Practice Essentials
Zenker diverticulum, a pulsion diverticulum of the hypopharynx, is a rare lesion that occurs in elderly populations. The condition results in a classic presentation of symptoms, with complications that include aspiration and pneumonia, and is managed by endoscopic [1, 2, 3] and open transcervical surgical repair. (See the image below.)
Zenker diverticula are lined with stratified squamous epithelium with a thin lamina propria. No muscular layer exists. Fibrosis surrounding the diverticulum is common.
Signs and symptoms of Zenker diverticulum
The history strongly suggests the diagnosis of Zenker diverticulum. The combination of the following signs and symptoms is nearly pathognomonic for the condition:
-
Dysphagia - Most patients (98%) present with some degree of dysphagia
-
Regurgitation of undigested food hours after eating
-
Sensation of food sticking in the throat
-
Special maneuvers to dislodge food
-
Coughing after eating
-
Aspiration of organic material
-
Unexplained weight loss
-
Fetor ex ore (halitosis)
-
Borborygmi in the neck
Workup in Zenker diverticulum
The criterion standard for confirmatory evaluation of Zenker diverticulum is a barium swallow with videofluoroscopy. Esophageal manometry is indicated if the contrast study suggests that achalasia or another esophageal motility disorder is present.
Endoscopic examinations include the following:
-
Flexible endoscopic evaluation of swallowing
-
Rigid/flexible esophagoscopy - Essential before surgical management to assess the nature of the mucosa of the Zenker diverticulum and to exclude the presence of squamous cell carcinoma (SCC) or carcinoma in situ
Management of Zenker diverticulum
Nonsurgical management
Botulinum toxin may be used to provide temporary relief of dysphagia symptoms. Symptomatic patients who are poor surgical risks and have small Zenker diverticula may be treated satisfactorily by this method.
Surgical management
Surgical approaches include the following:
-
Diverticulum invagination or imbrication with cricopharyngeal (CP) myotomy
-
Stapled or hand-sewn diverticulectomy with CP myotomy
-
Stapled or hand-sewn diverticulopexy with CP myotomy
-
Endoscopic division of the diverticular wall with an endoscopic stapler
-
Endoscopic division of the diverticular wall with a CO 2 laser or other cutting tool
A study by Schoeff et al suggested that patients with Zenker diverticulum often have a subjective voice handicap and that, as indicated on the voice handicap index, these patients frequently perceive postoperative voice quality improvement. In this study, surgery consisted of either transoral endoscopic diverticulotomy with laser assistance, or a transcervical approach with diverticulopexy or diverticulectomy. [4]
In a study of 139 patients with Zenker diverticulum who underwent transoral septum stapling, Siboni et al found, at median 38-month follow-up, statistically significant decreases in the following rates [5] :
-
Chronic cough - 36.8% versus 7.9%
-
Recurrent episodes of pneumonia - 6.6% versus 0.0%
-
Dysphagia - 78.9% versus 6.6%
-
Regurgitation - 67.1% versus 6.6%
Pathophysiology
The pathologic process in Zenker diverticulum involves herniation of the esophageal mucosa posteriorly between the cricopharyngeal (CP) muscle and the inferior pharyngeal constrictor muscles. Therefore, by strict definition, a Zenker diverticulum is a false diverticulum. The retention of food elements and secretions within the lesion’s pouch frequently leads to halitosis, regurgitation, aspiration, and dysphagia in patients. [6, 7]
Although Zenker proposed that a pulsion mechanism affects the pharyngeal mucosa above the CP muscle, no consensus exists regarding a unifying concept of the cause of Zenker diverticula. The specific abnormality of the CP muscle has not been elucidated. Hypothetical abnormalities include the following:
-
Abnormal timing of deglutition resulting in closure of the CP muscle when ideally it should be opening
-
Incomplete CP muscle relaxation
-
Elevated resting tone of the entire upper esophageal sphincter (UES)
-
Loss of CP muscle elasticity
-
CP muscle myopathy or denervation atrophy
-
Central nervous system (CNS) injury with a focal spastic CP muscle
-
CP muscle spasm in response to gastroesophageal reflux disease (GERD)
Studies to investigate the mechanism are scant. Cook histologically examined the CP muscle obtained at the time of diverticulectomy and found abundant fibrosis within the muscle. Whether this finding is a cause or a result of Zenker diverticulum is uncertain.
Kern determined that older individuals exhibit less anterior excursion of the larynx and hyoid with deglutition than younger subjects, resulting in higher hypopharyngeal intrabolus pressures in older subjects. [8] Whether this leads to Zenker diverticula over time is speculative. Van Overbeek suggested an anthropometric explanation. He felt individuals with longer necks had a larger Killian triangle, which predisposed them to formation of Zenker diverticulum. [9]
It is hypothesized that abnormal muscle activity in the cricopharyngeus results in a discoordination of the swallowing mechanism, [10, 11] which, when coupled with increased intraluminal pressure on the mucosa of the pharynx, results in the slow, progressive distention of the mucosa. As the weakest portion of this area is located posteriorly, this becomes the location of the pulsion diverticulum formation.
