Practice Essentials
Malignancies involving mesothelial cells that normally line the body cavities, including the pleura (see the images below), peritoneum, pericardium, and testis, are known as malignant mesothelioma. Asbestos, particularly the types of amphibole asbestos known as crocidolite and amosite asbestos, is the principal carcinogen implicated in the pathogenesis of malignant pleural mesothelioma.
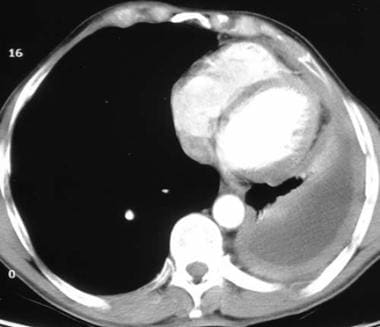 Computed tomography scan of a 58-year-old patient with mesothelioma and shortness of breath. This image shows the extensive pleural thickening that is characteristic of mesothelioma, effusion, and reduction in the volume of the affected hemithorax.
Computed tomography scan of a 58-year-old patient with mesothelioma and shortness of breath. This image shows the extensive pleural thickening that is characteristic of mesothelioma, effusion, and reduction in the volume of the affected hemithorax.
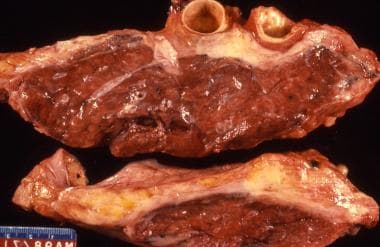 The classic description of malignant pleural mesothelioma is a thickening in the pleural space with encasement of the lung by a rindlike visceral pleura. These gross specimens are from an autopsy lung case with diffuse thickening of the pleura causing compression of the underlying lung tissue.
The classic description of malignant pleural mesothelioma is a thickening in the pleural space with encasement of the lung by a rindlike visceral pleura. These gross specimens are from an autopsy lung case with diffuse thickening of the pleura causing compression of the underlying lung tissue.
Signs and symptoms
Dyspnea and nonpleuritic chest wall pain are the most common presenting symptoms of malignant pleural mesothelioma, with at least 1 of these occurring in 60-90% of patients. Other common accompanying symptoms are as follows:
-
Chest discomfort
-
Pleuritic pain
-
Easy fatigability
-
Fever
-
Sweats
-
Weight loss
On physical examination, findings of pleural effusion are usually noted upon percussion and auscultation. Patients may also be asymptomatic, with evidence of a pleural effusion noted incidentally on physical examination or by chest radiograph.
See Presentation for more detail.
Diagnosis
Thoracentesis
-
More than 90% of patients with pleural mesothelioma present with pleural effusion that decreases after thoracentesis
-
Typically, the pleural fluid findings are nondiagnostic, with < 1000 leukocytes/μL, few erythrocytes, elevated protein levels, and normal lactate dehydrogenase levels
-
Pleural fluid cytologic findings are diagnostic in only 32% of patients and are suggestive in 56%
-
FISH to distinguish malignant mesothelioma from reactive mesothelial cells in effusions had 79% sensitivity [1]
Thoracoscopically guided biopsy
-
Indicated if mesothelioma is suggested
-
Diagnostic in 98% of cases
Routinely stained biopsy preparations are the most valuable diagnostic tool in malignant mesothelioma. Diagnostic features that distinguish malignant mesothelioma from adenocarcinoma include the following:
-
Negative results for periodic acid-Schiff stain, mucicarmine stain, carcinoembryonic antigen, and Leu M1
-
Positive test results for calretinin, vimentin, and cytokeratin
Serum biomarkers
-
Soluble mesothelin (the current reference biomarker)
-
Megakaryocyte potentiating factor
Chest radiographs
Findings in malignant pleural mesothelioma include one or more of the following:
-
Obliteration of the diaphragm
-
Nodular thickening of the pleura
-
Decreased size of the involved chest
-
Radiolucent, sheetlike encasement of the pleura
-
A loculated effusion (> 50% of patients), with opacification of a major portion of the pleura
See Workup for more detail.
