Background
Schistosomiasis is a parasitic disease caused by flukes (trematodes) of the genus Schistosoma. After malaria and intestinal helminthiasis, schistosomiasis is the third most devastating tropical disease in the world, being a major source of morbidity and mortality for developing countries in Africa, South America, the Caribbean, the Middle East, and Asia. (See Epidemiology and Prognosis.) [1]
More than 140 million people, 90% of who live in Africa, are infected with schistosomiasis. [2, 3] An estimated 700 million people are at risk for infection in 76 countries where the disease is considered endemic, as their agricultural work, domestic chores, and recreational activities expose them to infested water. [1, 4] Globally, 200,000 deaths are attributed to schistosomiasis annually. [5] Transmission is interrupted in some countries. [4] (See Etiology and Epidemiology.)
The World Health Organization (WHO) estimates that about 220.8 million people required preventive treatment for schistosomiasis in 2017. An estimated 102.3 million people were treated the same year. [3]
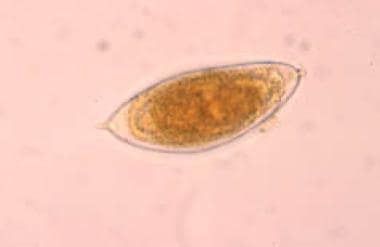
Sometimes referred to as bilharzias, bilharziasis, or snail fever, schistosomiasis was discovered by Theodore Bilharz, a German surgeon working in Cairo, who first identified the etiological agent Schistosoma hematobium in 1851. [6] A Schistosoma egg is seen below.
Most human schistosomiasis is caused by S haematobium, S mansoni, and S japonicum. Less prevalent species, such as S mekongi and S intercalatum, may also cause systemic human disease. Less importantly, other schistosomes with avian or mammalian primary hosts can cause severe dermatitis in humans (eg, swimmer's itch secondary to Trichobilharzia ocellata). (See Etiology.)
Over time transmission dynamics of schistosomiasis have changed. The eggs from human excreta and urine have been found in endemic water supplies. Cattle and other animals may have distinct schistosomes. Human and cattle both contaminate the water. This promotes mixing of different schistosomes. This has occurred in marshlands, leading to “hybrid” species of schistosomes, which have re-infected humans, usually from the same supply sources. [7]
Examples include the following:
-
The hybrid S haematobium- S guineenis species from 1996 in Cameroon. In 2003, a S mansoni-S rodhaini hybrid was found in snails, the intermediate host, in Kenya. With changes in the Senegal river basin and dams built on it, in 2009, S haematobium–S bovis hybrids were found in Senegal. [8]
-
Described from 2008 and published in a case report in 2019, a 14-year-old schoolboy from a Paris suburb visiting the Ivory Coast, his parents' native country, was found with hematuria and S haematobium–S mansoni hybrid infection. [9] Livestock and endemicity play a major role in schistosomiasis transmission. S haematobium, x S bovis, x S curassoni, and x S mattheei are other such species of cattle origin. Schistosomes are dioecous (ie, they have separate sexes).
Characteristics of schistosomiasis
Schistosomiasis is due to immunologic reactions to Schistosoma eggs trapped in tissues. Antigens released from the egg stimulate a granulomatous reaction involving T cells, macrophages, and eosinophils that results in clinical disease (see the image below). Symptoms and signs depend on the number and location of eggs trapped in the tissues. Initially, the inflammatory reaction is readily reversible. In the latter stages of the disease, the pathology is associated with collagen deposition and fibrosis, resulting in organ damage that may be only partially reversible. (See Pathophysiology, Etiology, and Presentation.)
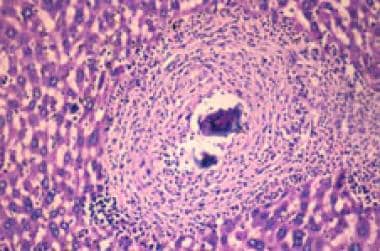 Granuloma in the liver due to Schistosoma mansoni. The S mansoni egg is at the center of the granuloma.
