Overview
Peripartum (postpartum) cardiomyopathy (PPCM) is the most common cardiomyopathy in pregnancy. [1] PPCM is defined as an idiopathic cardiomyopathy that presents with heart failure secondary to left ventricular (LV) systolic dysfunction toward the end of pregnancy or in the months after delivery, in the absence of any other cause of heart failure. [2, 3] PPCM is a diagnosis of exclusion, and the majority are diagnosed postpartum. Although the LV may not be dilated, the ejection fraction is nearly always reduced below 45%. [2, 3]
This definition specifically excludes women who develop LV dysfunction early in their pregnancy and explicitly notes that not all cases of PPCM present with LV dilatation. [4] Thus, it helps avoid misdiagnosis of other conditions that present with pulmonary edema in pregnancy, such as diastolic dysfunction from preeclampsia and other disorders (see the section on Differential Diagnosis).
PPCM is more common in women older than 30 years, black women, multiparous women, women with preeclampsia or hypertension, and those who smoke or are malnourished. [3] The overlap between PPCM and preeclampsia is clinically important, as patients with preeclampsia can present with noncardiogenic edema and the coexistence of these two conditions highlights potential shared pathogenic mechanisms.
The severity of symptoms in patients with PPCM can be classified by the New York Heart Association (NYHA) system as follows:
-
Class I - Disease with no symptoms
-
Class II - Mild symptoms/effect on function or symptoms only with extreme exertion
-
Class III - Symptoms with minimal exertion
-
Class IV - Symptoms at rest
Etiopathophysiology
Although many potential mechanisms for peripartum (postpartum) cardiomyopathy (PPCM) exist, its exact cause remains unknown [3] but is likely to be multifactorial. The timing of presentation argues against the hemodynamic changes of pregnancy for causing PPCM, because such changes usually peak by 28 weeks' gestation. Furthermore, the timing highlights a potential hormonal pathogenesis given that hormonal changes, such as the production of prolactin, continue in late pregnancy. Relatively recent research has focused on the “vasculo-hormonal hypothesis” with soluble Fms-like tyrosine kinase 1 (sFlt1) and prolactin as molecules involved in the pathophysiology of PPCM. [5]
Prolactin
Increased reactive oxygen species lead to secretion of cathepsin D by an mechanism that is currently not well understood. Cathepsin cleaves prolactin into a 16kDa prolactin. [6] 16kDa Prolactin induces endothelial cells to package miR-146 into exosomes which are then taken up by cardiomyocytes. [7] 16kDA Prolactin is associated with endothelial and myocyte apotosis.
sFLT1
A murine model of PPCM has implicated the loss of vascular endothelial growth factor (VEGF) in the pathogenesis of PPCM. [8] sFlt1 is a molecule secreted by the placenta in late gestation, and in higher levels in preeclampsia and twin gestations. sFlt1 neutralizes VEGF, thereby reducing circulating VEGF which is thought to contribute to PPCM. [9]
Taken together, the increased production of prolactin and placental secretion of sflt1 in late pregnancy could be toxic to both the vasculature and the cardiac myocytes. The shared role of sFlt1 in preeclampsia, twin pregnancies, and PPCM provides a biological rationale for the increased incidence of PPCM in patients with preeclampsia or twin gestations.
Other pathophysiologic mechanisms
Genetics
Critics argue that the recovery of left ventricular ejection fraction and the lack of recurrence in all subsequent pregnancies challenge the notion that PPCM is a solely genetically-mediated condition. However, a study that sequenced 43 genes with variants associated with dilated cardiomyopathy from 172 women with PPCM revealed 26 (15%) distinct, rare truncating variants in 8 genes among these women—with TTN (titan) truncating variants the most prevalent. [10] The prevalence of these truncating variants was significantly higher than that found in a reference population of 60,706 people (4.7%) but similar to those of patients with dilated cardiomyopathy (17%).
Myocarditis
The equal prevalence of myocardial inflammation [11] and viral genomes [12, 13] that has been noted in subjects and controls who underwent endomyocardial biopsy challenges the pathogenic role of myocarditis in PPCM.
Nutritional factors
Lower levels of selenium or iron have been proposed as causative factors. Patients with PPCM, particularly in Nigeria, have been noted to have low selenium levels. [14]
Microchimerism
Microchimersium, with fetal-derived cells in the maternal circulation, has been hypothesized as potential contributing factor to the development of PPCM. [5, 15]
Epidemiology
United States statistics
Reports estimating the incidence of peripartum (postpartum) cardiomyopathy (PPCM) in the United States vary widely, ranging from 1 case per 1000-4000 live births. Approximately 75% of cases are diagnosed within the first postpartum month, and 45% present in the first week. [4]
International statistics
The prevalence is reported to be 1 case per 6000 live births in Japan, 1 case per 1000 live births in South Africa, and 1 case per 350-400 live births in Haiti. A high prevalence in Nigeria has been attributed to the tradition of ingesting kanwa (dried lake salt) while lying on heated mud beds twice a day for 40 days postpartum.