Management
Currently, no therapy is considered standard. Treatment options for the management of localized malignant mesothelioma include the following:
-
Surgery (reasonable when disease is confined to the pleural space)
-
Radiation (provides significant palliation of chest pain and chest wall metastasis in 50% of patients)
-
Multimodality treatment
For metastatic disease, generally used metastatic disease chemotherapy/immunotherapy regimens include the following:
-
Cisplatin – For single-agent therapy
-
Cisplatin/pemetrexed
-
Cisplatin/pemetrexed/bevacizumab – A reasonable option if no contradindication to bevacizumab
-
Ipilimumab/nivolumab – New standard for metastatic disease
-
Pemetrexed/gemcitabine – For patients who cannot take cisplatin
-
Pemetrexed – For single-agent therapy
-
Cisplatin/gemcitabine
See Treatment and Medication for more detail.
Go to Oncology Decision Point for expert commentary on mesothelioma treatment decisions and related guidelines. For patient education information, see Mesothelioma and Mesothelioma in Children.
Background
Malignancies involving mesothelial cells that normally line the body cavities, including the pleura, peritoneum, pericardium, and testis, are known as malignant mesothelioma. The 3 major histologic types are sarcomatous, epithelial, and mixed. The condition may be localized or diffuse. (See Etiology.)
Primary sites for malignant mesothelioma include the pleura (87%), the peritoneum (5.1%), the pericardium (0.4%), and the right side of the thorax (more so than the left side, by a right-to-left ratio of 1.6:1). (See Etiology, Presentation, and Workup.)
Among patients with malignant pleural mesothelioma, 77% have previously been exposed to asbestos. [4] Diagnosis is difficult because results from fluid analysis of the tumor’s effusion are not usually diagnostic. Death from malignant mesothelioma is usually due to infection or respiratory failure from the progression of the disease. (See Etiology, Presentation, and Workup.)
Malignant pleural mesothelioma
Malignant pleural mesothelioma usually begins as discrete plaques and nodules that coalesce to produce a sheetlike neoplasm. Tumor growth usually starts at the lower part of the chest. The tumor may invade the diaphragm and encase the surface of the lung and interlobar fissures.
The tumor may also grow along drainage and thoracotomy tracts. As the disease progresses, it often extends into the pulmonary parenchyma, chest wall, and mediastinum. Malignant pleural mesothelioma may also extend into the esophagus, ribs, vertebra, brachial plexus, and superior vena cava.
Etiology
Asbestos
Asbestos, particularly the types of amphibole asbestos known as crocidolite and amosite asbestos, is the principal carcinogen implicated in the pathogenesis of malignant pleural mesothelioma. Exposure to chrysotile asbestos is also associated malignant mesothelioma, but at a lower incidence than occurs with the other types. (The rod-shaped amphiboles are more carcinogenic than the chrysotile.) [5]
Approximately 8 million people in the United States have been exposed to asbestos in the workplace. A substantial proportion of patients with malignant pleural mesothelioma were exposed to asbestos in asbestos mills, shipping yards, mines, or their homes. The crocidolite in asbestos is associated with mesothelioma in miners, manufacturers who use asbestos, and heating and construction workers. Family members of workers exposed to asbestos can also be at risk of exposure if asbestos becomes embedded in the workers’ clothing.