Granuloma in the liver due to Schistosoma mansoni. The S mansoni egg is at the center of the granuloma.
Eggs can end up in the skin, brain, muscle, adrenal glands, and eyes. As the eggs penetrate the urinary system, they can find their way to the female genital region and form granulomas in the uterus, fallopian tube, and ovaries. Central nervous system (CNS) involvement occurs because of embolization of eggs from the portal mesenteric system to the brain and spinal cord via the paravertebral venous plexus. [10, 11, 12]
Snail hosts
The different species of Schistosoma have different types of snails serving as their intermediate hosts; these hosts are as follows [13, 14, 15] :
-
Biomphalaria for S mansoni
-
Oncomelania for S japonicum
-
Tricula (Neotricula aperta) for S mekongi
-
Bulinus for S haematobium and S intercalatum
At-risk populations
Today, 120 million people are symptomatic with schistosomiasis, with 20 million having severe clinical disease. [1] More than 200,000 deaths per year are due to schistosomiasis in sub-Saharan Africa. [16] Women washing clothes in infested water are at risk. [17] Hygiene and playing in mud and water make children vulnerable to infection. Forty million women of childbearing age are infected. [18] Approximately 10 million women in Africa have schistosomiasis during pregnancy. [18] In endemic areas, the infection usually is acquired as a child. [4]
In Brazil and Africa, refugee movements and migration to urban areas are introducing the disease to new locations. Increasing population size and corresponding needs for power and water have led to increased transmission. Infections are not uniformly distributed within communities. It has been estimated that 5-10% of an endemic community may be heavily infected, and the remainder has mild to moderate infections. The risk for infection is highest amongst those who lived near lakes or rivers. [19] In Uganda, almost no transmission was found to have occurred at altitudes greater than 1400 m or where the annual rainfall was less than 900 mm. [19]
With the rise of tourism and travel, an increasing number of tourists are contracting it. Tourists often present with severe acute infection and unusual problems including paralysis.
The intensity and prevalence of infection rises with age and peaks usually between ages 15 and 20 years. In older adults, no significant change is found in the prevalence of disease, but the parasite burden or the intensity decreases. [13, 20, 21] The disease is not endemic in the United States.
Complications
Complications of schistosomiasis include the following:
-
Gastrointestinal (GI) bleeding
-
GI obstruction
-
Malnutrition
-
Schistosomal nephropathy
-
Renal failure
-
Pyelonephritis
-
Hematuria
-
Hemospermia
-
Squamous cell bladder cancer
-
Sepsis (Salmonella)
-
Pulmonary hypertension
-
Cor pulmonale
-
Neuroschistosomiasis - Transverse myelitis, paralysis, and cerebral microinfarcts
-
Infertility
-
Severe anemia
-
Low-birth-weight babies
-
Spontaneous abortion
-
Higher risk for ectopic pregnancies
-
End-organ disease
-
Portal hypertension
-
Obstructive uropathy
-
Pregnancy complications from vulvar or fallopian granuloma
-
Carcinoma of the liver, bladder, or gallbladder
Atypical schistosome eggs have been retrieved from children in Malawi. Molecular analysis has found hybrid schistosomes. Their analysis (eg, between Schistosoma haematobium and S bovis and S mattheel) reveals new hybrid species. Some atypical eggs have discordant genetic profiles. These factors indicate natural hybridization fron livestock; urogenital schistosomiasis control and epidemiology has changed due to these, and heightened urogenital and intestinal schistosomiasis surveillance are based on this. [22]
Subsaharan Africa has seen pulmonary schistosomiasis. All cases have been documented with CT scans. These may occur early, or late in disease. Patients have been exposed to multiple re-infections. Mostly they appear as ground glass or as nodules in the lungs. [23]
Pathophysiology
Acute schistosomiasis
Acute schistosomiasis (Katayama syndrome) is a systemic, serum sickness-like illness that develops after several weeks in some, but not most, individuals with new schistosomal infections. It may correspond to the first cycle of egg deposition and is associated with marked peripheral eosinophilia and circulating immune complexes. It is most common with S japonicum and S mansoni infections and is most likely to occur in heavily infected individuals after primary infection.
