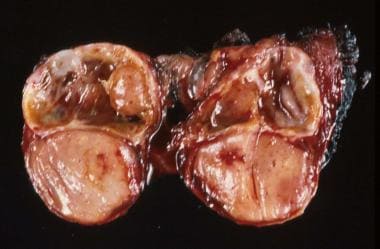 Surgical specimen of a thyroid lobe with papillary carcinoma, taken from a 12-year-old patient with an asymptomatic, palpable thyroid nodule; the nodule was noticed upon routine physical examination.
Surgical specimen of a thyroid lobe with papillary carcinoma, taken from a 12-year-old patient with an asymptomatic, palpable thyroid nodule; the nodule was noticed upon routine physical examination.
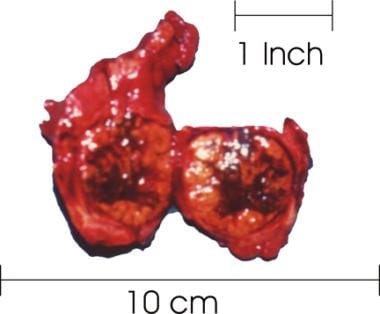 Thyroid with a large goiter. The right lobe of the thyroid was sectioned and reveals a large, solid nodule with necrotic and hemorrhagic areas. The histologic diagnosis was follicular thyroid carcinoma.
Thyroid with a large goiter. The right lobe of the thyroid was sectioned and reveals a large, solid nodule with necrotic and hemorrhagic areas. The histologic diagnosis was follicular thyroid carcinoma.
While nodular disease of the thyroid is common, malignancy of the thyroid occurs in only 7-15% of nodules. The incidence of both thyroid nodules and thyroid malignancy has increased rapidly in recent years. Most recent data for the US indicates approximately 63,000 new thyroid cancer cases/year. This increase is thought to largely be related to early detection by high resolution ultrasound and discovery of sub-clinical thyroid nodules. [2] The likelihood that the increased incidence of thyroid cancer being related to early detection is supported by evidence suggesting survival rates for thyroid cancer have remained fairly stable. [3]
While roughly 7-15% of thyroid nodules are malignant; the remainder represent a variety of benign diagnoses, including colloid nodules, degenerative cysts, hyperplasia, thyroiditis, or benign neoplasms. A rational approach to management of a thyroid nodule is based on the clinician's ability to distinguish the more common benign diagnoses from malignancy in a highly reliable and cost-effective manner.
See 10 Patients with Neck Masses: Identifying Malignant versus Benign, a Critical Images slideshow, to help identify several types of masses.
Clinical Outline
A comprehensive history and physical examination provides the foundation for decision making in the management of thyroid nodules. A number of features in the patient's history and physical examination significantly influence the statistical probability of malignancy in a thyroid nodule.
Factors suggesting a malignant diagnosis include the following:
-
Age younger than 20 years or older than 70 years
-
Male sex
-
Associated symptoms of dysphagia or dysphonia
-
History of neck irradiation
-
Prior history of thyroid carcinoma
-
Firm, hard, or immobile nodule
-
Presence of cervical lymphadenopathy
Factors suggesting a benign diagnosis include the following:
-
Family history of autoimmune disease (eg, Hashimoto thyroiditis)
-
Family history of benign thyroid nodule or goiter
-
Presence of thyroid hormonal dysfunction (eg, hypothyroidism, hyperthyroidism)
-
Pain or tenderness associated with nodule
-
Soft, smooth, and mobile nodule
Of importance, the factors mentioned above are only guidelines to assist in decision-making, and they do not provide absolute diagnostic information. For example, a historical axiom is that a multinodular goiter without a dominant nodule or a solitary cyst suggests a benign diagnosis. Data from contemporary studies, including those incorporating image-guided fine-needle aspiration biopsy (FNAB), have raised questions about this axiom. Furthermore, the ultrasonographic size of a solid thyroid nodule may have some diagnostic importance, because nodules larger than 3 cm are thought to have an increased risk of malignancy. However, findings suggest that nonpalpable nodules (incidentalomas) incidentally found on high-resolution ultrasonography may have a risk of malignancy comparable to that of palpable nodules.
Diagnostic Workup
Laboratory evaluation
The most important laboratory test is a sensitive thyroid-stimulating hormone (TSH) assay, which is used to screen for hypothyroidism or hyperthyroidism. In addition, obtaining serum thyroxine (T4) and triiodothyronine (T3) levels may be helpful (eg, when TSH levels are low-normal or high-normal). In most cases of solitary thyroid nodules, the TSH level is normal. In cases of a solitary thyroid nodule with a normal TSH value, no additional laboratory studies may be required in the diagnostic evaluation unless autoimmune disease (eg, Hashimoto thyroiditis) is suspected.