The industries associated with asbestos exposure include the following:
-
Mining
-
Ship building involving the use of asbestos
-
Asbestos cement manufacture
-
Ceramics
-
Paper milling
-
Auto parts (asbestos brake lining)
-
Railroad repair
-
Insulation
Environmental exposure to naturally occurring asbestos released by farming and urban development may also increase the incidence of mesothelioma. [6]
Erionite
Erionite is a fibrous mineral similar to amphibole asbestos that has been linked to malignant mesothelioma. [7] For example, in the Cappadocia region of Turkey, the use of erionite in building construction and road paving led to an epidemic of pulmonary mesothelioma. Deposits of naturally occurring erionite are also found elsewhere in the world, including at least 12 US states. In North Dakota, where erionite-containing gravel was used to surface more than 300 miles of roads, airborne erionite concentrations measured in transiting school buses have been similar to those measured in the affected Turkish villages. [8]
Other sources of mesothelioma
Interleukin-8 has direct growth-potentiating activity in mesothelial cell lines. Malignant mesothelioma has also been linked to therapeutic radiation using thorium dioxide and zeolite, a silicate in the soil.
An etiologic role for simian virus 40 in malignant mesothelioma has been suggested. However, although asbestos exposure alone has been associated with malignant mesothelioma, simian virus 40 alone has not. Thus, some epidemiologic evidence exists that simian virus 40 is a possible cocarcinogen. Its direct role at this point is still controversial. [9]
Alcohol, dietary factors, and tobacco smoke have no effect on the incidence of pleural mesothelioma.
Genetics
Most malignant mesotheliomas have complex karyotypes, with extensive aneuploidy and the rearrangement of many chromosomes. Loss of 1 copy of chromosome 22 is the single most common karyotypic change in malignant mesothelioma. Other chromosomal changes commonly observed include deletions in the chromosome arms 1p, 3p, 9p, and 6q. Several changes in the tumor suppressor genes p16 (CDKN2A) and p14 (ARF) and loss of function of neurofibromin-2 (NF2) have also been noted. [10]
Epidemiology
Occurrence in the United States
Approximately 3000 cases of malignant mesothelioma are diagnosed annually. In the absence of occupational exposure to asbestos, the incidence is 0.1-0.2 per 100,000 population in both sexes. The risk of mesothelioma is increased in polluted areas by 2-10 fold compared with nonpolluted areas. Of patients with malignant mesothelioma in the United States, 80% have been exposed to asbestos.
The annual number of deaths from malignant mesothelioma in the US increased by 4.8% from 1999 to 2015, from 2479 to 2579, according to the Centers for Disease Control and Prevention (CDC). However, over that period the age-adjusted mesothelioma death rate decreased nearly 22%, from roughly 14 to 11 per million population. From 199 to 2015, 45,000 deaths were attributed to malignant mesothelioma in people aged 25 and older. Deaths increased in people aged 85 and older, in men and women, and in whites, blacks, Asians/Pacific Islanders, and Hispanics. [11]
International occurrence
Incidence of malignant mesothelioma is 0.9 case per 100,000 persons annually. Marked variability exists in the incidence of malignant mesothelioma in different countries. In some countries, the incidence is low even though asbestos exposure is high. The reasons for these differences are not known.
Sex- and age-related demographics
Malignant mesothelioma is more common in men than in women, with a male-to-female ratio of 3:1. [12]
Malignant mesothelioma has a peak incidence 35-45 years after asbestos exposure. Two thirds of cases of malignant mesothelioma develop in the fifth to seventh decade of life.
Malignant mesothelioma also occurs in children; however, these cases are not thought to be associated with asbestos exposure.
Prognosis
Without treatment, malignant mesothelioma is fatal within 4-8 months. With trimodality treatment, some patients have survived 16-19 months. A few have survived as long as 5 years, with rates of 14% for all types and 46% for the epithelial type. However, numbers are small. [13, 14] The tumor recurrence rate is 50% for patients treated with surgery.
Median survival for patients with malignant mesothelioma is 11 months. It is almost always fatal. Median survival based on histologic type is 9.4 months for sarcomatous, 12.5 months for epithelial, and 11 months for mixed. Approximately 15% of patients have an indolent course.
