Practice Essentials
Atrial flutter is a cardiac arrhythmia characterized by atrial rates of 240-400 beats/min, usually with some degree of atrioventricular (AV) node conduction block. For the most part, morbidity and mortality are due to complications of rate (eg, syncope and congestive heart failure [CHF]). See the image below.
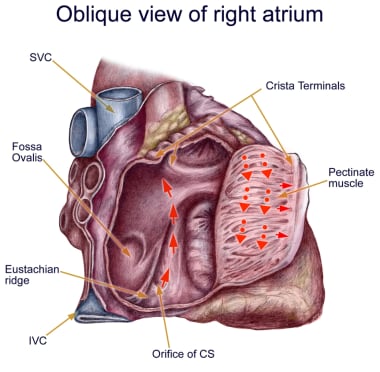 Anatomy of classic counterclockwise atrial flutter. This image demonstrates an oblique view of the right atrium and shows some crucial structures. The isthmus of tissue responsible for atrial flutter is seen anterior to the coronary sinus (CS) orifice. The eustachian ridge is part of the crista terminalis that separates the roughened part of the right atrium from the smooth septal part of the right atrium. IVC = inferior vena cava; SVC = superior vena cava.
Anatomy of classic counterclockwise atrial flutter. This image demonstrates an oblique view of the right atrium and shows some crucial structures. The isthmus of tissue responsible for atrial flutter is seen anterior to the coronary sinus (CS) orifice. The eustachian ridge is part of the crista terminalis that separates the roughened part of the right atrium from the smooth septal part of the right atrium. IVC = inferior vena cava; SVC = superior vena cava.
Signs and symptoms
Signs and symptoms in patients with atrial flutter typically reflect decreased cardiac output as a result of the rapid ventricular rate. Typical symptoms include the following:
-
Palpitations
-
Fatigue or poor exercise tolerance
-
Mild dyspnea
-
Presyncope
Less common symptoms include angina, profound dyspnea, or syncope. Tachycardia may or may not be present, depending on the degree of AV block associated with the atrial flutter activity.
Physical findings include the following:
-
The heart rate is often approximately 150 beats/min because of a 2:1 AV block
-
The pulse may be regular or slightly irregular
-
Hypotension is possible, but normal blood pressure is more commonly observed
Other points in the physical examination are as follows:
-
Palpate the neck and thyroid gland for goiter
-
Evaluate the neck for jugular venous distention
-
Auscultate the lungs for rales or crackles
-
Auscultate the heart for extra heart sounds and murmurs
-
Palpate the point of maximum impulse on the chest wall
-
Assess the lower extremities for edema or impaired perfusion
If embolization has occurred from intermittent atrial flutter, findings are related to brain or peripheral vascular involvement. Other complications of atrial flutter may include the following:
-
Embolization (arterial)
-
CHF
-
Severe bradycardia
-
Myocardial rate–related ischemia
See Presentation for more detail.
Diagnosis
The following techniques aid in the diagnosis of atrial flutter:
-
Electrocardiography (ECG): This is an essential diagnostic modality for atrial flutter
-
Vagal maneuvers: These can be helpful in determining the underlying atrial rhythm if flutter waves are not seen well
-
Adenosine: This drug can be helpful in making the diagnosis of atrial flutter by transiently blocking the AV node
-
Exercise testing: This can be utilized to identify exercise-induced atrial fibrillation and to evaluate the response of the ventricular rate to activity, as well as its interaction with symptoms including those of ischemic heart disease
-
Holter monitor: This can be used to help identify arrhythmias in patients with nonspecific symptoms, to identify triggers, and to detect associated atrial arrhythmias
Transthoracic echocardiography (TTE) is the preferred initial imaging modality for evaluating atrial flutter. It can evaluate right and left atrial size, as well as the size and function of the right and left ventricles, and this information facilitates diagnosis of valvular heart disease, left ventricular hypertrophy (LVH), and pericardial disease.
See Workup for more detail.
Management
General treatment goals for symptomatic atrial flutter are similar to those for atrial fibrillation. They include the following:
-
Control of ventricular rate: This can be achieved with drugs that block the AV node; intravenous (IV) calcium channel blockers (eg, verapamil and diltiazem) or beta blockers can be used, followed by initiation of oral agents. Concurrent control of resting and activity-associated rates is generally required, and may be difficult to achieve.
-
Restoration of sinus rhythm: This can be achieved by means of electrical or pharmacologic cardioversion or radiofrequency ablation (RFA); successful ablation reduces or eliminates the need for long-term anticoagulation and antiarrhythmic medications
-
Prevention of recurrent episodes or reduction in their frequency or duration: In general, the use of antiarrhythmic drugs in atrial flutter is similar to that in atrial fibrillation
-
Prevention of thromboembolic complications: Adequate anticoagulation, as recommended by the American College of Chest Physicians, has been shown to decrease thromboembolic complications in patients with chronic atrial flutter and in patients undergoing cardioversion
-
Minimization of adverse effects from therapy: Because atrial flutter is a nonfatal arrhythmia, carefully assess the risks and benefits of drug therapy, especially with antiarrhythmic agents
See Treatment and Medication for more detail.