When the patient's history and physical findings reveal a family history or raise clinical suspicion for Hashimoto thyroiditis, obtain serum antithyroid peroxidase (anti-TPO) antibody and antithyroglobulin (anti-Tg) antibody levels. A diagnosis of Hashimoto thyroiditis does not exclude the possibility of malignancy.
Additional laboratory studies are unnecessary in the routine initial diagnostic evaluation of a solitary thyroid nodule.
Imaging studies
Thyroid scintigraphy
In most centers, the routine initial diagnostic evaluation of a solitary thyroid nodule no longer includes nuclear imaging studies. In the past, radionuclide scanning was an important imaging study performed routinely in the initial assessment of a thyroid nodule. Nuclear imaging can be used to describe a nodule as hot, warm, or cold on the basis of its relative uptake of radioactive isotope. Hot nodules indicate autonomously functioning nodules, warm nodules suggest normal thyroid function, and cold nodules indicate hypofunctional or nonfunctional thyroid tissue. (Examples of hot and cold nodules are seen in the image below.) Hot nodules are rarely malignant; however, 5-8% of warm or cold nodules are malignant. [4]
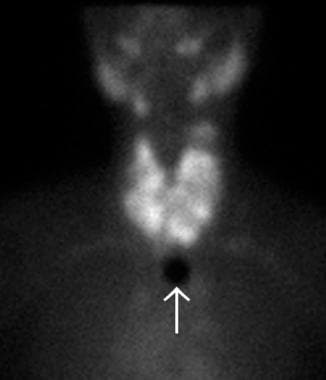 Technetium-99m (99mTc) thyroid scan of a large, nontoxic multinodular goiter. Multiple cold and hot nodules are observed in the enlarged thyroid gland. The white arrow indicates the sternal notch marker.
Technetium-99m (99mTc) thyroid scan of a large, nontoxic multinodular goiter. Multiple cold and hot nodules are observed in the enlarged thyroid gland. The white arrow indicates the sternal notch marker.
Ultrasonography
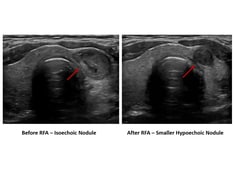
Because of advances in technology, ultrasonography is highly sensitive in determining the size and number of thyroid nodules. By itself, ultrasonography cannot reliably be used to distinguish a benign nodule from a malignant nodule. However, combining high-resolution sonography with Doppler and spectral analysis of the vascular characteristics of a thyroid nodule holds promise as a useful tool in screening thyroid nodules for malignancy. Studies have shown that the risk of malignancy is lower in nodules with a predominantly perinodular pattern than in nodules with an exclusively central vascular pattern. Furthermore, if the vascular characteristics of thyroid nodules are combined with their ultrasonographic parameters, including a halo, microcalcifications, cross-sectional diameter, and echogenicity, the predictive value of this imaging approach may increase. [4, 5, 6, 7]
Given the evidence suggesting the increase in thyroid nodule and thyroid cancer diagnosis is largely attributable to advances in high resolution ultrasonography as well as the evidence that such imaging can have predictive value in distinguishing benign disease from malignancy, efforts to standardize thyroid ultrasound reporting have been made. Su, H et al have published a recent consensus report by a multidisciplinary panel of specialists in which recommendations for standardized thyroid ultrasound reporting have been made. These recommendations outline characterization of both thyroid nodules and regional lymph nodes in the neck. [8]
Haugen et al developed the 2015 American Thyroid Association guidelines for management of thyroid nodules in which they have stratified the estimated risk of malignancy based on specific ultrasonographic characteristics of thyroid nodules and the recommendations for those nodules which warrant biopsy based on suspicious ultrasound patterns and nodule size. [1] Tessler et al expanded on these guidelines and proposed a risk-stratification system based on ultrasound thyroid-nodule characteristics (composition, echogenicity, shape, margin, and echogenic foci) to determine which thyroid nodules need biopsy. [9]
Thyroid ultrasonography can be helpful in certain cases when it is used to guide FNAB. Data have suggested that ultrasonography-guided FNAB may be preferable to palpation-guided FNAB. [10] Although sensitivity and specificity are not clearly and significantly between the approaches to FNAB, many authors consider image-guided FNAB to hold certain advantages. For example, image-guided FNAB may be particularly helpful in the assessment of nonpalpable or small nodules, nodules with cystic components, or nodules that are difficult to access (eg, posterior or substernal nodules). Ultrasonography-guided FNAB, combined with on-site cytologic verification of the adequacy of the specimen by a cytotechnologist or pathologist, may likely provide the highest sensitivity and specificity. Whether this is the most cost-effective approach for all thyroid nodules remains an issue.