Age- and race-related demographics
Although PPM can occur at any age, approximately 50% of cases occur in women older than 30 years. [16]
PPCM has been reported in white, Chinese, Korean, and Japanese women. Case series indicate that many cases occur in black women from the southern United States. A case control study in the United States found that, when compared to non–black women, black women had a 15.7-fold higher relative risk of PPCM. [17]
Prognosis
Mechanical support devices and transplantation
A study of 99 patients who received durable ventricular assist devices (VADs) betwen 2006 and 2012 reported better outcomes in patients with peripartum (postpartum) cardiomyopathy (PPCM) than for patients with other etiologies of cardiomyopathy. Approximately half of patients with VADs went on to have cardiac transplanation with only four of the patients having the VAD removed. Approximately 5% of transplants performed in women in the United States are in the context of PPCM. [5]
Mortality
In general, PPCM-related mortality ranges from less than 2% to 50%. [1] In-hospital mortality in the United States has been reported as 1.3%. Long-term mortality at 7-8 years has been reported as 11-16%. [5] Internationally, 6-month mortality has ranged from 2% in Germany to 12.6% in South Africa; 24-month data from Turkey reveal a 24% mortality. [3]
Left ventricular (LV) recovery
In the Investigations of Pregnancy Associated Cardimyopathy (IPAC) study of 100 women in the United States, the majority (71%) improved their LV ejection fraction (EF) to above 50% by 6 months post partum. [18] Approximately 13% of women had major events with persistent cardiomyopathy and an LVEF below 35%.
Predictors that suggest the LV will not recover include an initial LVEF below 30%, LV dilatation (LV end diastolic diameter [LVEDD] >60 mm) and black race. [18] In a Chinese study, both LVEF below 35% at presentation and an elevated B-type natriuretic peptide (BNP) level over 1860 pg/mL predicted persistent LV dysfunction. [19]
Black women in the United States have a worse prognosis, with lower rates of LV recovery and a higher incidence of mortality or transplantation. [20] Mortality is higher in South Africa (28% at 2 years) and Haiti (15% at 2 years).
Recurrence with subsequent pregnancies
In a study of 34 patients (predominantly black women) with subsequent pregnancies after a diagnosis of PPCM, the risk of relapse was 56%. [21] The risk of recurrence was higher in those with persistent LV dysfunction than in those with a normalized LVEF, with a study of 191 subsequent pregnancies demonstrating a relapse in 48% of patients with persistent LV dysfunction and 27% of patients with a normalized LVEF. [22] The incidence of mortality in recurrent PPCM in patients with persistent LV dysfunction prior to pregnancy has been reported as 19% [22] to 25%. [21] The risk of premature delivery and threatened abortions is also higher in patients with persistent LV dysfunction. [22]
Although assessment with stress echocardiography to demonstrate contractile reserve has been studied, the clinical revelance of adequate LV augmentation in subsequent pregnancies is not clear. [23]
The 2018 European Society of Cardiology (ESC) guidelines on the management of cardiovascular diseases and pregnancy categorize PPCM with persistent LV dysfunction (LVEF < 50%) as modified World Health Organization level IV (mWHO IV), a category defined as high maternal risk in which pregnancy should be considered contraindicated. [3] Prior PPCM with a normalized LVEF is categorized as mWHO III, a category defined as moderate maternal risk in which close monthly surveillance and delivery at a specalized center is recommended. [3] Prophylactic bromocriptine at the time of delivery in subsequent pregnancies in patients with prior PPCM is an evolving area of study. [3, 21, 24, 25, 26, 27]
Patient Education
The initial diagnosis of peripartum (postpartum) cardiomyopathy (PPCM) is most often unexpected. It is important to provide honest and clear advice about the potential for decompensation. Education should be provided about heart failure management including lifestyle modifications (salt and fluid restrictions), daily weights, the importance of medication compliance, and awareness of worrisome symptoms such as weight gain, worsening dyspnea, pedal edema, orthopnea, paroxysmal dyspnea, chest pain, palpitations, presyncope, or syncope.
Women should be advised to avoid conceiving until follow-up echocardiography has been performed to provide an informed discussion about the risks of subsequent pregnancies. Education should also include a discussion on the risks of recurrent decline in left ventricular (LV) ejection fraction, possible mortality, and potential fetal events in subsequent pregnancies, particularly in women with persistent LV dysfunction.
Presentation
History
Many presenting complaints in women with cardiac disease occur during a normal pregnancy. Dyspnea, dizziness, orthopnea, and decreased exercise capacity often are normal symptoms in pregnant women. Mild dyspnea upon exertion is particularly common in a normal pregnancy. The classic dyspnea of pregnancy is often described by the woman as feeling as if she is unable to get enough air in, unable to get a good deep breath, or both; this is thought to be due to the progesterone-mediated hyperventilation.
Note that early, rapid diagnosis of peripartum (postpartum) cardiomyopathy (PPCM) is not the norm. Often, patients do not show any indication of the syndrome until after delivery. In one study, it took 7 or more days to establish the diagnosis in 48% of women, and half of those women had major adverse events before the diagnosis was made. [28]
Many PPCM patients present with heart failure or a major adverse event (eg, stroke or respiratory failure) without any previous signs or symptoms to alert the clinician that a cardiomyopathy is going to develop; 19% of patients may present with the syndrome before the last gestational month. [4] Symptoms of PPCM are the same as those in nonpregnant patients with systolic dysfunction. New or rapid onset of the following symptoms requires prompt evaluation:
-
Cough
-
Orthopnea
-
Paroxysmal nocturnal dyspnea
-
Fatigue
-
Palpitations
-
Hemoptysis
-
Chest pain
-
Abdominal pain
Physical examination
In a normal pregnancy, as a result of the increase in endogenous progestins, respiratory tidal volume is increased and patients have a tendency to hyperventilate. However, the rate of respiration should be normal. Normal pregnancy is characterized by an exaggerated x and y descent of the jugular venous waveform, but the jugular venous pressure should be normal.
