Practice Essentials
Prosthetic heart valves are increasingly being used for dysfunctional native valves requiring intervention. Broadly, they can be classified into three categories: mechanical heart valves, bioprosthetic valves, and homograft. The goal of an artificially placed valve is to function like a native one in terms of hemodynamics and with minimal side effects (low thrombogenicity).
Bioprosthetic valves (see the image below) generally offer functional properties (eg, hemodynamics, resistance to thrombosis) similar to that of native valves, but longevity is limited relative to mechanical valves. Mechanical heart valves have subpar hemodynamics with increased thrombogenicity; they also require anticoagulation but have greater long-term durability. [1]
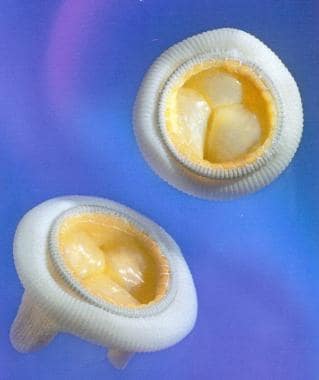 Prosthetic Heart Valves. The Hancock M.O. II aortic bioprosthesis (porcine). Reproduced with permission from Medtronic, Inc.
Prosthetic Heart Valves. The Hancock M.O. II aortic bioprosthesis (porcine). Reproduced with permission from Medtronic, Inc.
Replacement of diseased valves with prosthetic heart valves reduces the morbidity and mortality associated with native valvular disease, but it comes at the expense of risking complications related to the implanted prosthetic device. Even when promptly recognized and treated, acute prosthetic valve failure is associated with a high mortality.
Signs and symptoms
Signs and symptoms of prosthetic heart valve malfunction depend on the type of valve, its location, and the nature of the complication. Presentations may include the following:
-
Acute prosthetic valve failure: Acute heart failure signs such as sudden onset dyspnea, syncope, precordial pain, new heart murmur, and lung crackles on auscultation
-
Subacute valvular failure: Symptoms of gradually worsening congestive heart failure
-
Embolic complications (thrombotic or infectious): Symptoms related to the site of embolization (eg, stroke, myocardial infarction [MI], signs and symptoms of visceral or peripheral embolization)
-
Anticoagulant-related hemorrhage: Site-specific hemorrhage, gastrointestinal (GI) bleed, muscle hematoma, cerebral hemorrhage
-
Prosthetic valve endocarditis: Fever, chills, fatigue, malaise, night sweats, signs and symptoms of heart failure if there is disrupted valvular integrity, dyspnea, cough, pleuritic chest pain, new or changed heart murmur, splenomegaly, septic shock, septic emboli
-
Hemolysis secondary to prosthetic valve: Symptoms secondary to anemia and/or thrombocytopenia, such as pallor, dyspnea, bleeding, and petechiae
On physical examination, normal prosthetic heart valve sounds include the following:
-
Mechanical valves: Loud, high-frequency, metallic closing sound; soft opening sound (tilting disc and bileaflet valves); low-frequency opening and closing sounds of nearly equal intensity (caged ball valves)
-
Tissue valves: Similar closing to those of native valves, low-frequency early opening sound in the mitral position
Prosthetic heart valve murmurs noted include the following:
-
Aortic prosthetic valves: Some degree of outflow obstruction with a resultant systolic ejection murmur (loudest in caged ball and small porcine valves); low-intensity diastolic murmur (tilting disc and bileaflet valves)
-
Mitral prosthetic valves: Low-grade systolic murmur (caged ball valves); short diastolic murmur (bioprostheses and, occasionally, St. Jude bileaflet valves)
Additional findings may include the following:
-
Acute valvular failure: Evidence of poor tissue perfusion; hyperdynamic precordium and right ventricular impulse (50% of cases); absence of a normal valve closure sound or presence of an abnormal regurgitant murmur
-
Subacute valvular failure: Rales and jugular venous distention; signs of right-side failure; a new regurgitant murmur or absence of normal closing sounds; a new or worsening hemolytic anemia (may be the only presenting abnormality)
See Presentation for more detail.
Diagnosis
Laboratory studies that may be useful include the following:
-
Complete blood cell (CBC) count
-
Blood urea nitrogen (BUN) and creatinine levels
-
Urinalysis
-
Blood cultures
-
Prothrombin time (PT) or international normalized ratio (INR)
-
Peripheral smear
-
Lactate dehydrogenase (LDH) and haptoglobin
-
Liver function tests
Imaging studies that may be helpful include the following:
-
Chest radiography: This modality can help in delineating the valvular morphology and determining whether the valve and occluder are intact; each of the most commonly used valve types has its own characteristic radiographic appearance
-
Echocardiography (transthoracic Doppler, transesophageal [the study of choice for a suspected prosthetic valve complication])
-
Cinefluorography: This study may detect impaired occluder movement but often cannot readily determine the etiology
-
Computed tomography (CT) scanning: The Society of Cardiovascular Computed Tomography (SCCT) states that CT scanning should be performed as part of the evaluation of all patients being considered for transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR), except those in whom CT is contraindicated. [2, 3] CT images should be interpreted with a member of the TAVI/TAVR team or reviewed with the operator before the procedure.