Zenker diverticula extend into the left neck 90% of the time. This is likely due to the slight convexity of the cervical esophagus to the left side and to the more laterally positioned carotid artery on the left side, creating a potential space for the sac.
Pressure measurements
Esophageal manometry has been used to elucidate the pathophysiology of the upper esophagus, which is responsible for the diverticular formation. However, upper esophageal manometry is technically difficult to perform. Results are confounded by the asymmetry of the upper esophageal sphincter. Pressures can be very high, but they last for only a fraction of a second, resulting in difficulty obtaining equipment sensitive enough to demonstrate these pressures accurately.
To further confound the problem, the process of obtaining measurements stimulates the swallowing reflex, resulting in the catheter being displaced and the data lost. Because of these limitations, very few studies have been performed to describe the manometric aspects of Zenker diverticulum. Manometry is certainly not useful in routine patient evaluation.
The studies that have been performed show upper esophageal sphincter pressures that can be either normal or decreased. Some patients have abnormal premature relaxation and contractions of the upper esophageal sphincter, while others have pharyngeal contractions against a closed sphincter. [10, 12, 13, 14]
Etiology
A complete understanding of the etiology of Zenker diverticulum formation is not available. Further studies focused on the function of the CP muscle are likely to be fruitful.
Zenker diverticula occur in a muscular dehiscence that is present most commonly between the oblique muscle fibers of the inferior constrictor muscle and the transverse fibers of the CP muscle. This area is known as the Killian triangle. Other areas of muscular dehiscence occur between the oblique and transverse fibers of the CP muscle (ie, Killian-Jamieson area) and between the CP muscle and the esophageal muscles (ie, Laimer triangle). More inferiorly positioned Zenker diverticula may occur in one of these latter sites. (See the image below.)
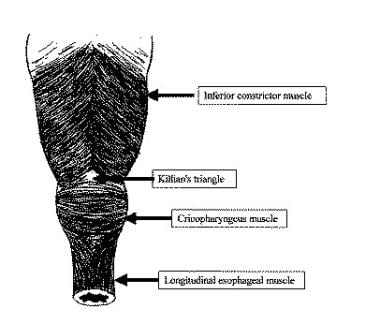 Posterior view of the hypopharynx and proximal esophagus showing the Killian triangle (dehiscence between the inferior constrictor muscle and the cricopharyngeus [CP] muscle), the Killian-Jamieson area (dehiscence between the oblique and transverse fibers of the CP muscle), and the Laimer triangle (dehiscence between the CP and esophageal muscles).
Posterior view of the hypopharynx and proximal esophagus showing the Killian triangle (dehiscence between the inferior constrictor muscle and the cricopharyngeus [CP] muscle), the Killian-Jamieson area (dehiscence between the oblique and transverse fibers of the CP muscle), and the Laimer triangle (dehiscence between the CP and esophageal muscles).
Staging
Zenker diverticula may be staged in 1 of the following 3 systems, as assessed by means of barium swallow videofluoroscopy:
Lahey system
Criteria of the Lahey staging system are as follows:
-
Stage I - A small mucosal protrusion is present
-
Stage II - A definite sac is present, but the hypopharynx and esophagus are in line
-
Stage III - The hypopharynx is in line with diverticulum, and the esophagus is indented and pushed anteriorly.
Morton system
Criteria of the Morton staging system are as follows:
-
Small sacs are less than 2 cm in length
-
Intermediate sacs are 2-4 cm in length
-
Large sacs are greater than 4 cm in length
Van Overbeek system
Criteria of the van Overbeek system are as follows:
-
Small sacs are less than 1 vertebral body in length
-
Intermediate sacs are 1-3 vertebral bodies in length
-
Large sacs are greater than 3 vertebral bodies in length
Epidemiology
Occurrence in the United States
The prevalence of Zenker diverticulum in the United States ranges from 0.01-0.11% of the population. [15] It is more common in males and in the elderly, with a peak incidence in the seventh to ninth decades.
International occurrence
Zenker diverticula occur more commonly in certain parts of the world. They are observed most often in northern European countries and in countries whose population has a northern European heritage (eg, United States, Canada, Australia). Zenker diverticulum is rarely observed in Japan and Indonesia. The prevalence in high-risk countries is 2 cases per 100,000 people. It has a male-to-female ratio of 1.5:1 and is observed almost exclusively in older individuals.