Background
Atrial flutter is a cardiac arrhythmia characterized by atrial rates of 240-400 beats/min, usually with some degree of atrioventricular (AV) node conduction block. In the most common form of atrial flutter (typical atrial flutter), electrocardiography (ECG) demonstrates a negative sawtooth pattern in leads II, III, and aVF.
Typical (or classic) atrial flutter involves a single reentrant circuit with circus activation in the right atrium around the tricuspid valve annulus. The electrical wavefront most often propagates in a counterclockwise direction. Atypical atrial flutter follows a different circuit; it may involve the right or the left atrium. (See Pathophysiology.)
Atrial flutter is associated with a variety of cardiac disorders. In most studies, approximately 60% of patients with atrial flutter have coronary artery disease (CAD) or hypertensive heart disease; 30% have no underlying cardiac disease. Uncommon forms of atrial flutter have been noted during long-term follow-up in as many as 26% of patients with surgical correction of congenital cardiac anomalies. (See Etiology.)
Symptoms in patients with atrial flutter typically reflect decreased cardiac output as a result of the rapid ventricular rate. The most common symptom is palpitations. Other symptoms include fatigue, dyspnea, and chest pain. (See Presentation.) ECG is essential in making the diagnosis. Transthoracic echocardiography (TTE) is the preferred initial imaging modality for evaluating atrial flutter. (See Workup.)
Intervening to control the ventricular response rate or to return the patient to sinus rhythm is important. Consider immediate electrical cardioversion for patients who are hemodynamically unstable. Consider catheter-based ablation as first-line therapy in patients with typical atrial flutter if they are reasonable candidates. Ablation is usually performed as an elective procedure; however, it can also be performed when the patient is in atrial flutter. (See Treatment.)
Atrial flutter is similar to atrial fibrillation in many respects (eg, underlying disease, predisposing factors, complications, and medical management), and some patients have both atrial flutter and atrial fibrillation. However, the underlying mechanism of atrial flutter makes this arrhythmia amenable to cure with percutaneous catheter-based techniques.
Pathophysiology
In humans, the most common form of atrial flutter (typical) involves a single reentrant circuit with circus activation in the right atrium around the tricuspid valve annulus (most often in a counterclockwise direction), with an area of slow conduction located between the tricuspid valve annulus and the coronary sinus ostium (subeustachian isthmus). A 3-dimensional electroanatomic map of typical atrial flutter is shown in the video below.
Animal models have been used to demonstrate that an anatomic block (surgically created) or a functional block of conduction between the superior vena cava and the inferior vena cava, similar to the crista terminalis in the human right atrium, is key to initiating and maintaining the arrhythmia.
The crista terminalis acts as another anatomic conduction barrier, similar to the line of conduction block between the two venae cavae required in the animal model. The orifices of both venae cavae, the eustachian ridge, the coronary sinus orifice, and the tricuspid annulus complete the barrier for the reentry circuit (see the image below). Typical atrial flutter is often referred to as isthmus-dependent flutter. The rhythm is due to macroreentry, there is an excitable gap, and the rhythm can be entrained.
 Anatomy of classic counterclockwise atrial flutter. This image demonstrates an oblique view of the right atrium and shows some crucial structures. The isthmus of tissue responsible for atrial flutter is seen anterior to the coronary sinus (CS) orifice. The eustachian ridge is part of the crista terminalis that separates the roughened part of the right atrium from the smooth septal part of the right atrium. IVC = inferior vena cava; SVC = superior vena cava.
Anatomy of classic counterclockwise atrial flutter. This image demonstrates an oblique view of the right atrium and shows some crucial structures. The isthmus of tissue responsible for atrial flutter is seen anterior to the coronary sinus (CS) orifice. The eustachian ridge is part of the crista terminalis that separates the roughened part of the right atrium from the smooth septal part of the right atrium. IVC = inferior vena cava; SVC = superior vena cava.
Typical counterclockwise atrial flutter has caudocranial activation (ie, activation counterclockwise around the tricuspid valve annulus when viewed in the left antero-oblique fluoroscopic view) of the atrial septum (see the image below).
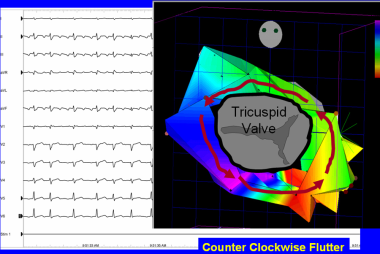 Typical counterclockwise atrial flutter. This 3-dimensional electroanatomic map of a tricuspid valve and right atrium shows the activation pattern displayed in color format. Red is early and blue is late, relative to a fixed point in time. Activation travels in counterclockwise direction.