In a study of 261 patients undergoing surgical evaluation for thyroid disease, Mazzaglia investigated whether office-based, surgeon-performed ultrasonographic examination significantly affected operative treatment of the patients even though all of these individuals had previously undergone ultrasonographic thyroid examination. Mazzaglia reported that treatment plans for 46 patients (17.6%) were altered because of significant differences between outside and surgeon-administered ultrasonograms. In 12 patients, for example, previously unidentified nonpalpable, enlarged lymph nodes were found in the surgeon-administered ultrasonograms, with biopsy revealing metastatic thyroid cancer in 3 of these patients. Mazzaglia concluded that surgeon-performed ultrasonographic examinations can be used to make necessary changes in surgical treatment and to avoid unnecessary surgery. [11]
Computed tomography (CT) scanning, magnetic resonance imaging (MRI), and positron emission tomography (PET) scanning
CT scanning or MRI is generally not cost-effective in the initial evaluation of solitary thyroid nodules. Such studies may be useful in the assessment of thyroid masses that are largely substernal. Also, in some cases CT scan–guided FNAB may be helpful. PET scanning with 18F-fluorodeoxyglucose is at present primarily an investigational tool, but it might have some role in thyroid imaging in the future, particularly in the evaluation of metastatic disease. [12, 13] However, a study by Deandreis et al found PET scanning to offer no additional diagnostic benefit in the evaluation of a thyroid nodule that has been found to have indeterminate cytopathology on FNAB. [14]
In the past, nuclear imaging studies of the thyroid, often combined with ultrasonography, were routinely performed in initial assessment of thyroid nodules. Because only 10% of solitary thyroid nodules are hot and because 90% of cold nodules are not malignant, nuclear imaging with or without ultrasonography typically offers a low yield of cancer diagnoses in surgical specimens when their results are used as the main guides for referral to a surgeon.
Fine-needle aspiration biopsy
FNAB has emerged as the most important step in the diagnostic evaluation of thyroid nodules. [15] Data from numerous studies have established FNAB as highly accurate, with mean sensitivity higher than 80% and mean specificity higher than 90%. The accuracy of FNAB in diagnosing thyroid conditions highly depends on the cytopathologist's expertise and experience and the technical skill of the physician performing the biopsy. In addition, FNAB is highly cost-effective compared with traditional workups that heavily depended on nuclear imaging and ultrasonography. Routine use of FNAB in the evaluation of thyroid nodules can reduce the need for diagnostic thyroidectomy by 20-50% while increasing the yield of cancer diagnoses in thyroid specimens by 15-45%.
When FNAB of a thyroid nodule provides adequate cellular material for analysis, the specimen can be assigned into one of several different diagnostic classifications. In an effort to improve the communication and clarity of thyroid cytopathology, the National Cancer Institute convened a conference in 2007 to address the current status of FNAB of thyroid nodules. This conference developed a consensus for terminology known as the Bethesda System for Reporting Thyroid Cytopathology. The recommended thyroid FNAB diagnostic categories in this system include benign, atypia of undetermined significance, follicular neoplasm, suspicious for malignancy, malignancy, and nondiagnostic. [16]
The respective risk of malignancy associated with each diagnostic category is as follows:
-
Benign - < 1%
-
Atypia (AUS) - 5-10%
-
Follicular neoplasm - 20-30%
-
Suspicious for malignancy - 50-75%
-
Malignant - 100%
The main weakness of FNAB involves hypocellular aspirates and aspirates with high follicular cellularity. Hypocellular aspirates may be observed in cystic nodules, or they may be related to biopsy technique. The addition of ultrasonography to guide FNAB sometimes reduces technical errors. Furthermore, ultrasonography-guidance combined with on-site verification of the adequacy of the specimen by a cytotechnologist or a pathologist is likely to reduce the rate of nondiagnostic specimens.
See the image below
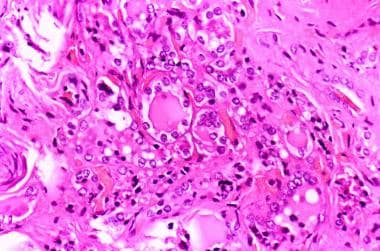 Histologic pattern of a mildly differentiated follicular thyroid carcinoma (250 X). Image courtesy of Professor Pantaleo Bufo at University of Foggia, Italy.