Symptoms usually resolve over several weeks, but the syndrome can be fatal. Early treatment with cidal drugs may exacerbate this syndrome and necessitate concomitant glucocorticoid therapy. Tourists and travelers are most likely to present to emergency departments (EDs) in the United States with this syndrome. A history of the patient’s contact with fresh water, such as through swimming, boating, rafting, or water skiing, should be obtained.
Mild, maculopapular skin lesions may develop in acute infection within hours after exposure to cercariae. Significant dermatitis is rare with the major human schistosomal pathogens, probably because the invading and developing cercariae are minimally immunogenic. However, abortive human infection with schistosomal species that rely on other primary hosts may cause marked dermatitis or swimmer's itch. This self-limited process may recur more intensely with subsequent exposures to the same species.
Chronic schistosomiasis
The pathology of chronic schistosomiasis, which is far more common than the acute form of the infection, results from egg-induced immune response, granuloma formation, and associated fibrotic changes. Although cercarial and adult worms are minimally immunogenic, schistosomal eggs are highly immunogenic and induce vigorous circulating and local immune responses. (Eggs may require an intense immune response to aid their migration through the body.) Adult worms can absorb host proteins. If not attacked by the immune system, they can live for years in the bloodstream as they are coated with host antigens.
Egg retention and granuloma formation in the bowel wall (usually S mansoni or S japonicum) may cause bloody diarrhea, cramping, and, eventually, inflammatory colonic polyposis. Patients with heavy bowel wall involvement have an increased rate of recurrent Salmonella infection, generally with positive blood cultures and negative stool cultures. Chronic intestinal schistosomiasis can present with acute complications of appendicitis, [24, 25] perforation, and bleeding long after travel-related (or endemic) exposure. Rectal perforation caused by S haematobium has also been described in a case report. [26]
Eggs can penetrate the bowel adjacent to mesenteric vessels where adult worms are residing. Unshed eggs, which are swept back to the portal circulation, lodge there and induce granulomatous reactions in the portal tracts.
Heavy infestations are more likely to produce hepatic disease. Eventually, severe periportal fibrosis in a characteristic pipestem pattern (Symmers pipestem fibrosis) may occur. Although hepatocellular function is spared, periportal fibrosis can lead to portal hypertension with the usual potential sequelae, including splenomegaly, ascites, esophageal variceal bleeding, and development of portosystemic collaterals. Through these collaterals (or directly from the inferior vena cava in the case of bladder wall schistosomiasis), eggs can reach the pulmonary circulation. The resulting pulmonary granulomatosis and fibrosis can lead to pulmonary hypertension and frank cor pulmonale with a high mortality rate.
In 1 series, pulmonary hypertension was found in 18.5% of patients with known hepatosplenic schistosomiasis. [27] Co-infection with hepatitis B or hepatitis C can accelerate hepatic dysfunction and raise the risk for hepatocellular carcinoma beyond that seen with hepatitis alone. In addition, gallbladder cancer may be associated with schistosomal infection.
Egg retention and granuloma formation in the urinary tract (S haematobium) can lead to hematuria, dysuria, bladder polyps and ulcers, and even obstructive uropathies. S haematobium infection is also associated with an increased rate of bladder cancer, usually squamous cell rather than transitional cell. [28]
Ectopic egg deposition can lead to additional clinical syndromes, including involvement of skin, lungs, brain, muscles, adrenal glands, genitalia, and eyes. CNS involvement can result in transverse myelitis (best described for S haematobium and S mansoni) and/or cerebral disease (most common with S japonicum infection). Local tissue invasion of eggs brings about the release of toxins and enzymes and provokes a TH-2–mediated immune response. [29]
Etiology
Human beings become infected with schistosomiasis when larval forms of the parasite, released by freshwater snails, penetrate their skin during contact with infested water. In the body, the larvae develop into adult schistosomes. Adult worms live in the blood vessels, where the females release eggs. Some of the eggs are passed out of the body in the feces or urine to continue the parasite life cycle. Others become trapped in body tissues, causing an immune reaction and progressive damage to organs.