Typical counterclockwise atrial flutter. This 3-dimensional electroanatomic map of a tricuspid valve and right atrium shows the activation pattern displayed in color format. Red is early and blue is late, relative to a fixed point in time. Activation travels in counterclockwise direction.
Typical atrial flutter can also have the opposite activation sequence (ie, clockwise activation around the tricuspid valve annulus). Clockwise atrial flutter is much less common. When the electric activity moves in a clockwise direction, the electrocardiogram (ECG) will show positive flutter waves in leads II, III, and aVF, and they may appear somewhat sinusoidal. This arrhythmia is still considered typical, isthmus-dependent flutter; it is usually called reverse typical atrial flutter.
Atypical atrial flutters are less extensively studied and electroanatomically characterized. Atypical atrial flutters may originate from the right atrium, as a result of surgical scars (ie, incisional reentry), or from the left atrium, specifically the pulmonary veins (ie, focal reentry) or mitral annulus (see the image below). Left atrial flutter is common and often problematic after left atrial linear ablation procedures (for atrial fibrillation). Thus, tricuspid isthmus dependency is not a prerequisite for atypical atrial flutter.
Etiology
Atrial flutter is associated with a variety of cardiac disorders. In most studies, approximately 30% of patients with atrial flutter have coronary artery disease, 30% have hypertensive heart disease, and 30% have no underlying cardiac disease. Rheumatic heart disease, congenital heart disease, pericarditis, and cardiomyopathy may also lead to atrial flutter. Rarely, mitral valve prolapse or acute myocardial infarction has been associated with atrial flutter.
In addition, the following conditions are also associated with atrial flutter:
-
Hypoxia
-
Chronic obstructive pulmonary disease
-
Pulmonary embolism
-
Hyperthyroidism
-
Pheochromocytoma
-
Diabetes
-
Electrolyte imbalance
-
Alcohol consumption
-
Obesity
-
Myotonic dystrophy in childhood (rare) [1]
Atrial flutter may be a sequela of open heart surgery. After cardiac surgery, atrial flutter may be reentrant as a result of natural barriers, atrial incisions, and other cardiac scars. Some patients develop atypical left atrial flutter after pulmonary vein isolation procedures for atrial fibrillation.
Although there are no clearly defined genetic conditions that cause atrial flutter, in many cases there is likely an underlying genetic susceptibility to acquiring it. Genome-wide association studies have identified genes associated with atrial flutter. [2]
The PITX2 (paired-like homeodomain 2) gene on chromosome locus 4q25 is known to play a major role in left-right asymmetry of the heart and has been found to have a strong association with atrial fibrillation [3] and an even stronger association with typical atrial flutter. [4] There are not yet any clinically available genetic tests that can identify persons at increased risk for atrial flutter.
Epidemiology
United States statistics
Atrial flutter is much less common than atrial fibrillation. Of the patients admitted to US hospitals with a diagnosis of supraventricular tachycardia between 1985 and 1990, 77% had atrial fibrillation and 10% had atrial flutter. On the basis of a study of patients referred to tertiary care centers, the incidence of atrial flutter in the United States is estimated to be approximately 200,000 new cases per year. [5]
Sex- and age-related demographics
In a study of 100 patients with atrial flutter, 75% were men. In another study performed at a tertiary care study, atrial flutter was 2.5 times more common in men.
Patients with atrial flutter, as with atrial fibrillation, tend to be older adults. In one study, the average age was 64 years. The prevalence of atrial fibrillation increases with age, as follows:
-
25-35 years: 2-3 cases per 1000 population
-
55-64 years: 30-90 cases per 1000 population
-
65-90 years: 50-90 cases per 1000 population
Prognosis
The prognosis for atrial flutter depends on the patient’s underlying medical condition. Any prolonged atrial arrhythmia can cause a tachycardia-induced cardiomyopathy. Intervening to control the ventricular response rate or to return the patient to sinus rhythm is important. Thrombus formation in the left atrium has been described in patients with atrial flutter (0-21%). Thromboembolic complications have also been described. [6]
Because of the conduction properties of the atrioventricular (AV) node, many people with atrial flutter will have a faster ventricular response than those with atrial fibrillation. The heart rate is often more difficult to control with atrial flutter than with atrial fibrillation, because of increased concealed conduction in those with atrial fibrillation.
For the most part, morbidity and mortality result from complications of rate (eg, syncope and congestive heart failure [CHF]). In patients with atrial flutter, the risk of embolic occurrences approaches that seen in atrial fibrillation. Patients with Wolff-Parkinson-White syndrome who develop atrial flutter can develop life-threatening ventricular responses and therefore should be considered for catheter ablation of their accessory bypass tract.