History and Physical Examination
The history strongly suggests the diagnosis of Zenker diverticulum. The combination of the following signs and symptoms is nearly pathognomonic for the condition:
-
Dysphagia - Most patients (98%) present with some degree of dysphagia
-
Regurgitation of undigested food hours after eating
-
Sensation of food sticking in the throat
-
Special maneuvers to dislodge food
-
Coughing after eating
-
Aspiration of organic material
-
Unexplained weight loss
-
Fetor ex ore (halitosis)
-
Borborygmi in the neck
Symptoms may last from months to years. The most common life-threatening complication in patients with a Zenker diverticulum is aspiration. Other complications include massive bleeding from the mucosa or from fistulization into a major vessel, esophageal obstruction, and fistulization into the trachea. Coexistent hiatal hernia, esophageal spasm, achalasia, and esophagogastroduodenal ulceration are common. Although the diverticulum can reach sizes of 15 cm or more, it is rarely palpable. [16]
Squamous cell carcinoma (SCC) within a Zenker diverticulum is extremely rare, occurring in 0.3% of Zenker diverticula worldwide. A Mayo Clinic review suggests an incidence of 0.48% in the United States. Approximately 50 cases of invasive SCC and carcinoma in situ are reported in the literature. This possibility should be considered when evaluating patients with cervical metastatic SCC with an unknown primary cancer. [17, 18]
Barium Swallow With Videofluoroscopy
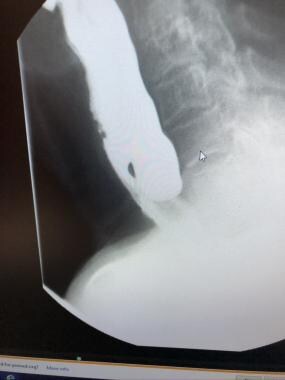
The criterion standard for confirmatory evaluations is the barium swallow with videofluoroscopy. This study provides information about the size, location, and character of the mucosal lining of the Zenker diverticulum, as depicted in the image below. Certain radiographic features of the diverticulum neck may predict the likelihood of success of the endoscopic stapling approach. [19]
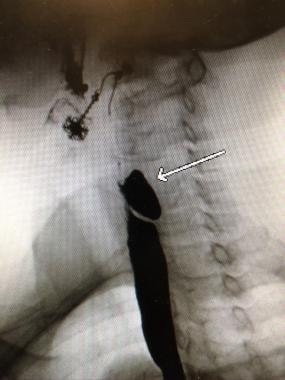 Very prominent cricopharyngeal muscle creating obstruction immediately inferior to the diverticulum.
Very prominent cricopharyngeal muscle creating obstruction immediately inferior to the diverticulum.
Patients with symptomatic disease usually have a posterior midline pouch greater than 2 cm in diameter arising just above the CP muscle. No other study is required if no other abnormality is present. [12, 13]
Esophageal manometry
Esophageal manometry is indicated if the contrast study suggests that achalasia or another esophageal motility disorder is present.
Endoscopic Assessment
As previously mentioned, fibrosis surrounding a diverticulum is common. The fibrotic tissue may limit the spread of any extravasated material from the diverticulum during endoscopic procedures and therefore reduce the likelihood of local abscess formation.
Flexible endoscopic evaluation of swallowing
Flexible endoscopic evaluation of swallowing (FEES) provides information that may suggest the presence of a Zenker diverticulum, but this test has not supplanted the barium swallow in most surgeons' practices. Pooling in the esophageal introitus may be ascertained in individuals with a Zenker diverticulum by using cream or other readily visible forms of food of various consistencies. This test may help to select patients for whom barium swallow is appropriate.
Rigid/flexible esophagoscopy
Rigid or flexible esophagoscopy is essential before surgical management to assess the nature of the mucosa of the Zenker diverticulum and to exclude the presence of SCC or carcinoma in situ. Care must be taken with rigid esophagoscopy to avoid perforating the Zenker diverticulum. [9]
Treatment Indications and Contraindications
Lesion size
Zenker diverticula require intervention only if they produce symptoms. In general, small (ie, < 2 cm) lesions found incidentally require no intervention. However, some surgeons contend that because these lesions are likely to become larger with time, intervention ought to be considered in younger, healthier, asymptomatic patients with Zenker diverticula.
Small lesions are satisfactorily treated with a CP myotomy with or without an invagination procedure. Intermediate and large diverticula (ie, 2-6 cm) are best managed with open diverticulectomy with CP myotomy or by endoscopic diverticulotomy. Very large diverticula (ie, >6 cm) are best managed with excision with CP myotomy or a diverticulopexy with CP myotomy, depending on the health of the patient. On one occasion, the authors placed a gastrostomy as the sole form of intervention for an ill patient aged 95 years with a 20-cm Zenker diverticulum.
Nonoperative intervention
Nonoperative management may be undertaken in patients with diverticula of under 1 cm (the surgeon may elect to follow these patients so that they can be assessed for the development of symptoms or enlargement of the diverticulum) or in patients with medical comorbidities precluding surgery. [20]
As with CP achalasia, botulinum toxin may be used to provide temporary relief of dysphagia symptoms. Symptomatic patients who are poor surgical risks and have small Zenker diverticula may be treated satisfactorily by this method.