Cardiac auscultation reveals a systolic ejection murmur at the lower left sternal edge, over the pulmonary area, or both, in 96% of women during pregnancy. [29] This pulmonic arterial flow murmur tends to become quieter during inspiration. Diastolic murmurs warrant further evaluation. The first heart sound (S1) may be exaggerated, and the second heart sound (S2) split may be more prominent due to an increased right-sided flow. An S3 has been described as a normal finding in pregnancy. Peripheral edema occurs in approximately one third of healthy gravid women. However, clinicians should be alert to sudden changes in swelling late in pregnancy, which can be abnormal and should be investigated.
In a patient with PPCM, signs of heart failure are the same as those in nonpregnant patients with systolic dysfunction. Tachycardia and decreased oxygen saturation could be present. Blood pressure may be normal. Elevated blood pressures (systolic >140 mm Hg and/or diastolic >90 mm Hg) and hyperreflexia with clonus suggest preeclampsia. Elevated jugular venous pressure, cardiomegaly, a third heart sound, a loud pulmonic component of the second heart sound, mitral or tricuspid regurgitation, pulmonary rales, worsening of peripheral edema, ascites, arrhythmias, embolic phenomenon, and hepatomegaly may be present.
Differential Diagnosis
As noted earlier, peripartum (postpartum) cardiomyopathy (PPCM) is a diagnosis of exclusion in which an idiopathic cardiomyopathy presents with heart failure secondary to left ventricular systolic dysfunction toward the end of pregnancy or in the months after delivery, in the absence of any other cause of heart failure. [2, 3]
Clinically, the most common differential diagnosis considered is that of preeclampsia. Preeclampsia should be excluded on the basis of the history, the physical examination, and blood work. New headaches, visual disturbances, right-sided abdominal pain, and new swelling of the hands or face may be present. Retinal vasospasm, a fourth heart sound (S4) heard on cardiac auscultation, hyperreflexia/clonus, right upper quadrant tenderness, and face or hand edema may be present
The differential diagnosis includes the following:
-
Noncardiogenic pulmonary edema during pregnancy: Pregnancy is a state of low oncotic pressure, reflected in decreased serum albumin (expected values, ~3.2 mg/dL); consequently, when other stressors are present, pulmonary edema can occur with normal cardiac filling pressures; the most common triggers include pyelonephritis and other infections, corticosteroids, and tocolytics such as beta agonists and magnesium sulfate
-
Preeclampsia (toxemia of pregnancy)
Other problems to be considered include the following:
-
Arrhythmogenic right ventricular dysplasia
-
Cardiomyopathy, diabetic heart disease
-
Infectious, toxic, or metabolic disorders
Laboratory Studies
Cardiac biomarkers
Creatinine phosphokinase (CPK) levels can be elevated after normal delivery due to release from the uterus, and they may be elevated after cesarean section due to release from the uterus and/or skeletal muscle. An elevated CPK level is not diagnostic of peripartum (postpartum) cardiomyopathy (PPCM), because it can be elevated for many other reasons, including normal delivery, skeletal muscle disorders, and viral myocarditis. The CPK from the placenta routinely has a CPK-MB (muscle/brain) fraction of 6% or more. [30] Therefore, without an obvious clinical presentation and electrocardiographic (ECG) findings to suggest myocardial infarction, the use of this test in the puerperium is very limited.
Troponin-I elevations are more likely to indicate true myocardial disease, whether it is inflammatory or due to infarction. They are certainly useful in diagnosing acute myocardial infarction. One study found that a cardiac troponin T level greater than 0.04 ng/mL, measured within 2 weeks of diagnosis, was 60% sensitive at identifying women more likely to have persistent ventricular dysfunction at 6 months after the diagnosis. Given the poor sensitivity, the clinical use of this test is not entirely clear; these women should be placed on maximal medical therapy and undergo serial ECG assessments regardless of the troponin result. [31]
Levels of B-type natriuretic peptide (BNP) remain in the normal range in uncomplicated pregnancies. [32] Elevations in BNP and N-terminal (NT)-pro hormone (proBNP) can be seen in PPCM; however, these findings can also be seen in the setting of preeclampsia. [33]
Preeclampsia laboratory studies should be obtained. Abnormalities found with preeclampsia include the following:
-
Serum creatinine level greater than 0.8 mg/dL
-
Hemoglobin level above 13 g/dL (due to leaky capillaries and hemoconcentration)
-
Elevated liver enzymes
-
Thrombocytopenia
-
Urine dipstick test results indicating more than “1+” protein
-
Decreased 24-hour urine creatinine clearance (normally 150% above the nonpregnant level, or approximately 150 mL/min)
-
More than 300 mg of proteinuria evident on a 24-hour collection
On urinalysis, trace or 1+ proteinuria can be normal. Proteinuria 2+ or higher suggests preeclampsia. Exclude infection with urine cultures.
Other causes of cardiomyopathy
Serologic testing may help identify known causes of cardiomyopathy, including infections (eg, viral, rickettsial, human immunodeficiency virus [HIV], syphilis, Chagas disease, diphtheria toxin). Exclude toxic etiologies such as ethanol and cocaine. When indicated, exclude systemic disorders such as collagen vascular diseases, sarcoidosis, thyrotoxicosis, pheochromocytoma, and acromegaly.
ECG and rhythm monitoring
Left-axis deviation, right-axis deviation, small Q waves in lead III, T-wave inversions or an increased R/S ratio in leads V1 and V2 can be findings of normal pregnancy. ECGs in patients with PPCM may show sinus tachycardia or, rarely, atrial fibrillation. [34] Other nonspecific findings include low voltage, left ventricular hypertrophy, and nonspecific ST-segment and T-wave abnormalities.