Data from the Framingham study suggest that patients with atrial fibrillation do not live as long as patients without atrial fibrillation (ie, control subjects). No data are available on atrial flutter.
The prognosis for patients with typical atrial flutter who undergo catheter ablation is excellent, with a very low recurrence rate. The picture is not as clear for patients with both atrial flutter and atrial fibrillation. Some reports have documented fewer episodes of atrial fibrillation after successful flutter ablation; others have not. It is possible that atrial fibrillation may be more responsive to antiarrhythmic agents after atrial flutter has been eliminated.
Bohnen et al performed a prospective study to assess the incidence and predictors of major complications from contemporary catheter ablation procedures. [7] Major complication rates ranged from 0.8% (supraventricular tachycardia, applicable to ablation of typical atrial flutter) to 6% (ventricular tachycardia associated with structural heart disease), depending on the ablation procedure performed. Renal insufficiency was the only independent predictor of a major complication.
Numerous reports indicate that patients with atrial fibrillation who are given class IC antiarrhythmic agents may convert to atrial flutter with faster ventricular rates, even if the flutter rate is "relatively slow." Thus, patients receiving type IC agents (eg, flecainide) should also receive an AV node−blocking drug such as a beta blocker or calcium channel blocker. In patients with atrial flutter in one study, the relative risk for development of stroke was 1.4 in comparison to control subjects. [8]
A multicenter report by Stiell et al that prospectively evaluated management and 30-day outcomes in 1091 emergency department (ED) patients with recent-onset atrial fibrillation (84.7%) or atrial flutter (15.3%) found that oral anticoagulants were underprescribed and that patients discharged from the ED in sinus rhythm had a smaller likelihood of experiencing an adverse event. [9] More than 10% of all patients had adverse events within 30 days, including one case of stroke, but no deaths were reported. Potential risk factors for adverse events included longer duration from arrhythmia onset, radiographic evidence of pulmonary congestion, previous history of stroke/transient ischemic attack, and ED discharge without being in sinus rhythm. [9]
Patients with concurrently diagnosed new rapid atrial fibrillation or atrial flutter and new reduced left ventricular ejection fraction (LVEF) appear to have a high rate prevalence of left atrial appendage thrombi (LAAT). [10] However, the presence of LAAT does not appear to be prognostic for eventual improvement.
Patient Education
Educating patients regarding medications and diet is important. Patients taking warfarin should avoid making major changes in their diet until they have consulted with their healthcare providers. Specifically, a sudden change in the consumption of green leafy vegetables, which are sources of vitamin K, can affect coagulation in patients taking warfarin, which inhibits vitamin K synthesis. This education is not needed with newer drugs that avoid these drug-drug or drug-food interactions, but these drugs lack the monitoring that warfarin can provide.
For patient education information, see the Heart Health Center, as well as Atrial Flutter, Arrhythmias (Heart Rhythm Disorders), Stroke, Supraventricular Tachycardia (SVT, PSVT), and Palpitations.
-
Anatomy of classic counterclockwise atrial flutter. This image demonstrates an oblique view of the right atrium and shows some crucial structures. The isthmus of tissue responsible for atrial flutter is seen anterior to the coronary sinus (CS) orifice. The eustachian ridge is part of the crista terminalis that separates the roughened part of the right atrium from the smooth septal part of the right atrium. IVC = inferior vena cava; SVC = superior vena cava.
-
Typical counterclockwise atrial flutter. This 3-dimensional electroanatomic map of a tricuspid valve and right atrium shows the activation pattern displayed in color format. Red is early and blue is late, relative to a fixed point in time. Activation travels in counterclockwise direction.
-
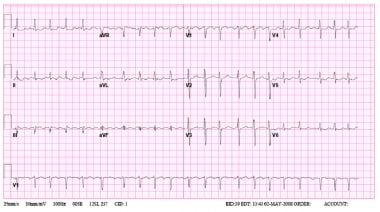
A 12-Lead electrocardiogram of typical atrial flutter. Note the negative sawtooth pattern of the flutter waves in leads II, III, and aVF.
-
Electrocardiogram of atypical left atrial flutter.
-
3-Dimensional electroanatomic map of typical atrial flutter. Colors progress from blue to red to white and represent the relative conduction time in the right atrium (early to late). An ablation line (red dots) has been created on the tricuspid ridge extending to the inferior vena cava. This ablation line interrupts the flutter circuit. CSO = coronary sinus os; IVC = inferior vena cava; RAA = right atrial appendage; TV = tricuspid valve annulus.
-
Rhythm strips demonstrating typical atrial flutter unmasked by adenosine (Adenocard).