Surgical intervention
There are a variety of approaches in managing patients with Zenker diverticulum. Familiarity by the treating surgeon with all approaches will allow for optimal decision-making for each patient. [21, 22]
Indications for the repair of Zenker diverticulum are broad. The diverticulum can frequently be the etiology for aspiration and pneumonia and should therefore be repaired in patients capable of tolerating the operative procedure. [23]
However, the overall health of people in the generally older population of patients with Zenker diverticulum may not allow a significant surgical undertaking. [24] Recognition of this problem is essential in designing the ideal treatment plan. However, the range of effective treatment options allows treatment for essentially all symptomatic patients.
A retrospective study by Uoti et al indicated that in the surgical treatment of Zenker diverticulum, the occurrence of complications is associated with patient age, the surgical technique used, and the clinician’s surgical specialty. The report, which involved 1044 patients, 606 of whom were without preoperative comorbidities, found the complication and mortality rates to be 6.4% and 0.9%, respectively. Patient age (particularly age over 90 years), surgical approach, and surgical specialty correlated with length of stay, while an association was revealed between reoperation and both initial surgical approach and surgical specialty. [25]
From an anatomic perspective, the most common open procedure (diverticulectomy with CP myotomy) has no contraindications. The endoscopic approach (diverticulotomy) may not be performed if the patient has significantly reduced cervical extension or marked trismus.
With regard to improvement of dysphagia and regurgitation, a literature review by Howell et al did not find that open transcervical diverticulectomy for Zenker diverticula was superior to endoscopic laser diverticulectomy or endoscopic stapler-assisted diverticulectomy. [26]
A study by Rudler et al indicated that in the treatment of Zenker diverticulum, flexible endoscopy seems to be similar to open surgery with regard to safety and efficacy, while rigid endoscopy appears to be less effective than the other two procedures. In the report, the clinical success rates of open surgery and flexible endoscopy were 97% and 90%, respectively, compared with 79% for rigid endoscopy. Moreover, the frequency of technical failure was greater in rigid endoscopy than in the other procedures. While the endoscopies were associated with statistically shorter median procedure time, median time to feeding resumption, and time to hospital discharge, than was open surgery, fewer recurrences (and fewer reinterventions) were found in open surgery patients than in the endoscopy group. [27]
CP Myotomy
The 2 key elements for the successful surgical management of a Zenker diverticulum are division of the CP muscle to eliminate the potentially elevated pressure zone and elimination of the diverticular pouch as a reservoir of food and secretions. Presently, the goal of myotomy is to reduce the septum to less than 1 cm in length. [28, 29]
Surgical approaches include the following: (1) stapled or hand-sewn diverticulectomy with CP myotomy, (2) stapled or hand-sewn diverticulopexy with CP myotomy, (3) endoscopic division of the diverticular wall with an endoscopic stapler, and (4) endoscopic division of the diverticular wall with a CO2 laser or other cutting tool. [30, 31, 32, 33, 34, 35, 36, 37, 38, 39] . There have been reports concerning newer tools used for diverticular wall surgery, including the LigaSure device and the Harmonic scalpel. [40, 41]
The 2 most commonly performed surgical procedures are diverticulectomy with CP myotomy and endoscopic diverticulotomy with a stapler. (The latter procedure is discussed in the next section.)
CP myotomy alone
This procedure was described in several reports during the 1970s as a sole form of intervention for Zenker diverticulum. Although the rationale for and the benefits of the procedure are generally well recognized, myotomy alone has been associated with persistent symptoms in up to 30% of patients, [30] as well as with more frequent complications. In addition, recurrence requiring repeat surgery has been necessary more frequently with myotomy alone than with other procedures.Therefore, CP myotomy, when performed through a transcervical approach, is most commonly used for CP achalasia alone. When a diverticulum is present, a diverticulectomy is performed. On occasion, a high-risk patient may be treated with open CP myotomy without diverticulectomy to avoid the risk of fistula formation and the attendant morbidity.
A more commonly employed approach for CP myotomy is the endoscopic transmucosal CP myotomy, which is usually carried out with rigid instrumentation. An example of an endoscopic transmucosal CP myotomy using a carbon dioxide (CO2) laser may be seen below. This approach is appropriate for CP achalasia alone or in patients who have developed small Zenker diverticula.
Diverticulum invagination or imbrication with CP myotomy
This surgical approach is a reasonable option for small or intermediate-sized, symptomatic Zenker diverticula that cannot be managed with an endoscopic approach. With this technique, the diverticulum is pushed into the hypopharynx, and the muscle and adjacent fibrous tissue are oversewn. A CP myotomy is usually combined with this technique.
Diverticulectomy with CP myotomy
With a stapled or hand-sewn diverticulectomy and CP myotomy, the pouch neck is either oversewn or stapled, and the pouch is excised. The CP muscle is divided longitudinally no less than 5 cm. This is typically performed through a left neck incision and is primarily closed with a closed-suction drain in place.