Patients admitted to the hospital should be monitored with telemetry. Rhythm monitoring with a Holter monitor or Ziopatch should be considered for outpatients with palpitations.
Echocardiography
All women in whom the diagnosis of peripartum (postpartum) cardiomyopathy (PPCM) is being considered should undergo echocardiographic evaluation to assess left ventricular function, valve structure, chamber size, and wall motion. Cardiac chambers do enlarge slightly during pregnancy, usually within normal limits. Normal function suggests a pulmonary process or noncardiogenic pulmonary edema, and diastolic dysfunction can be observed in patients with severe preeclampsia.
See the images below.
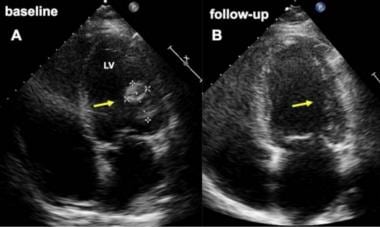 Pericardial cardiomyopathy. Baseline (A) and follow-up (B) four-chamber view echocardiograms in a woman who developed cardiomyopathy 4 weeks after an elective cesarean delivery. Image A demonstrates a large thrombus (arrow) attached to the lateral wall of the left ventricle (LV) at baseline (A), which completely resolved after two months (B). The patient's younger sister also experienced peripartum cardiomyopathy. Courtesy of BioMed Central Ltd, Springer Nature (Meyer GP, Labidi S, Podewski E, et al. Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep. 2010 Mar 4;4:80. Online at https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-4-80).
Pericardial cardiomyopathy. Baseline (A) and follow-up (B) four-chamber view echocardiograms in a woman who developed cardiomyopathy 4 weeks after an elective cesarean delivery. Image A demonstrates a large thrombus (arrow) attached to the lateral wall of the left ventricle (LV) at baseline (A), which completely resolved after two months (B). The patient's younger sister also experienced peripartum cardiomyopathy. Courtesy of BioMed Central Ltd, Springer Nature (Meyer GP, Labidi S, Podewski E, et al. Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep. 2010 Mar 4;4:80. Online at https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-4-80).
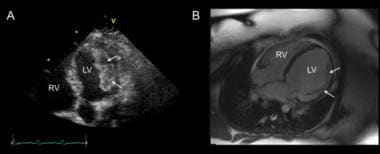 Peripartum cardiomyopathy. These images were obtained in a young woman with peripartum cardiomyopathy and multiple left ventricular (LV) thrombi. (A) Apical five-chamber view on transthoracic echocardiography demonstrating a large layered echodense mass attached to the LV lateral wall consistent with thrombi. (B) Four-chamber view of a balanced steady-state free precession cardiac magnetic resonance imaging acquisition 4 days after anticoagulation with parenteral heparin demonstrating near resolution of the thrombus, leaving behind only the underlying muscular trabeculations on this view (arrows). RV = right ventricle. Courtesy of BioMed Central Ltd, Springer Nature (Altuwaijri WA, Kirkpatrick ID, Jassal DS, Soni A. Vanishing left ventricular thrombus in a woman with peripartum cardiomyopathy: a case report. BMC Res Notes. 2012 Oct 2;5:544. Online at https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-5-544).
Peripartum cardiomyopathy. These images were obtained in a young woman with peripartum cardiomyopathy and multiple left ventricular (LV) thrombi. (A) Apical five-chamber view on transthoracic echocardiography demonstrating a large layered echodense mass attached to the LV lateral wall consistent with thrombi. (B) Four-chamber view of a balanced steady-state free precession cardiac magnetic resonance imaging acquisition 4 days after anticoagulation with parenteral heparin demonstrating near resolution of the thrombus, leaving behind only the underlying muscular trabeculations on this view (arrows). RV = right ventricle. Courtesy of BioMed Central Ltd, Springer Nature (Altuwaijri WA, Kirkpatrick ID, Jassal DS, Soni A. Vanishing left ventricular thrombus in a woman with peripartum cardiomyopathy: a case report. BMC Res Notes. 2012 Oct 2;5:544. Online at https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-5-544).
Chest Radiography
When evaluating new onset dyspnea, tachycardia, or hypoxia in a pregnant woman, immediately obtain a chest radiograph with abdominal shielding to detect pulmonary edema. Concerns regarding fetal redation should not interfere with obtaining a chest x-ray. When chest radiography is performed with abdominal shielding, the fetal radiation exposure is well below the accepted limit of fetal radiation during pregnancy.
Patchy infiltrates in the lower lung fields, with vascular redistribution/cephalization, cardiomegaly, and pleural effusions, indicate congestive heart failure.
Bilateral lower lobe infiltrates without vascular redistribution suggest either an atypical pneumonia or noncardiogenic pulmonary edema (see the image below) resulting from the low oncotic state of pregnancy combined with various stressors or preeclampsia.
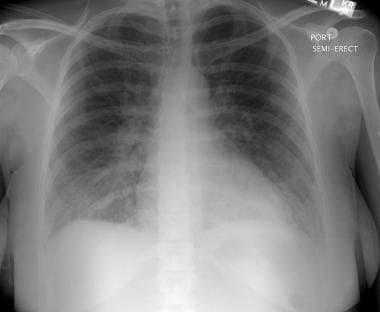 Peripartum cardiomyopathy. This radiograph reveals noncardiogenic pulmonary edema in patient with preeclampsia, due to a capillary leak that can be a primary component of preeclampsia. Note the diffuse increase in lung markings without the cephalization or vascular redistribution seen in patients with pulmonary edema from systolic dysfunction. The patient had rapid clinical improvement after only 10 mg of intravenous furosemide.