Two major forms of schistosomiasis exist: intestinal and urogenital. These are caused by 5 main species, listed in Table 1.
Table 1. Parasitic Species and Geographical Distribution of Schistosomiasis [1, 13] (Open Table in a new window)
Species |
Geographical distribution |
|
Intestinal schistosomiasis |
Schistosoma mansoni (mesenteric venules of the colon) |
Africa, the Middle East, the Caribbean, and South America |
Schistosoma japonicum (mesenteric venules of the small intestine) |
Asia only: China, Indonesia, the Philippines, and Thailand |
|
Schistosoma mekongi (mesenteric venules of the small intestine) |
Several districts of Cambodia and the Lao People’s Democratic Republic. 200-km area of Mekong river basin; now extending toward northern provinces |
|
Schistosoma intercalatum (mesenteric venules of the colon) and related S guineensis |
Rain forest areas of Central and West Africa |
|
Urogenital schistosomiasis |
Schistosoma haematobium (vesical venous plexus) |
Africa, the Middle East, India, and Turkey |
Life cycle
The geographic distribution and etiology of schistosomiasis reflect the unique life cycle of Schistosoma species. Schistosomes infect susceptible freshwater snails in endemic areas, usually with specific species of schistosomes infecting specific species of snails. The infected snails release cercariae 4-6 weeks after infection. The cercariae are fork-tailed, free-swimming larvae approximately 1mm in length. They can survive in fresh water up to 72 hours, during which time they must attach to human skin or to that of another susceptible host mammal or die.
Successful cercariae attach to human hosts, utilizing oral and ventral suckers. They then migrate through intact skin to dermal veins and, over the next several days, to the pulmonary vasculature. During this migration, the cercariae metamorphose, shedding tails and outer glycocalyces while developing double-lipid-bilayer teguments that are highly resistant to host immune responses.
The organisms, now called schistosomula, incorporate host proteins, including major histocompatibility complexes (MHCs) and blood group antigens, into their integuments. Their metabolism shifts to glycolysis. The worms then migrate through the pulmonary capillaries to the systemic circulation, which carries them to the portal veins, where they mature. The adult worms are small, 12-26mm long and 0.3-0.6mm wide, and vary with the different species.
Within the portal vasculature, male and female adults pair off, with the thin female entering and remaining in the gynecophoric canal of the stockier 8mm male worm. Together they migrate along the endothelium, against portal blood flow, to the mesenteric (S mansoni, S japonicum) or vesicular (S haematobium) veins, where they begin to produce eggs.
The microscopic appearance of the egg allows diagnostic differentiation of the 5 species. An adult S haematobium produces 20-200 round, terminally spined eggs per day (see the image below); S mansoni produces 100-300 ovoid, laterally spined eggs per day; and S japonicum produces 500-3500 small, round, laterally spined eggs per day. The eggs of S intercalatum have prominent, terminal spines, and those of S mekongi have small, lateral spines.
The eggs, which are highly antigenic and can induce an intense granulomatous response, migrate through the bowel or bladder wall to be shed via feces or urine. During this time (approximately 10d), the organisms begin to mature into miracidia.
Eggs that are not shed successfully may remain in the tissues or be swept back to the portal circulation (from the mesenteric vessels) or to the pulmonary circulation (from the vesicular vessels via the inferior vena cava). Eggs can end up in the skin, brain, muscle, adrenal glands, and eyes. As the eggs penetrate the urinary system, they can find their way to the female genital region and form granulomas in the uterus, fallopian tube, and ovaries. CNS involvement occurs because of embolization of eggs from the portal mesenteric system to the brain and spinal cord via the paravertebral venous plexus. [10, 11]
The free-swimming miracidia that are shed into fresh water survive 1-3 weeks, during which time they must infect a susceptible snail to complete the life cycle. Within the infected snail, 2 generations of sporocysts multiply, mature into free-swimming cercariae, and exit the snail to seek a human host and begin a new cycle.