In a review of 64 patients undergoing pleurectomy, the overall survival rate was 43%, 28%, and 10% at 1, 2, and 3 years, respectively. The overall median survival with epithelial histology was 21.7 months (n=56 patients); it was 5.8 months for patients with sarcomatous or mixed type mesothelioma (n=28 patients). The causes of morbidity include atrial fibrillation, wound infection, prolonged intubation, pulmonary emboli, myocardial infarction, respiratory failure, deep vein thrombosis, and postoperative bleeding.
In rare cases, malignant mesothelioma manifests as cord compression, brachial plexopathy, Horner syndrome, or superior vena cava syndrome.
Prognostic factors
Based on many clinical factors, 2 separate groups, the Cancer and Leukemia Group B and the European Organization for Research and Treatment of Cancer, identified the following poor prognostic factors [15, 16] :
-
Performance status of 2 or greater
-
Nonepithelial histology
-
Chest pain
-
Age older than 75 years
-
Male sex
-
High platelet count
-
Lactate dehydrogenase greater than 500 IU/L
-
Low hemoglobin concentration
-
High white count
-
Weight loss
Nodal metastasis
The pattern of nodal metastasis is different from that of lung cancer. The mechanism of spread of the disease to the hilar nodes may be through lung invasion and not due to spread directly from the pleura. In a study of 49 patients who underwent surgery, only 7 had no lung invasion and none had positive hilar nodes. In the postpneumonectomy patients, 6 of 14 had positive hilar node and mediastinal nodes.
-
Positron emission tomography (PET) scan in a male patient with known mesothelioma. Although PET scanning is not standard for the evaluation of mesothelioma, this image illustrates the extent of the disease into the mediastinum and peritoneum.
-
Chest radiograph of a 58-year-old patient with mesothelioma and shortness of breath. This image reveals diffuse, left-sided pleural thickening, a pleural effusion, and ipsilateral volume loss.
-
Computed tomography scan of a 58-year-old patient with mesothelioma and shortness of breath. This image shows the extensive pleural thickening that is characteristic of mesothelioma, effusion, and reduction in the volume of the affected hemithorax.
-
Computed tomography scan of the chest. This image demonstrates mesothelioma that extends into the chest wall. Note the concentric left pleural thickening, pleural effusion, reduction in volume of the left hemithorax, and the tumor nodules within the chest wall.
-
Magnetic resonance imaging (MRI) scan in a 72-year-old Veterans Administration patient with left-sided mesothelioma. Note that the MRI scan well delineates the soft tissues and, in particular, the thoracoabdominal interface at the diaphragm.
-
Computed tomography (CT) scan in a male Veterans Administration patient with a history of asbestos exposure and an enlarging abdominal girth. This upper CT scan slice reveals the calcified pleural plaques along the diaphragmatic surface that are associated with asbestos exposure. Ascites is seen lateral to the liver. Aspiration of the ascitic fluid demonstrated mesothelioma.
-
The soft-tissue window setting of this chest computed tomography (CT) scan shows the envelope-like mass along the pleural surface surrounding the lung. This was a mesothelioma.
-
The classic description of malignant pleural mesothelioma is a thickening in the pleural space with encasement of the lung by a rindlike visceral pleura. These gross specimens are from an autopsy lung case with diffuse thickening of the pleura causing compression of the underlying lung tissue.
-
This histologic section stained with hematoxylin and eosin shows papillary structures on the right and a transition to a more solid pattern on the left.
-
A solid pattern of mesothelioma on the right of this histologic section transitions to a spindle cell morphology on the left of the image in this predominantly sarcomatoid malignant mesothelioma.
-
Well differentiated papillary mesothelioma is characterized with a single layer of bland cuboidal cells lining fibrovascular cores, as demonstrated in this image.
-
Immunohistochemistry helps to demonstrate that the atypical mesothelial cells in this reactive proliferation are in fact in one roughly linear layer, an important criterion supporting a benign process.