Histologic pattern of a mildly differentiated follicular thyroid carcinoma (250 X). Image courtesy of Professor Pantaleo Bufo at University of Foggia, Italy.
Aspirates characterized by high follicular cellularity suggest follicular neoplasm; however, FNAB cannot be used reliably to distinguish a benign follicular neoplasm from a malignant neoplasm. In addition, aspirates that are highly cellular with Hürthle cells can be observed with benign or malignant Hürthle-cell neoplasms and with some cases of Hashimoto thyroiditis. [17]
Advances in cytologic analysis may increase the predictive value of FNAB of thyroid nodules. For example, the incorporation of immunocytochemical studies, as well as genetic and molecular profiling of aspirates, may improve the accuracy of minimally invasive diagnostic techniques. In the specific case of aspirates revealing cytology of indeterminant significance or follicular lesions, the use of molecular testing such as the Afirma gene expression classifier can aid in decision making regarding recommendations for surgery. [18]
In those cytopathologic classifications where risk of malignancy is relatively indeterminate (Atypia of undetermined significance, follicular neoplasm), improvement in the assessment of the risk of malignancy might be achieved by viewing the cytopathologic results in the context of ultrasonographic characteristics suspicious for malignancy.
An Italian study compared the effectiveness of FNAB with that of fine-needle nonaspiration biopsy or "capillary technique" (FNNAB) in the evaluation of thyroid nodules. [19] The 2 techniques were performed on the same 104 patients who were known to have a uninodular or multinodular goiter. No statistically significant difference was found between the adequacy of samples obtained through FNAB and those collected through FNNAB in the diagnosis of colloid, follicular, or malignant nodules. The only significant difference was in the percentage of samples yielding inadequate results (16.3% and 5.8% for FNAB and FNNAB, respectively). The authors suggested that the frequency of inadequate samples was lower for FNNAB because the technique allows better-quality specimens to be collected. Otherwise, the investigators found both techniques to be useful and cost-effective.
Ultrasonography-guided FNAB has become increasingly more common. Clinicians need adequate sampling during biopsies to provide an accurate diagnosis and to avoid repeating the procedure. Insufficient experience with the technique of ultrasonography-guided FNAB is an important factor in the yield of this procedure. One study found, not surprisingly, that physicians who have more experience in performing ultrasonography-guided FNAB have lower rates of inadequate samples. [20] Although the inexperienced group had smaller size nodules, this probably does not affect the results given the obvious difference in competency rates.
Management of Thyroid Nodules
In addition to the clarification of terminology in cytopathologic reporting, the Bethesda conference also established a consensus for the indications to perform FNAB of thyroid nodules, as well as post-FNAB management options. The current state of the art in thyroid FNAB is nicely outlined in a review by Layfield et al. [21]
The most important routine aspects of the diagnostic evaluation of solitary thyroid nodules include thorough history-taking and physical examination, measurement of the serum TSH level, ultrasound-imaging, and FNAB of the nodule. Subsequent management of a solitary thyroid nodule largely depends on the diagnosis from FNAB.
Using the Bethesda system, the follicular neoplasm, suspicious for malignancy, and malignant classifications each warrant surgical consultation. Patients with follicular cytopathology on FNAB should be referred to a surgeon because 20-30% of such nodules are malignant. Exceptions may be made in the case of malignant lymphoma, which is typically not managed surgically, and in cases of anaplastic carcinoma, in which surgical intervention may be futile.
For the atypia of undetermined significance category, management options include the following:
-
Repeat FNAB in 3-6 months; if repeat ultrasonography-guided biopsy is again atypical, surgical consultation is warranted
-
Surgical consultation if, in addition to atypia, worrisome characteristics on ultrasound are also noted, such as hypoechogenicity, irregular borders, calcifications, or hypervascularity. [17]
Nodules classified as benign can be safely followed with ultrasound at 6-18 month intervals with further intervention based on imaging features such as increased growth. Most thyroid nodules associated with benign cytopathology on FNAB can be managed without routine surgical referral, provided that adequate follow-up is possible. Although the incidence of false-negative results with FNAB is low, some physicians recommend repeat FNAB for confirmation 6-12 months after an initial diagnosis of a benign lesion or if the characteristics of the nodule change on follow-up examination. When a benign diagnosis is confirmed, referral to a surgeon is reasonable for patients with symptoms, such as dysphagia or discomfort, or concerns about cosmesis.
