Practice Essentials
In 1951, Gardner described the occurrence of familial adenomatous polyposis (FAP) with the extracolonic manifestations of intestinal polyposis, desmoids, osteomas, and epidermoid cysts (ie, Gardner syndrome [1] ).
FAP, formerly known as familial polyposis coli (FPC) and hereditary adenomatosis of the colon and rectum, is an autosomal dominant condition caused by a mutation in the adenomatous polyposis coli tumor-suppressor gene (APC). [2, 3] The incidence of FAP is approximately 1 case per 7500 live births. In approximately 20% of patients with multiple colonic polyps, a family history of the disease is lacking, indicating that a spontaneous mutation of APC has occurred. [4]
The defining features of FAP are the presence of multiple adenomatous large bowel polyps in childhood and adolescence and the inevitable development of colorectal carcinoma.
There is an attenuated form of FAP in which patients develop fewer adenomas at a later age. One reason behind this change in the condition appears to be that the germline mutation responsible for the attenuated phenotype increases the likelihood that a further somatic mutation—one causing loss of function—will occur in the affected APC. The increased likelihood of mutation in the affected gene and the chance of sporadic mutation in the other APC of that cell eventually lead to the development of the attenuated phenotype.
In 1882, Cripps was the first to observe colorectal polyps in a familial setting. Dukes was the first to postulate that carcinomas of the colon and rectum are derived from adenomas. Following this, Jackson and Mayo were the first to describe the adenoma-to-carcinoma sequence in 1951.
FAP was first linked to extracolonic manifestations in 1923, when Nichols commented on the association between FAP and desmoid tumors. A number of other syndromes have since been described.
Patients with colorectal cancer and FAP appear to be more at risk for other cancers, including the cribriform variant of papillary thyroid cancer. In women younger than 35 years, the risk is 160 times greater than normal. Other malignant tumors include periampullary adenocarcinoma, hepatoblastoma, and multifocal cholangiocarcinomas.
Benign lesions that may be associated with FAP are desmoids, osteomas, epidermal cysts, and gastric fundic gland polyps.
The authors believe that the commonality among the different entities is colon polyps, with the extracolonic manifestations being determined by the location of the defect on the APC gene and the possible contribution of environmental factors. Inheritance of FAP is mendelian dominant.
The presence of colonic polyps carpeting the colon is an indication for surgical treatment. Prophylactic surgery is the only curative treatment. Choices are as follows:
-
Proctocolectomy with ileostomy
-
Proctocolectomy, mucosectomy, and ileal pouch–anal anastomosis (IPAA)
-
Total colectomy with ileorectal anastomosis (IRA)
Anatomy
The colon is approximately 1.5 m long, and the rectum is approximately 15 cm long. The cecum is normally 7-8 cm in diameter, whereas the sigmoid colon is 5 cm in diameter.
The colon is embryologically derived from the midgut and hindgut and has a close association with the yolk sac and cloaca. Because of the embryologic derivation of the midgut (ie, ampulla of Vater to middle of transverse colon) and hindgut (ie, middle of transverse colon to anus), the superior and inferior mesenteric arteries provide the colon's arterial supply.
The right colon is supplied by the middle colic artery (a branch of the superior mesenteric artery) and the right colic artery (a branch of the ileocolic artery). The left colon is supplied by the sigmoidal, left colic, and superior rectal branches of the inferior mesenteric artery. Collateral circulation is via the marginal artery of Drummond and the arc of Riolan or the meandering mesenteric artery.
Pathophysiology
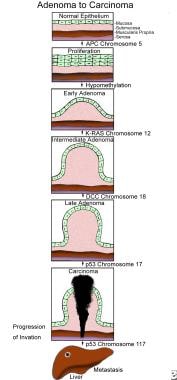
The APC gene located on chromosome 5 is the first mutation in the adenoma-to-carcinoma sequence and is believed to initiate the sequence. (See the image below.) APC is composed of 2844 codons and 8532 nucleotides arranged in 15 exons. The gene is altered by the same mechanism that alters many other genes: (1) nonsense or stop codon, (2) missense transitions, and (3) frameshift mutation due to the addition or deletion of one or several base pairs.
The point at which the APC mutation occurs determines the manifestations and severity of Gardner syndrome. Specific codon mutations correlate with specific extracolonic manifestations and the number, time frame, and malignant degeneration of adenomas. The most consistent description of a mutation on codon 1309 (exon 15) is associated with increased extracolonic manifestations, an increase in the number of polyps, a younger age at the onset of cancer, and the risk of cancer in prophylactically resected specimens.
FAP is generally characterized by the presence of more than 100 polyps in the colon, with a left-side predominance. The number of polyps can range from no detectable polyps at colonoscopy to more than 7000 observed on resected specimens. Patients with more than 1000 polyps have been proved to have 2.3 times the cancer risk of patients with fewer than 1000 polyps, independent of age. [5] The cancer risk increases 2.4 times for each 10-year time frame. The mutations in codons 233, 835, 1179, 1323, and 1407 also correspond to an increased number of polyps and, therefore, the increased risk of cancer.