Peripartum cardiomyopathy. This radiograph reveals noncardiogenic pulmonary edema in patient with preeclampsia, due to a capillary leak that can be a primary component of preeclampsia. Note the diffuse increase in lung markings without the cephalization or vascular redistribution seen in patients with pulmonary edema from systolic dysfunction. The patient had rapid clinical improvement after only 10 mg of intravenous furosemide.
Magnetic Resonance Imaging
The role of cardiac magnetic resonance imaging (CMRI) in peripartum (postpartum) cardiomyopathy (PPCM) continues to evolve. The presence of late gadolinium enhancement (LGE) in a nonischemic pattern has been inconsistently identified in patients with PPCM. [35, 36] In a study of 10 women with PPCM, the presence of LGE was associated with a worse prognosis and persistent decrease in left ventricular ejection fraction. [36] Importantly, these scans were performed in postpartum patients, as most centers do not presently administer gadolinium during pregnancy due to a lack of data on the long-term fetal effects of gadolinium exposure.
Stress Testing
Stress testing may be considered in a woman with peripartum (postpartum) cardiomyopathy (PPCM) to exclude coronary ischemia when it is suspected clinically. Stress echocardiography is the test of choice to evaluate for coronary artery disease during pregnancy. A submaximal exercise protocol with fetal monitoring is recommended. [34]
Nuclear stress testing should be avoided in the first trimester due to the risk of teratogenesis. Although nuclear imaging is perhaps safer in the second and third trimesters, it is not without risks—including intrauterine growth restriction, central nervous system abnormalities, and an increased risk of malignancy. [34]
Cardiac Catheterization and Invasive Hemodynamic Monitoring
Cardiac catheterization can be safely performed in pregnancy, with careful attention to limiting fetal radiation by the use of abdominal shielding, brachial or radial access, and minimal fluorscopy. [34]
Right-side heart catheterization can be performed if the hemodynamic data will change management. However, close attention to vital signs, volume status, urine output, and oxygenation is more likely to detect clinically important changes. These assessments allow treatment decisions to be guided by the global assessment of a patient’s unique physiology rather than a standard response to a single number. Hemodynamic data should be interpreted in the context of expected changes in pregnancy, including an increase in cardiac output (by 50% at 28 weeks) and a decrease in systemic vascular resistance (SVR) (mean 850 dynes/sec/cm-5).
The filling pressures can distinguish peripartum (postpartum) cardiomyopathy (PPCM) from preeclampsia, with lower filling pressures seen in preeclampsia and higher filling pressures seen in PPCM. Both conditions may have lower than expected cardiac output and higher than expected SVR.
Tissue Analysis and Histologic Findings
Endomyocardial biopsy in the setting of peripartum (postpartum) cardiomyopathy (PPCM) is controversial, in that it has not yet been demonstrated to offer information that can significantly alter the plan of care.
Findings at autopsy have included a dilated heart, pale myocardium, endocardial thickening, and pericardial fluid. Biopsy specimens may show myofiber hypertrophy or degeneration, fibrosis, edema, or lymphocytic infiltration. Ventricular thrombi can be seen. Lymphocytic myocarditis has been found in some series, but its clinical significance is not clear because there is no convincing evidence to support immunosuppressive therapy if this result is obtained. [37]
General Treatment Approach
An overarching principle to treating patients with peripartum (postpartum) cardiomyopathy (PPCM) is to remember that a healthy fetus depends on a healthy mother. Formulate the care plan and consider the consequences of not treating the mother before addressing the potential or theoretical effects of a test/treatment on the fetus. This approach will help clarify the best plan for clinicians who infrequently address medical issues during pregnancy.
Transfer
Consider rapid transfer to an intensive care unit (ICU) for monitoring of maternal status. Consider transfer to a center that offers tertiary care services for both the mother and the fetus. If the mother is at less than 37 weeks’ gestation, transfer her to a center with a neonatal ICU (NICU).
Nonpharmacologic managment
The patient should follow a low-sodium (2 g/day sodium chloride) diet. Strict bedrest may increase the risk of venous thromboembolism and is no longer recommended as a mainstay of therapy. Activity should be limited only by the patient’s symptoms. In severe cases of true PPCM, bedrest may promote better uteroplacental perfusion.
Pharmacologic management
Medications should be used when there is clear benefit to the mother. The US Food and Drug Administration (FDA) has outlined a classification system for medications in pregnancy, with medications in category X clearly having more risks to the fetus than benefitis. Any medication in class A through D may be used when the potential benefit justifies the potential risk. Organogenesis is completed by 13 weeks’ gestation. Although some medications may have direct effects on the fetus, no risk of teratogenesis is present after the first trimester.
Medical therapy for patients with systolic dysfunction during pregnancy is similar to that for the nonpregnant patient. The mainstays of medical therapy are digoxin, loop diuretics, and beta-adrenergic blockade with carvedilol or metoprolol succinate, as they have been shown to decrease all-cause mortality and hospitalization in those with systolic dysfunction. Afterload reduction is typically accomplished with hydralazine and nitrates, because angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) are contraindicated in pregnancy owing to fetal renal dysgenesis and death. When the ejection fraction (EF) is less than 35%, anticoagulation is recommended due to a high risk of venous and arterial thrombosis. [38] Warfarin, low-molecular weight heparin (LMWH), or unfractionated heparin (UFH) may be considered for anticoagulation, with determinants of choice of anticoagulant the gestational age, risk of fetal toxicity of warfarin, and renal function.
Device therapy
Use intraaortic balloon pumps when indicated.