In the case of S japonicum, which may be the species with the highest risk for complications, the life cycle may include domesticated animals (eg, cattle) and wild animals (including rodents). This creates a complex and durable reservoir of disease that often thwarts control efforts based on treating human infection and reducing snail populations.
Epidemiology
Occurrence in the United States
Acute and chronic schistosomiasis infections are not common in the United States. Although it is estimated that 400,000 infected persons have immigrated to this country, neither susceptible snail species nor chronically infected human reservoirs sufficient to infest fresh water exist. However, pathogenic schistosomes can survive and replicate in human hosts for years and even decades. Therefore, persons who have traveled or immigrated may present to EDs with active cases of acute or chronic schistosomiasis and/or associated end-organ complications. Most infected patients remain asymptomatic. Acute symptoms are more common in nonimmune travelers. This is because of a severe immune response following exposure.
International occurrence
Globally, schistosomiasis is a major source of morbidity and mortality. The unique schistosomal life cycle limits endemic areas to tropical and subtropical zones, but these areas exist around the world and may even increase with some agricultural practices. Although freshwater lakes and streams are usually identified as the source of the disease, man-made reservoirs and irrigation systems are increasingly implicated in some countries. Indeed, geographic spread continues because of water resource engineering issues in developing countries and the migration of infected populations.
Intestinal schistosomiasis caused by S mansoni occurs in 52 nations, including Caribbean countries (ie, Saint Lucia, Antigua, Montserrat, Martinique, Guadeloupe, Dominican Republic, Puerto Rico), eastern Mediterranean countries, South American countries (ie, Brazil, Venezuela, Surinam), and most countries in Africa. [30]
Other Schistosoma species that can cause intestinal symptoms and diseases include S intercalatum, S japonicum, and S mekongi.S intercalatum is found in 10 countries within the rain forests of central Africa. S japonicum is endemic in 4 countries in the western Pacific region (ie, China, Philippines, Indonesia, Thailand). S mekongi infection occurs in the Mekong River area of Southeast Asia (ie, Kampuchea, Laos, Thailand). Urinary schistosomiasis caused by S haematobium affects 54 countries in Africa and the eastern Mediterranean.
More than 207 million people in at least 74 countries have active schistosomal infection. [31] Of this population, approximately 60% have disease symptoms, including organ-specific complaints and problems related to chronic anemia and malnutrition from the infection; more than 20 million are severely ill. Disease prevalence is heterogeneous in vulnerable locales and tends to be worse in areas with poor sanitation, increased freshwater irrigation usage, and heavy schistosomal infestation of human, animal, and/or snail populations.
However, targeted interventions combining snail control, improved water supply quality, and treatment of infected persons, particularly children, have shown success in diverse endemic areas, including China, Brazil, Egypt, and some areas of sub-Saharan Africa. Interventions based on treating infections annually in humans and domesticated animals while controlling snail populations have decreased disease burden in some areas by an order of magnitude in the last 50 years, but these efforts have plateaued.
A controlled study of enhanced community intervention performed in rural Chinese villages demonstrated significant improvements in human, snail, and wild mouse schistosomiasis infection rates. Along with the preexisting programs for the annual treatment of farmers and cattle, efforts were made to optimize animal grazing sites, sewage management, drinking water supplies, and health education with regard to schistosomiasis. [32, 33]
According to the WHO, the global distribution of schistosomiasis has changed in that it has been eradicated from Japan and the Lesser Antilles islands; transmission has been stopped in Tunisia; and transmission is very low in Morocco, Saudi Arabia, Venezuela, and Puerto Rico.
Nevertheless, the human cost of schistosomal infections remains high, and the disease contributes to comorbidity with other infections, including hepatitis, human immunodeficiency virus (HIV), and malaria, in endemic regions. [34]
In China, despite mutifaceted control over decades indirectly transmitted Scistosoma japonicum remains endemic because of animal resorvoirs. [35] Host samples were tested for miracidia hatching, in cattle, goats, pigs, dogs, and cats. Intermediate host snails were also tested. Water buffalo, for example, maintained transmission in marshland, and removal of these could achieve local elimination of transmission. [35]
Race-related demographics
The frequency of infection among individuals of specific races is based on the geographic distribution of endemic schistosomiasis in large tropical and subtropical regions of Africa, Asia, the Middle East, and the Caribbean. However, all humans appear equally susceptible if exposed to infested fresh water.