For many years, diverticulectomy was considered the standard approach. The endoscopic approaches, however, have come to be favored in the United States. The open approach nonetheless remains a useful option for patients who remain symptomatic after an endoscopic approach or for those patients who wish to have more definitive removal of the sac. (See the image below.)
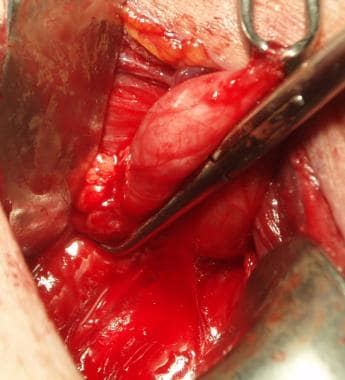 View of a Zenker diverticulum with transverse fibers of the cricopharyngeus muscle below the diverticulum, as viewed through the left side of the neck.
View of a Zenker diverticulum with transverse fibers of the cricopharyngeus muscle below the diverticulum, as viewed through the left side of the neck.
Preoperative details
Precede this surgery with direct laryngoscopy and rigid esophagoscopy, principally to assess the mucosa. Many surgeons find placement of a Foley catheter in the Zenker diverticulum helpful to facilitate locating the Zenker diverticulum later in the procedure. Other maneuvers include placing a Maloney dilator or nasogastric (NG) tube in the esophagus to facilitate later dissection.
Intraoperative details
Diverticulectomy with CP myotomy is carried out as follows:
-
Make a through transverse incision at the level of the cricoid, extending laterally to the sternocleidomastoid (SCM) muscle
-
Retract the SCM muscle and carotid sheath contents laterally; retract the thyroid and thyroid cartilage medially, and turn them slightly away from the dissection
-
The recurrent laryngeal nerve (RLN) is not routinely identified
-
Perform the dissection on the diverticulum if readily apparent; if the sac is not apparent, begin dissection posteriorly in the midline at the level of the inferior constrictor muscle, extending inferiorly until the sac is encountered
-
Then transect the sac neck either sharply or with a stapler/cutter device
-
Perform CP myotomy
-
If the sac neck is sharply transected, perform closure with 3-0 or 4-0 absorbable suture placed in a Connell closure with 2-3 layers
-
Place an NG tube
-
Place a nonsuction drain
Postoperative details
Postoperative care following diverticulectomy with CP myotomy includes the following:
-
Place an NG tube for 3-4 days
-
If a stapler/cutter such as a GIA device is used, an NG tube may not be necessary
-
Start alimentation slowly
-
Closely monitor temperature; obtain a chest radiograph
Diverticulopexy with CP myotomy
Using this technique, the diverticulum is inverted and sutured to the prevertebral fascia, and the CP muscle is divided. The pouch is not excised in this procedure. Older patients with very large Zenker diverticula (>6 cm) who cannot tolerate a diverticulectomy are the best candidates for diverticulopexy with CP myotomy because there is no division of the esophagus, pharynx, or diverticulum, and there is no suture line. [42]
Endoscopic Diverticulotomy
A study by Venkatesan found that after successful endoscopic diverticulotomy in patients with Zenker diverticulum, videofluoroscopic swallow study (VFSS) showed a 50.9% reduction in diverticulum size and a 33.6% reduction in the pharyngeal constriction ratio, as well as a 61.6% increase in the mean pharyngoesophageal segment opening in the lateral view and a 40.0% increase in the anteroposterior view. In addition, the mean value for the Eating Assessment Tool-10 (EAT-10) was reduced by 72.1%, indicating improved swallowing. [43]
In 1917, Mosher first described an endoscopic approach to diverticulotomy but abandoned it because of complications. This approach was reintroduced by Dohlman and Seiffert in 1958. [44] Although these authors reported few complications, other surgeons experienced complications such as mediastinitis and abscess formation, and the technique did not gain widespread acceptance. A cautery unit was used to perform the diverticulotomy. [39, 45, 46, 47, 48]
Laser technique
During the 1980s, the CO2 laser and potassium-titanyl-phosphate (KTP) laser were used to perform the incision in diverticulotomy. The CO2 laser technique remains a favored procedure in Europe and the United States. This technique is particularly useful for small (< 2 cm) and moderate (2-4 cm) diverticula because a stapler may not be able to satisfactorily grasp the "party wall" between the esophagus and diverticulum.
The risk of cervical emphysema is higher with the laser technique over the stapling technique. The laser technique, however, has a distinct advantage over the stapling technique because of the ease of transection of the "party wall." The stapler is bulky and is difficult to insert, particularly in small female patients.