Cardiac transplantation and left ventricular (LV) assist devices have been used to treat PPCM. These should be considered for women with progressive LV dysfunction or deterioration despite medical therapy. Most centers will need to consider transfer of affected patients to a heart-transplant center for such therapy. However, LV function in most of these patients improves over time, and surgical therapy should be delayed if possible.
Women with PPCM with severely reduced LVEF (≤35%) appear to have an increased risk of ventricular tachyarrhythmias early after diagnosis. [39] Given the potential for recovery of LV function, use of the wearable cardioverter-defibrillator (WCD) may be preferred over implantable cardioverter-defibrillator (ICD) in affected women during the first 6 months after initiation of heart failure therapy. [39]
Route of delivery
Provided the gravida is hemodynamically stable, a spontaneous vaginal delivery with early epidural placement and assisted second stage of labor is appropriate. Vaginal deliveries are preferred because they are associated with much lower rates of complications, such as endometritis and pulmonary embolism, 75% of which occur in association with cesarean delivery. Vaginal deliveries are associated with less of the postoperative third-spacing of fluid that occurs after cesarean deliveries. This third-spaced fluid reverses after approximately 48 hours, leading to intravascular volume overload and possible maternal decompensation.
Pushing during the second stage of labor increases the body's metabolic demands and increases the afterload, both of which are ideally avoided in patients with significant LV dysfunction. Furthermore, cardiac output can increase by 15%-20% with each contraction and as high as 45% from baseline. Pain with contractions can also increase the metabolic demands through increased sympathetic stress with tachycardia and hypertension. Initiating early regional anestheisa and assisting the second stage of labor with forceps or vacuum can overcome these issues.
If the patient is hemodynamically unstable, requires inotropic agents, or requires mechanical assistive devices, then urgent delivery is indicated. A cesarean section may be preferable for critically ill women receiving inotropes.
After delivery, the contracted uterus squeezes 300-500 mL of blood into the systemic circulation. This autotransfusion, along with the release of inferior vena cava compression by the gravid uterus, leads to an increase in cardiac output as high as 10%-20% over predelivery levels. Therefore, worsening of heart failure is anticipated typically at 2-3 days post partum due these fluid shifts, the loss of the low-resistance placental bed, and the decrease in the vascular compliance that was maintained by the hormonal changes of pregnancy.
Other considerations
After delivery, patients on hydralazine/nitrates should be placed on an ACEI, and the dose should be maximized. Captopril and enalapril are thought to be compatible with breastfeeding.
Postdelivery breastfeeding is an important consideration. The risks and benefits of breastfeeding must be weighed. Breastfeeding increases metabolic demands. Prolactin remains elevated during breastfeeding, and this may contribute to the ongoing LV dysfunction. Furthermore, administration of bromocriptine inhibits prolactin secretion, effectively limiting breast milk production.
However, breastfeeding has a number of benefits to the newborn, particularly in developing countries where unsafe water supply limits formula use. Interestingly, an Internet-recruited study from the United States demonstrated improved outcomes in patients with PPCM who breastfed, which challenges the assertion that breastfeeding could prevent LV recovery by increasing metabolic demands and persistent prolactin production. [40]
At present, breastfeeding is discouraged in patients with severe LV dysfunction and New York Heart Association (NYHA) class 3-4 symptoms. [3] Recommendations for patients with less severe LV dysfunction or NYHA class 1-2 status are less well defined.
An echocardiogram should be ordered as indicated by new clinical findings or by a decline in function. If an echocardiogram reveals abnormal systolic function during pregnancy, a repeat study should be obtained approximately 2 months after delivery. If the results of that study show that systolic function has improved but has not returned to normal levels, another study should be obtained within the year to determine the patient’s new baseline.
Genetic testing for familial cardiomyopathy may be considered in patients with persistent LV dysfunction and a family history of cardiomyopathy.
For women considering pregnancy or those who desire an evaluation to estimate the risk of a future pregnancy, recovery of systolic function is a prime concern (see Prognosis). In women with a normalized LVEF, consideration could be made for weaning off one cardiac medication at a time with continued close clinical and echocardiographic surveillance throughout the discontinuaton process. Women with persistent systolic dysfunction should be maintained on vasodilators, nitrates, and diuretics as tolerated and indicated.
Consultations
Many internists do not have extensive exposure to diagnosing and treating medical disorders during pregnancy and therefore feel uncomfortable doing so. The best way to address this is consultation with an obstetric internist, a perinatologist, or a medical subspecialist. Their experience allows them to quickly help assess which treatments offer the best risk-to-benefit ratio. In most situations, the benefit of maximizing maternal well-being with the usual therapies outweighs the potential effects on the fetus, which may make some clinicians feel uneasy.
Consultations depend on which specialties are available locally and may include the following:
-
Internist with expertise in medical disorders in pregnancy (obstetric internist, pulmonary/critical care specialist, cardiologist)
-
High-risk obstetrician (maternal-fetal medicine/perinatologist)
-
Anesthesiologist: Neuraxial anesthesia is preferred to avoid myocardial depression from inhaled anesthetics; for this reason, as the mother nears delivery, LMWH should be used with caution.
Pharmacologic Therapy
In the treatment of systolic dysfunction in peripartum (postpartum) cardiomyopathy (PPCM), the principles of treating systolic dysfunction outside of pregnancy with diuretics, afterload reduction, and beta blockers are applied. Historically, hydralazine and nitrates are effective agents for reducing preload and afterload and have been the medications of choice during pregnancy.
Diuretics
Use diuretics when indicated to manage the maternal volume status, with close monitoring of electrolytes. Avoid maternal volume depletion that could lead to uteroplacental hypoperfusion.