However, it is not always the case that the presence of few or no polyps correlates the absence of cancer risk. Reports documented four individuals with a mutation only at codon 1962 who had colorectal cancer without any evidence of polyps. Although families that demonstrate a mutation at codon 1982 or 1983 show a significantly less severe form of FAP or Gardner syndrome, they develop fewer polyps later in life and thus have a later progression to cancer. [6]
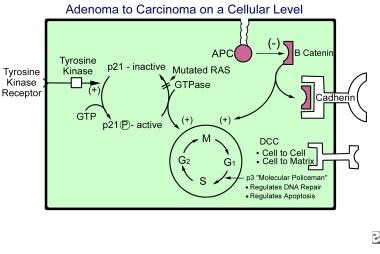
The initiating step in the adenoma-to-carcinoma sequence is the mutation in the APC gene that allows normal colonic epithelium to proliferate. [7] The epithelial cells increase in number, and the mucosa becomes thickened. A normal APC gene exerts its effects intracellularly by binding to beta-catenin. The actions of beta-catenins are twofold. First, they bind to cadherins, thus promoting cell adhesion and cell-to-cell interaction. Second, they send intracellular signals to the nucleus, promoting proliferation. [2]
The second step in the adenoma-to-carcinoma sequence is the loss of DNA methylation or hypomethylation. This results in the conversion of proliferating epithelium into an adenoma. The adenoma then undergoes further transformation to an intermediate adenoma by an RAS mutation and progresses to a late adenoma by the deletion of DCC.
RAS is located on chromosome 12 and is responsible for coding GTPase (an enzyme that hydrolyzes guanosine triphosphate [GTP] to guanosine diphosphate [GDP]), which is very important in intracellular signaling. GTPase is responsible for converting active p21 to inactive p21 by dephosphorylating the p21 protein. The p21 is converted to its active form by tyrosine kinase via phosphorylation, in the form of GTPase. Active p21 then stimulates the nucleus for the synthesis of DNA.
A mutated RAS gene unable to dephosphorylate p21 produces continuous stimulation to the nucleus by active p21, which causes the "on switch" to stay on. This overstimulation of the nucleus leads to an increased risk of mutation and, therefore, the progression of the adenoma (see the image below).
The deleted colon cancer gene (DCC) is located on chromosome 18 and is active at the cell surface. DCC is responsible for cell-to-cell and cell-to-matrix adhesion and works via contact inhibition. Accordingly, if DCC is deleted, contact inhibition is absent and the progression to a late adenoma occurs.
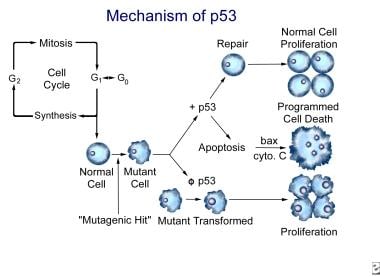
The TP53 gene, located on chromosome 17, is an antioncogene that is important in DNA repair and apoptosis (programmed cell death). TP53 is responsible for arresting the cell cycle between the G1 phase and the synthesis phase. When the cell has received a mutagenic hit, TP53 codes for the repair of DNA or codes for the cell to be destroyed via apoptosis.
Although TP53 does not actually perform apoptosis, it does regulate cell destruction. Apoptosis is initiated by cytochrome c of the mitochondria. The release of cytochrome c from the mitochondria is stimulated by the BAX gene, which opens channels in the mitochondria. These same channels are closed by the BCL2 gene formed by a translocation of chromosome 18 to chromosome 14.
TP53 has been coined the "molecular policeman" for its role in repairing mutant cells and destroying cells that cannot be repaired. When TP53 is mutated, the regulation of DNA repair and apoptosis is lost, and, therefore, cells with a mutagenic hit undergo transformation to malignant cells. Proliferation of these cells then follows as the cell cycle progresses (see the image below). This represents the final step in the adenoma-to-carcinoma sequence.
In a 2018 report that used whole‑exome sequencing of two affected individuals from a Chinese family with Gardner syndrome, Lv et al identified a mutL homolog (MLH)1 missense mutation, thereby adding to the mutated genes associated with Gardner syndrome. [8]
Etiology
The causes of FAP are as follows:
-
Mutation of APC, located on chromosome 5
-
Loss of DNA methylation
-
Mutation of RAS, located on chromosome 12
-
Deletion of DCC, located on chromosome 18
-
Mutation of TP53, located on chromosome 17
Epidemiology
The incidence of FAP is 1 case in 7500 live births and is due to congenital inheritance in a mendelian dominant fashion in 80% of patients. The remaining 20% represent spontaneous mutations, with no family history reported. The polyposis predominantly affects the left colon (80-90% of cases). In cases of attenuated FAP, a right-side predominance is often present.
Prognosis
The 5-year survival rate for patients older than 45 years who do not receive operative management is 0%. The 5-year survival rate for patients who undergo proctocolectomy and mucosectomy with ileal pouch–anal anastomosis (IPAA) is nearly 100%.
The recurrence rate in 20 years after total colectomy with ileorectal anastomosis (IRA) is 30%. The recurrence rate in 30 years after total colectomy with IRA is 45%.
Patient Education
The first objective of the surgeon is an informed and educated patient. Ensuring that the patient receives adequate unformation and education is the responsibility of the surgeon. The second objective is management decisions that are made jointly by the patient and the physician.
-
Adenoma-to-carcinoma sequence on a cellular level.
-
The mechanism of TP53 for normal function and for mutational effects.
-
Pictorial representation of the adenoma-to-carcinoma sequence.