A study by Kos et al of 229 endoscopic diverticulotomies (in 189 patients) indicated that using a combination of CO2 laser and AcuSpot in the endoscopic procedure provides better results than does employing endoscopic diverticulotomy with electrocautery or with a CO2 laser alone. [49] The investigators reported the following postsurgical results for the occurrence of dysphagia and repeat surgery:
Results from endoscopy with a CO2 laser were as follows:
-
Dysphagia - Absent following 78.4% of procedures
-
Repeat surgery - Required following 19.6% of procedures
Results from endoscopy with electrocautery were as follows:
-
Dysphagia - Absent following 72% of procedures
-
Repeat surgery - Required following 24.3% of procedures
Results from endoscopy with a CO2 laser and AcuSpot were as follows:
-
Dysphagia - Absent following 84.6% of procedures
-
Repeat surgery - Required following 13% of procedures
The ultrasonic scalpel is an alternate tool for transecting the “party wall” between the esophagus and diverticulum. [34]
Stapling technique
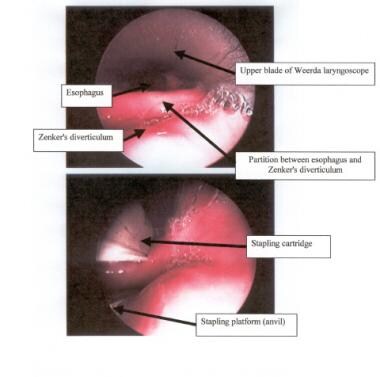
In endoscopic diverticulotomy, a double-bladed rigid endoscope is placed into the pharynx, with one blade positioned in the esophagus and the other in the diverticulum. An articulating endoscopic linear stapler is introduced into the pharynx with one jaw of the stapler in the pouch and one jaw in the esophagus. The stapler is locked across the common septum of the 2 and is fired. If necessary, this is repeated until the bottom of the pouch is reached. [50]
This results in an opening of the pouch and a division of the CP muscle, with the pouch wall becoming incorporated as a wall of the esophagus. This technique should not be used for diverticula less than 3 cm in length, owing to the fact that the stapler blade is too long for the common wall. [42]
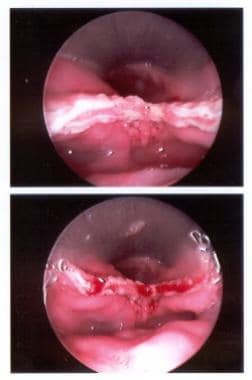
The endoscopic stapling technique consists of adhering the mucosal edges by stapling them together while cutting across the "party wall" at the same time. It has become the preferred endoscopic approach for most American and British surgeons. Few complications have been reported with this procedure. Patients with good flexibility, favorable dentition, and larger diverticula are the best candidates. A stapling diverticulotomy is demonstrated below. [51, 52]
Using traction sutures to pull the “party wall” into the stapler may improve long-term outcomes by increasing the length of the incision. However, this may also increase the likelihood of perforation of the diverticulum while engaging the stapler, so this additional technique should be used with caution. [52, 53]
(A study by Wilmsen et al reported that the use of flexible endoscopy in stapler-assisted diverticulotomy offers a safe and efficient means of treating Zenker diverticulum. The investigators found that in 11 out of 17 patients (64.7%), complete septum division was accomplished using the stapler. In the remaining patients, stapling was unsuccessful because the septum was too thick or the head was not sufficiently reclined. [54] )
Preoperative details
Proper instrumentation, including the following, is essential for the performance of this technique:
-
Three different-sized Weerda laryngoscopes, including the diverticuloscope (anatomic differences require different scopes)
-
Hopkins endoscopes (0 and 30°, 4 mm)
-
Dental protection
-
Vascular 35-mm stapler, preferably with an articulating arm
Intraoperative details
An endoscopic diverticulotomy with stapler proceeds as follows:
-
Intubate the patient with an endotracheal tube that is smaller than standard (ie, 6-mm outside-diameter tube for 70-kg patient)
-
Place dental protection on the upper and lower dentition
-
Inspect the larynx and postcricoid region with an anterior commissure laryngoscope
-
Position and suspend an appropriate-sized Weerda laryngoscope
-
Inspect the mucosa of the sac
-
Define the partition, or "party wall," between the sac and the esophagus and ascertain the size of the sac
-
Hopkins endoscopes are used to guide the stapler into place
-
Place the straight part of the stapler (containing the staples and blade) in the esophagus; place the angled part (anvil) in the sac, as depicted in the image below
-
Perform a second stapling if residual partition remains
Postoperative details
Postoperative care following an endoscopic diverticulotomy with stapler includes the following (which can be safely performed on an outpatient basis) [30, 55] :
-
Administer ice chips the night of the procedure and a soft diet the following day
-
Closely monitor temperature
-
Obtain a chest radiograph
Postoperative radiologic assessment of the surgical site with barium swallow videofluoroscopy must be interpreted with understanding of the surgical intent. Findings that indicate a successful result include the following:
-
Reduced height of the partition wall
-
Easy passage of barium into the esophagus
-
Reduced height of barium in the residual sac
Comparison of laser versus stapling procedures
A study by Adam et al examining the efficacy of CO2 laser versus stapling technique in the endoscopic repair of Zenker diverticulum found that although both procedures were effective in treating the condition, the laser technique was more effective. The retrospective review, in which 148 patient charts were examined, found lower recurrence rates in patients treated by laser, as well as better outcomes in these patients with regard to dysphagia and regurgitation. [56]