When pulmonary edema is diagnosed, loop diuretics should be the first-line treatment. Start with 10 mg of furosemide, as pregnant women have an increased glomerular filtration rate (GFR) that facilitates secretion of the drug into the loop of Henle.
Spironolactone has been shown to decrease morbidity and improve survival when administered to nonpregnant outpatients with systolic dysfunction. The use of spironolactone may be considered for patients with reduced left ventricular (LV) systolic function in the postpartum period. Spironolactone is contraindicated during pregnancy due to its teratogenic effects. Bumetanide may be used when clinically indicated, and a thiazide may be added cautiously to a loop diuretic for a synergistic effect in diuretic-resistant patients.
Diuretics should be used very cautiously in women with preeclampsia, because intravascular volume depletion is a hallmark of preeclampsia.
Hydralazine and nitrates
Nitrates may be used to decrease maternal preload when indicated; they are safe for the mother and fetus and are compatible with breastfeeding. As with any medication that alters maternal hemodynamics, a drop in blood pressure can result in fetal hypoperfusion and distress. Very slowly titrate intravenous (IV) drips, and maintain maternal intravascular euvolemia.
Hydralazine, in combination with nitrates, is the first choice for afterload reduction and vasodilatation during pregnancy.
Although hydralazine in combination with nitrates is the preferred regimen during pregnancy, women should be switched to an angiotensin-converting enzyme inhibitor (ACEI) after delivery.
Beta-blockers
Metoprolol tartrate has been most commonly used for PPCM during pregnancy. Atenolol is specifically avoided due to the increased risk of intrauterine growth restriction. Carvedilol remains an alternative to metoprolol, given its potential antitocolytic activity (through beta2 blockade). These drugs may be used safely as second-line agents during pregnancy when clinically indicated. Monitor fetal growth during the second and third trimesters of pregnancy.
Digoxin and inotropes
Digoxin could be considered in women with an abnormal ejection fraction. Consider IV inotropes should when hypotension and/or cardiogenic shock are/is present. Improving cardiac output ensures adequate uteroplacental perfusion. Consider invasive hemodynamic monitoring to gauge the response to therapy. Digoxin is safe with breastfeeding. Although IV inotropes are safe in breastfeeding patients, as noted under the previous section "General Treatment Approach: Other considerations," breastfeeding is currently discouraged in patients with severe LV dysfunction and/or cardiogenic shock.
Anticoagulants
PPCM is associated with a high rate of thromboembolic complications, with rates of 7% reported in a cohort of US patients. [38] The risk is likely related to the degree of chamber enlargement, systolic dysfunction, and the presence of atrial fibrillation. Because pregnancy is a hypercoagulable state, once the diagnosis of PPCM is established, prophylactic anticoagulation should be considered during pregnancy and for 2 months postpartum in women with an LV ejection fraction (EF) below 35%.
Warfarin, low-molecular weight heparin (LMWH) or unfractionated heparin (UFH) are possible options for anticoagulation.
Warfarin may be used safely in the second and third trimester, and then switched to heparin before delivery. Warfarin is the drug of choice post partum. This agent crosses into breast milk, but studies have shown that it does not affect the newborn coagulation system; therefore, it is compatible with breastfeeding. However, warfarin does carry a risk of spontaneous fetal cerebral hemorrhage in the second and third trimesters. Women may prefer oral warfarin to one to two heparin injections a day.
UFH is preferred over LMWH in patients who are near term. Due to the occurrence of epidural hematomas, the American Society of Anesthesiology recommends that women on full-dose LMWH not receive spinal or epidural anesthesia for 24 hours after the last injection. LMWH is not predictably reversed with protamine. The therapeutic dose of UFH can also be monitored with activated partial thromboplastin times (aPTTs).
At present time, direct anticoagulants (DOACs) are not recommended in pregnant or breastfeeding patients.
Bromocriptine
Bromocriptine may be considered in patients with severe LV dysfunction. This drug inhibits prolactin secretion, which may disrupt the ongoing effects of prolactin on the vascular and cardiac systems.
On the basis of inferences from an animal model of PPCM, [41] a pilot study reported randomizing women to bromocriptine therapy after the diagnosis of PPCM. [24] Twenty PPCM patients in Africa were randomized to open-label bromocriptine for 8 weeks or standard care; those receiving bromocriptine had more recovery of LV function and a reduction in clinical endpoints relative to standard care. [24] Further observational studies from Germany(N=115) [42] and Canada (N=76) [26] have also observed an increased LV recovery in women who received bromocriptine compared to those who did not receive bromocriptine. Critics of these studies highlight the nonblinded, nonrandomized nature of the studies.