The frequency of some complications appears to vary geographically during infection with the same worm species (eg, ascites is more common in the Middle East than in Brazil).
Sex-related demographics
Schistosomiasis is more common in males, most likely because of increased exposure to infected water via bathing, swimming, and agricultural activities. S haematobium causes genital lesions in 30% of women who are infected. Vulval lesions may increase the risk for HIV transmission.
Age-related demographics
The prevalence and severity of schistosomal infections vary with age. Children and adolescents are infected most often and are infested most heavily. Infection rates and severity may vary with gender-specific activity at all ages. (Congenital infection has been defined; Schistosomiasis has been detected in the placenta, and newborns have been diagnosed with the disease, thus confirming congenital infection. [6, 36]
Globally, infections peak in individuals aged 10-19 years. In some areas, the prevalence in this group may approach 100%.
In persons older than age 19 years living in endemic areas, the prevalence of active infection and egg counts slowly declines. (End-stage complications may persist or worsen.) This decline in active infection may reflect that individuals have an increasing host immune response or a decreasing exposure to contaminated water as they age. Reinfection, particularly after high exposure, is possible.
Age distribution differs somewhat in infected travelers, with young adults most likely to be exposed and infected.
Prognosis
Early disease usually improves with treatment. Surprisingly, patients with hepatic and urinary disease, even with fibrosis, may improve significantly over months or years following therapy. Renal and intestinal pathology also improves with treatment, as, usually, do brain lesions (depending on their location and size). [37]
Hepatosplenic schistosomiasis carries a relatively good prognosis because hepatic function is preserved until the end of the disease (unless variceal bleeding occurs).
Although treatment is indicated for patients with end-stage complications of portal hypertension and severe pulmonary hypertension, these patients are much less likely to benefit from it. Indeed, cor pulmonale usually does not improve significantly with treatment.
Spinal cord schistosomiasis carries a guarded prognosis. Praziquantel should be administered as soon as possible. Praziquantel can safely be given to pregnant and lactating individuals; it decreases the disease burden and improves pregnancy and fetal outcomes. Programs targeting women during reproductive years will improve their reproductive life and well-being. [38, 37]
Co-infection of schistosomiasis along with hepatitis, HIV, and malaria can raise the risk for hepatocellular carcinoma and increase the risk for mortality.
Patients with heavier worm burdens are less likely to improve and are more likely to require re-treatment.
Morbidity and mortality
Acute schistosomiasis is associated with a mortality rate of up to 25% in some series. Although most individuals with chronic schistosomiasis have few or no symptoms, significant morbidity can develop.
Complaints are difficult to quantitate because of the geographic distribution of this infection in developing nations and the frequency of comorbid conditions such as viral hepatitis. Hepatosplenic disease with portal hypertension is the most common long-term, serious outcome, followed by cardiopulmonary involvement, obstructive nephropathy, bacteremia, and malignancy. Female genital infection can contribute to pregnancy complications, including reports of related ectopic pregnancy. Urogenital schistosomiasis is considered to be a risk factor for HIV infection, especially in women. [1]
End-stage hepatosplenic disease with variceal bleeding, pulmonary hypertension with cor pulmonale, and central nervous system disease are associated with high mortality rates. Carcinoma of the urinary tract, liver, and gallbladder may cause death. Although effective antihelminthic treatment exists, it may not reverse fibrosis and may not be readily available in endemic areas.
Reinfection is extremely common in persons who live in, or return to, endemic areas. Repetitive treatment is necessary to prevent disease progression in this situation.
Acute schistosomiasis (Katayama syndrome)
Tissue migration of schistosomal larvae may cause a hypersensitivity reaction. Any species of Schistosoma can cause it. Although most clinical manifestations are benign, some are severe and may require hospitalization. If acute schistosomiasis is not suspected clinically and treated appropriately, it can result in severe morbidity or death. Nonimmune travelers are especially prone to this disease manifestation. After a single exposure to a freshwater pond in Tanzania, 86% of tourists developed acute schistosomiasis. Symptoms include cough, fever, and fatigue. [39] Symptoms usually appear 2-12 weeks after exposure.