In another study of the two techniques, Parker and Misono found that the CO2 laser and stapling procedures were similar with regard to most, but not all, types of complications in Zenker diverticulum treatment. The report, a meta-analysis of 391 procedures from seven retrospective, uncontrolled studies, found no differences in the prevalence of dental complications, major complications, overall complications, or revision surgery, as well as none in the length of time that patients were nil per os. However, nondental complications were more frequent in patients who underwent the CO2 laser procedure. [57]
A retrospective study by Hering and Wiegand indicated that in the treatment of Zenker diverticula, the systemic inflammatory response in the early period after endoscopic surgery is greater subsequent to CO2 laser treatment than it is following surgery with a linear stapling device. In the first postoperative days, the investigators found leukocyte counts and C-reactive protein levels to be significantly greater in the laser-surgery patients than in the stapler group. [58]
In a study by Barton et al of 106 patients, endoscopic laser- and stapler-assisted diverticulotomies were both found to be effective in short- and long-term symptom management and to be associated with high levels of patient satisfaction. Reviewing historical data for the stapler-assisted procedure alone at the single institution where the study was carried out, the investigators found that the addition of the laser-assisted technique produced a decline in the endoscopic diverticulotomy failure rate, with no rise in serious complications. [59]
A study by Visser et al indicated that laser-assisted and stapler-assisted endoscopic CP myotomy are both effective in treating Zenker diverticulum, with 45 out of 75 patients (60%)—23 out of 42 stapler patients (55%) and 22 out of 33 laser patients (67%)—experiencing complete resolution of symptoms after their initial treatment. Complications and recurrence rates did not significantly differ between the two endoscopic techniques in the study, although the investigators suggested that patients who undergo the stapler-assisted procedure nonetheless are at greater risk of requiring reintervention and of suffering more severe complications. [60]
Flexible endoscopic diverticulotomy with cautery
Peroral endoscopic myotomy (POEM) with flexible instrumentation and cautery has gained popularity in some centers and can play a role in the management of some patients. [41]
Surgical Advantages, Disadvantages, and Complications
Diverticulectomy with CP myotomy
Advantages of this procedure include the following:
-
Removes the diverticulum
-
Provides tissue for pathologic review
-
Highly effective
Disadvantages include the following:
-
Longer surgical intervention
-
Longer hospitalization
-
Delayed alimentation
Immediate symptom relief occurs in 90-100% of patients.
Long-term symptom recurrence
The long-term symptom recurrence rate is 2-33% for diverticulectomy with CP myotomy. Gutschow compared the success rates with open versus endoscopic techniques and found that in sacs that measured less than 3 cm, the open technique achieved 98% long-term success, while the endoscopic approach achieved only a 57% rate. With sacs larger than 3 cm, the success rate was 97% and 88%, respectively. [39]
Morbidity and mortality
The mortality rate for diverticulectomy with CP myotomy is 0-9.5%. Morbidity, which occurs in 4-47% of patients, includes the following:
-
Recurrent laryngeal nerve (RLN) paralysis
-
Esophageal stenosis
-
Mediastinitis
-
Pharyngocutaneous fistula
-
Hematoma
-
Esophageal perforation
A literature review by Bhatt et al indicated that in the surgical management of Zenker diverticulum, open diverticulectomy with CP myotomy has less association with recurrent or persistent postoperative symptoms than does endoscopic laser-assisted diverticulotomy or endoscopic stapler-assisted diverticulotomy. Evidence suggested that the likelihood or such symptoms in the open technique is approximately 20% that of the other two procedures. However, because the comparison-based confidence intervals were fairly wide, the reduction in symptom likelihood may actually be smaller. [61]
Endoscopic diverticulotomy with stapler
A study by Bonavina et al indicated that transoral stapling is a safe and effective treatment for Zenker diverticulum, with dysphagia and regurgitation scores significantly improving over a median follow-up period of 63 months and the median number of annual pneumonia episodes being significantly reduced. The operation, performed on 100 patients in the study, had an overall long-term success rate of 76%; the success rate was higher in patients aged 70 years or older (who tended to have larger diverticula) and in cases in which traction sutures were used on the septum. [62]
A prospective study by Van Abel et al assessed short-term quality-of-life changes and functional outcomes following transoral diverticulotomy with CP myotomy, finding improvement in both of these measures. The changes were evaluated in 18 patients at 3 months postprocedure through the Reflux Symptom Index (improvement from 27 preprocedure to 5), the Functional Outcome of Swallowing Scale (improvement from 2 preprocedure to 0), and the 10-item Eating Assessment Tool (improvement from 21.5 preprocedure to 0). [63]
Advantages of endoscopic diverticulotomy with stapler include the following:
-
Short surgical time
-
Easily repeated
-
Quick return to oral alimentation
-
Short hospitalization
-
Less tissue trauma
-
Highly effective
Disadvantages include the following:
-
Unable to perform in some patients
-
Does not remove the diverticulum
-
No tissue for pathologic review
Immediate symptom relief occurs in 94-100% of patients.