In a mutlicenter, blinded study of 63 German patients with PPCM and an LVEF below 35% who were randomized participants to receive either 1 week or 8 weeks of bromocriptine in addition to standard medical therapy for heart failure, [27] LVEF, as measured by magnetic resonance imaging (MRI), increased in both groups at 6 months, with no significant difference in the increase (28% to 49% in the 1-week group and 27% to 51% in the 8-week group) or the frequency of full recovery, defined as LVEF above 50% (52% in 1-week group vs 68% in 8-week group). [27] Although no control group was included in this study, the authors compared a subset of their cohort with LVEF less than 30% (n=37) to the IPAC (Investigations of Pregnancy Associated Cardimyopathy) cohort, [18] in which patients with LVEF below 30% received standard therapy for heart failure without bromocriptine. Persistent LV dysfunction was noted in 37% of the patients in the IPAC cohort and only 14% in the subset of patients with LVEF less than 30% in the current trial. [27]
Bromocriptine can be given as 2.5 mg daily for 1 week in uncomplicated cases. Higher doses (2.5 mg bid for 2 weeks, followed by 2.5 mg daily for 6 weeks) are recommended in patients with complicated course (eg, LVEF < 25% or cardiogenic shock). Note: Treatment with bromocriptine must always be accompanied by anticoagulation, given the increased risk of myocardial infarction and stroke with this drug. [25, 3]
Antiplatelet agents
In an open-label clinical trial assessing pentoxifylline 400 mg three times daily in a group of women with PPCM who were treated with diuretics, digoxin, enalapril, and carvedilol, a combined end-point of poor outcome—defined as death, failure to improve the LVEF more than 10 absolute points, or NYHA functional class III or IV at latest follow-up—occurred in 27% of patients treated with pentoxifylline and in 52% of those on usual therapy. [43]
A randomized trial (N=39) of pentoxifylline has shown that this agent may improve symptoms, LV function (by 5%), and lower levels of inflammatory cytokines (eg, tumor necrosis factor alpha). [44, 45] However, not all studies found a beneficial effect. Given the poor prognosis of persistent cardiac dysfunction and, on the assumption that the patients do not experience side effects from the medication, it seems reasonable to consider adding pentoxifylline to the standard regimen, as long as both the clinician and the patient understand that the available data were obtained from underpowered studies.
Other agents
Oxytocin is used to augment labor and may increase pulmonary arterial pressures. It also can control postpartum bleeding or hemorrhage. The pressor effect of sympathomimetics may increase when used concomitantly with oxytocic drugs, causing postpartum hypertension. Oxytocin has an intrinsic antidiuretic effect that, when administered by continuous infusion to a patient receiving fluids by mouth, can cause water intoxication.
Immunosuppression should not be used empirically, and current evidence does not support the routine use of immunosuppressive agents for myocarditis.
Levosimendan has also been considered to treat PPCM. A systematic review of two prospective studies found that bromocriptine and pentoxifylline, but not levosimendan, could be potentially useful agents to improve LV function and outcomes in PPCM. [43]
Complications
Maternal complications of peripartum (postpartum) cardiomyopathy (PPCM) may include the following:
-
Hypoxia
-
Thromboembolism: Small case series have reported the incidence to be as high as 50%, but they are limited by selection bias
-
Progressive cardiac failure
-
Arrhythmias
-
Misinterpretation of hemodynamic data obtained from right-heart catheterization as a result of failure to consider the normal physiologic alterations of pregnancy (see the section on Cardiac Catherization and Invasive Hemodynamic Monitoring)
-
Inadequate treatment or testing because of exaggerated concern about the effect on the fetus
-
Misdiagnosis of preeclampsia: Patients with preeclampsia experience depletion of intravascular volume and should receive low doses of diuretics only when they have pulmonary edema
Fetal complications of PPCM may include the following:
-
Distress due to maternal hypoxia
-
Distress due to placental hypoperfusion as a result of poor cardiac output, maternal hypovolemia due to excessive diuresis, or hypotension from aggressive afterload reduction
-
Peripartum cardiomyopathy. This radiograph reveals noncardiogenic pulmonary edema in patient with preeclampsia, due to a capillary leak that can be a primary component of preeclampsia. Note the diffuse increase in lung markings without the cephalization or vascular redistribution seen in patients with pulmonary edema from systolic dysfunction. The patient had rapid clinical improvement after only 10 mg of intravenous furosemide.
-
Pericardial cardiomyopathy. Baseline (A) and follow-up (B) four-chamber view echocardiograms in a woman who developed cardiomyopathy 4 weeks after an elective cesarean delivery. Image A demonstrates a large thrombus (arrow) attached to the lateral wall of the left ventricle (LV) at baseline (A), which completely resolved after two months (B). The patient's younger sister also experienced peripartum cardiomyopathy. Courtesy of BioMed Central Ltd, Springer Nature (Meyer GP, Labidi S, Podewski E, et al. Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep. 2010 Mar 4;4:80. Online at https://jmedicalcasereports.biomedcentral.com/articles/10.1186/1752-1947-4-80).
-
Peripartum cardiomyopathy. These images were obtained in a young woman with peripartum cardiomyopathy and multiple left ventricular (LV) thrombi. (A) Apical five-chamber view on transthoracic echocardiography demonstrating a large layered echodense mass attached to the LV lateral wall consistent with thrombi. (B) Four-chamber view of a balanced steady-state free precession cardiac magnetic resonance imaging acquisition 4 days after anticoagulation with parenteral heparin demonstrating near resolution of the thrombus, leaving behind only the underlying muscular trabeculations on this view (arrows). RV = right ventricle. Courtesy of BioMed Central Ltd, Springer Nature (Altuwaijri WA, Kirkpatrick ID, Jassal DS, Soni A. Vanishing left ventricular thrombus in a woman with peripartum cardiomyopathy: a case report. BMC Res Notes. 2012 Oct 2;5:544. Online at https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-5-544).
Tables
What would you like to print?
- Overview
- Etiopathophysiology
- Epidemiology
- Prognosis
- Patient Education
- Presentation
- Differential Diagnosis
- Laboratory Studies
- Echocardiography
- Chest Radiography
- Magnetic Resonance Imaging
- Stress Testing
- Cardiac Catheterization and Invasive Hemodynamic Monitoring
- Tissue Analysis and Histologic Findings
- General Treatment Approach
- Pharmacologic Therapy
- Complications
- Show All
- Media Gallery
- References