Chronic schistosomiasis
Most patients are asymptomatic or mildly symptomatic and do not require medical attention. Only a small proportion of an endemic population harbors a heavy worm burden that later leads to clinical complications.
Gastrointestinal schistosomiasis
The most common complication of GI schistosomiasis is periportal fibrosis, also termed Symmers pipestem fibrosis. This leads to portal hypertension and GI hemorrhage. Liver failure is uncommon, except in persons with concomitant chronic hepatitis or cirrhosis. Among persons with S mansoni, S japonicum, and, possibly, S mekongi, 4-8% develop hepatosplenic disease. [27]
People co-infected with either hepatitis B or C and S mansoni have been shown to have rapid progression of liver disease.
Urinary tract schistosomiasis
This can lead to renal failure due to obstructive uropathy, pyelonephritis, or bladder carcinoma (occurring usually 10-20y after the initial infection). In addition, immune complexes that contain worm antigens may deposit in the glomeruli, leading to glomerulonephritis and amyloidosis.
Female genital schistosomiasis
S haematobium causes lesions in the female lower genital tract (ie, cervix, valva, vagina). Female genital schistosomiasis has been identified as a major social and medical problem that may facilitate the spread of some sexually transmitted diseases, such as HIV and human papillomavirus (HPV). [40]
Coexistence of sexually transmitted infection and urogenital schistosomiasis
One study found that among women with S haematobium infection in Madagascar, 35% may have co-existing sexually transmitted infections, such as Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, or Trichomonas vaginalis, as compared with 17% of the men. This is found to be more common in younger populations (aged 15-24 y) than in older populations. The association became stronger with greater parasite burden. [41] Increased HIV replication and cytokine dysregulation occur when schistosomiasis is present in HIV-positive individuals. In patients on antiretroviral treatment, immune restoration syndrome has been described with symptomatic schistosomiasis.
Pulmonary arterial hypertension in schistosomiasis
This is an important complication that develops in about 7.7% of patients with hepatosplenic disease in S mansoni, S japonicum, and possibly S mekongi infections. [27] The prevalence of the disease worldwide is estimated to exceed 270,000 individuals. [27]
CNS schistosomiasis
Because of its smaller egg size, S japonicum causes 60% of all Schistosoma brain infections, [42] with CNS involvement occurring in 2-4% of all S japonicum infections. One million people in China are estimated to be infected with S japonicum. [43] Nodular, enhancing cerebellar lesions can occur as well with this species. [43]
However, CNS schistosomiasis can also occur with other species. Spinal schistosomiasis usually presents as transverse myelitis and is primarily due to S mansoni infection because of the larger egg size. [42] S haematobium can infect the brain or spinal cord. [42]
The distribution of S mekongi is limited to the Mekong River basin in Laos and Cambodia, where some 140,000 people are estimated to be at risk for this infection. Temporal mass causing paraesthesias of the arm and leg with dysphasia has been described with S mekongi infection. [11] Neurologic symptoms can develop months after the infection. Cauda equina syndrome, anterior spinal artery syndrome, and quadriparesis can occur. Most of the lower spinal cord is affected. [42]
Schistosomiasis in pregnancy
This has been associated with anemia and low birth weight. [18]
Schistosomiasis in HIV
As an anecdote, in a study among 674 men aged 18-50 years in rural Tanzania between 2014-2016, 63.6 % had schistosome infection identified by schistosome circulating antigen, or parasitic eggs in urine or stool or both. The odds of HIV infection was 1.3 in those with any schistosome infection and 1.4 in men with S hematobium infection. The researchers concluded that schistosome infection does not appear to have any association with HIV infection in men. [44]
-
Egg of Schistosoma hematobium, with its typical terminal spine.
-
Granuloma in the liver due to Schistosoma mansoni. The S mansoni egg is at the center of the granuloma.