When findings from the aspirate are nondiagnostic, repeat the aspiration, possibly with ultrasonographic guidance. Nodules for which aspirates are repeated nondiagnostic may ultimately require surgical management. [22]
Special Considerations
Incidentally discovered thyroid nodules
Advances in imaging technology have increased the potential for the incidental discovery of nonpalpable thyroid nodules. When the history and physical findings result in a low index of suspicion for malignancy, periodic follow-up evaluation with high-resolution ultrasonography is appropriate. Specific guidelines regarding such evaluation have not been established, but findings have raised concern that the incidence of malignancy in nonpalpable nodules may approach that of palpable nodules. For this reason, if sequential sonograms (eg, obtained at 6-mo intervals) reveal an increase in nodular size, ultrasonography-guided FNAB may be appropriate, even if the nodule remains nonpalpable.
Autonomously functioning thyroid nodules
Patients with solitary thyroid nodules associated with suppressed TSH levels, with overt or subclinical hyperthyroidism, do not require routine FNAB. In such cases, the patient may be referred to an endocrinologist to discuss iodine-131 treatment versus surgical intervention.
Guidelines
Pediatric Thyroid Nodules and Differentiated Thyroid Carcinoma Clinical Practice Guidelines
Clinical practice guidelines on the management of pediatric thyroid nodules and differentiated thyroid carcinoma from the European Thyroid Association were published in 2022. [23]
Ultrasonography of the neck is recommended to evaluate thyroid nodules. For pediatric patients with nodules suspected of being malignant, fine needle biopsy is recommended.
For the preoperative evaluation of a pediatric patient with differentiated thyroid carcinoma (DTC), neck palpation, comprehensive neck ultrasonography, and laboratory workup are recommended. Additional genetic testing or imaging studies are suggested for patients who have familial or extensive disease.
Total thyroidectomy is the suggested treatment for children with DTC.
It is suggested that in patients with incidentally found, very small thyroid carcinoma and nonaggressive histologic features, hemithyroidectomy may be an option.
It is suggested that prophylactic central lymph node dissection be reserved for patients with advanced thyroid cancer (extracapsular extension, vascular invasion, distant metastases).
It is suggested that radioactive iodine (I-131) therapy is indicated for all children following total thyroidectomy.
Serum thyroglobulin measurement and neck ultrasonography are recommended for follow-up after treatment for DTC during childhood. Monitoring of thyroid-stimulating hormone levels and suppression to low-normal values are suggested.
For children with persistent or recurrent cervical disease, it is suggested that surgery or I-131 therapy is indicated, depending on the size, tumor load, and degree of progression.
I-131 therapy is recommended as the first-line treatment for patients with pulmonary metastases.
Postoperative monitoring of laryngeal nerve and parathyroid gland function is recommended. Continued follow-up is suggested for at least 10 years after surgery.
Questions & Answers
Overview
How are history and physical findings used in the management of thyroid nodules?
Which factors suggest a malignant diagnosis in patients with thyroid nodules?
Which factors suggest a benign diagnosis in patients with thyroid nodules?
What is the role of lab testing in the evaluation in of thyroid nodules?
What is the role of thyroid scintigraphy in the diagnostic workup of thyroid nodules?
What is the role of ultrasonography in the diagnostic workup of thyroid nodules?
What is the role of CT scanning, MRI and PET scanning in the diagnosis of thyroid nodule?
What is the role of fine-needle aspiration biopsy (FNAB) in the diagnosis of thyroid nodule?
What is the risk of malignancy associated with each diagnostic category of thyroid nodules?
What is the efficacy of FNAB in the diagnosis of thyroid nodules?
How are solitary thyroid nodules diagnosed?
When is surgery indicated for treatment of a thyroid nodule?
How are thyroid nodules of undetermined significance treated?
How are benign thyroid nodules treated?
How are incidentally discovered thyroid nodules managed?
How are autonomously functioning thyroid nodules treated?
-
A 12-year-old patient with an asymptomatic, palpable thyroid nodule, which was noticed upon routine physical examination.
-
Surgical specimen of a thyroid lobe with papillary carcinoma, taken from a 12-year-old patient with an asymptomatic, palpable thyroid nodule; the nodule was noticed upon routine physical examination.
-
Technetium-99m (99mTc) thyroid scan of a large, nontoxic multinodular goiter. Multiple cold and hot nodules are observed in the enlarged thyroid gland. The white arrow indicates the sternal notch marker.
-
Thyroid with a large goiter. The right lobe of the thyroid was sectioned and reveals a large, solid nodule with necrotic and hemorrhagic areas. The histologic diagnosis was follicular thyroid carcinoma.
-
Histologic pattern of a mildly differentiated follicular thyroid carcinoma (250 X). Image courtesy of Professor Pantaleo Bufo at University of Foggia, Italy.