Long-term symptom recurrence
The long-term symptom recurrence rate for patients who undergo endoscopic diverticulotomy with stapler is 0-47%. According to Gutschow, the recurrence rate with any endoscopic approach is greater if the sac is smaller. [39]
Recurrences may be successfully managed with a repeat endoscopic procedure or by using an open approach. When performing the open approach, the surgeon needs to recognize the altered anatomy present as a result of the prior endoscopic procedure.
Morbidity and mortality
The mortality rate for this procedure is 0-1%. Morbidity, which occurs in 10-31% of patients, includes the following:
-
RLN paresis/paralysis
-
Bleeding
-
Mediastinitis
-
Dental injury
-
Esophageal perforation
-
Diverticulum perforation - This can occur when placing the angled end of the stapler in a small to moderate-sized sac while it is stretched tautly by the Weerda diverticuloscope; repairing this perforation with endoscopically placed sutures has been reported, but open drainage remains the preferred approach
-
Cervical emphysema
Questions & Answers
Overview
What is the pathophysiology of Zenker diverticulum?
What is the role of esophageal manometry in the workup of Zenker diverticulum?
Which esophageal manometry findings are characteristic of Zenker diverticulum?
What causes Zenker diverticulum?
How is Zenker diverticulum staged?
What is the Lahey system for staging Zenker diverticulum?
What is the Morton system for staging Zenker diverticulum?
What is the van Overbeek system for staging Zenker diverticulum?
What is the prevalence of Zenker diverticulum in the US?
What is the global prevalence of Zenker diverticulum?
What are the signs and symptoms of Zenker diverticulum?
What are the possible complications of Zenker diverticulum?
What is the prevalence of squamous cell carcinoma (SCC) within a Zenker diverticulum?
What is the role of barium swallow with videofluoroscopy in the workup of Zenker diverticulum?
When is esophageal manometry performed in the workup of Zenker diverticulum?
How does fibrotic tissue affect the performance of endoscopic procedures for Zenker diverticulum?
What is the role of esophagoscopy in the workup of Zenker diverticulum?
How are small lesions in Zenker diverticulum treated?
What are the indications for medical treatment of Zenker diverticulum?
When is surgery indicated for Zenker diverticulum?
What are the elements of successful surgical management of Zenker diverticulum?
What are the approaches to surgery for Zenker diverticulum?
What are the most common surgical treatments of Zenker diverticulum?
How is CP myotomy performed for the treatment of Zenker diverticulum?
How is diverticulectomy with CP myotomy performed for the treatment of Zenker diverticulum?
How is diverticulopexy with CP myotomy performed for the treatment of Zenker diverticulum?
What is the efficacy of endoscopic diverticulotomy in the treatment of Zenker diverticulum?
What is the role of lasers in the treatment of Zenker diverticulum?
How is endoscopic diverticulotomy performed in the treatment of Zenker diverticulum?
-
Images obtained during barium swallow videofluoroscopy demonstrating an intermediate-sized Zenker diverticulum.
-
Posterior view of the hypopharynx and proximal esophagus showing the Killian triangle (dehiscence between the inferior constrictor muscle and the cricopharyngeus [CP] muscle), the Killian-Jamieson area (dehiscence between the oblique and transverse fibers of the CP muscle), and the Laimer triangle (dehiscence between the CP and esophageal muscles).
-
Endoscopic view of the partition between the esophagus (anteriorly) and the Zenker diverticulum (posteriorly); the stapler is in place in the lower view.
-
Endoscopic view of the stapled and cut edges of the partition between the esophagus and the Zenker diverticulum.
-
Zenker diverticulum. Demonstration of stapling diverticulotomy using a 35 mm vascular stapler with an articulating arm.
-
Cricopharyngeal myotomy. Demonstration of a transmucosal cricopharyngeal myotomy using a CO2 laser. The buccopharyngeal fascia layer is meticulously preserved.
-
View of a Zenker diverticulum with transverse fibers of the cricopharyngeus muscle below the diverticulum, as viewed through the left side of the neck.
-
Illustrated barium swallow demonstrates the pouch retaining contrast and its connection to the esophagus immediately inferior and posterior to the larynx.
-
Barium swallow showing posteriorly positioned, moderate-sized Zenker diverticulum.
-
Very prominent cricopharyngeal muscle creating obstruction immediately inferior to the diverticulum.
Tables
What would you like to print?
- Practice Essentials
- Pathophysiology
- Etiology
- Staging
- Epidemiology
- History and Physical Examination
- Barium Swallow With Videofluoroscopy
- Endoscopic Assessment
- Treatment Indications and Contraindications
- CP Myotomy
- Endoscopic Diverticulotomy
- Surgical Advantages, Disadvantages, and Complications
- Questions & Answers
- Show All
- Media Gallery
- References