See Workup for more detail.
Management
In patients with acute valvular failure, diagnostic studies must be performed simultaneously with resuscitative efforts.
Treatment approaches to primary valve failure include the following:
-
Emergency valve replacement
-
Concomitant adjunctive therapy
-
Afterload reduction and inotropic support
-
In selected cases, intra-aortic balloon counterpulsation
Treatment approaches to prosthetic valve endocarditis (PVE) include the following:
-
Administration of intravenous (IV) antibiotics as soon as two sets of blood cultures are drawn
-
Cessation of warfarin until central nervous system involvement is ruled out and invasive procedures are determined to be unnecessary [4]
-
Consideration of IV heparin instead of oral anticoagulation in the acute setting
-
Consideration of emergency surgery in patients with large, mobile vegetations; persistence of septicemia for more than 2 days after being on an effective antibiotic treatment; moderate to severe heart failure; or with an unstable prosthesis noted on echocardiography or fluoroscopy
Treatment approaches to thromboembolic complications include the following:
-
Anticoagulation (if it has not already been initiated or if the patient has a subtherapeutic INR)
-
Assessment of valve function
Treatment approaches to prosthetic valve thrombosis include the following:
-
Surgery (historically the mainstay of treatment but associated with a high mortality)
-
Thrombolytic therapy (appropriate for selected patients with thrombosed prosthetic valves): Should always be performed in conjunction with cardiovascular surgical consultation
-
In cases of major anticoagulant-related hemorrhage, reversal of anticoagulation
See Treatment and Medication for more detail.
Background
Implantation of prosthetic cardiac valves to treat hemodynamically significant valvular disease has become an increasingly common procedure. According to a study by iData Research, it is estimated that 180,000 patients undergo heart valve replacement in the United States every year, [5] and the numbers are expected to increase further with the advent of newer modalities and with growing evidence of appropriate clinical indications for heart valve replacement. For example, indications for transcatheter aortic valve replacement (TAVR) have been expanded several times since the initial Food and Drug Administration (FDA) approval for prohibitive-risk surgical patients in 2011. [6] In 2019, following the results of the Placement of Aortic Transcatheter Valves (PARTNER)-3 trial, the FDA further expanded the indication for TAVR valves to include low-risk patients with severe aortic stenosis. [7, 8]
Broadly, these devices can be classified into three categories: mechanical heart valves, bioprosthetic valves, and homograft. The goal of an artificial valve is to function like a native one in terms of hemodynamics, with minimal side effects (low thrombogenicity). Bioprosthetic valves generally offer functional properties (eg, hemodynamics, resistance to thrombosis) similar to those of native valves, but they are at risk of structural damage and thus limiting their long-term usage. Mechanical heart valves have subpar hemodynamics and an increased level of thrombogenicity that requires anticoagulation, but they usually have long-term durability. [1]
Replacement of diseased valves reduces the morbidity and mortality associated with native valvular disease, but this comes at the expense of risking complications related to the implanted prosthetic device. These complications include primary valve failure, prosthetic valve endocarditis (PVE), prosthetic valve thrombosis (PVT), thromboembolism, and mechanical hemolytic anemia. In addition, because many of the affected patients require long-term anticoagulation, anticoagulant-related hemorrhage may occur.
More than 80 models of artificial valves have been introduced since 1950. In day-to-day clinical practice, however, it is necessary to be familiar with a few basic types. Prosthetic valves are either created from synthetic material (mechanical prosthesis) or fashioned from biological tissue (bioprosthesis). The choice of prosthesis is determined by the anticipated longevity of the patient and his/her ability to tolerate anticoagulation. [9]
Selecting the best prosthetic heart valve for a patient can be difficult owing to the lack of industry standards on the sizing, placement, and hemodynamic and structural performance of these devices. In October 2020, the European Association for Cardio-Thoracic Surgery (EACTS), the Society of Thoracic Surgeons (STS), and the American Association for Thoracic Surgery (AATS) Valve Labelling Task Force released their recommendations for providing surgical heart valve physical dimensions, intended implant position, and hemodynamic performance in a transparent, consistent manner. [10] The task force also advocates for a standardized chart to assess the probability of prosthesis–patient mismatch and calls for manufacturers to provide essential information required for valve choice on standardized charts.
Design Features
Mechanical valves
Three main designs of mechanical valves exist: caged ball valve, tilting disc (single leaflet) valve, and bileaflet valve. The only Food and Drug Administration (FDA)–approved caged ball valve is the Starr-Edwards valve (see the image below).
 Prosthetic Heart Valves. Starr-Edwards Silastic ball valve mitral Model 6120. Reproduced with permission from Baxter International, Inc.
Prosthetic Heart Valves. Starr-Edwards Silastic ball valve mitral Model 6120. Reproduced with permission from Baxter International, Inc.
Tilting disc (single leaflet) valves
Tilting disc valve models include the Medtronic Hall valve (see the image below), Omnicarbon (Medical CV) valves, Monostrut (Alliance Medical Technologies), and the discontinued Bjork-Shiley valves (Shiley Laboratories).
 Prosthetic Heart Valves. Medtronic Hall mitral valve. Reproduced with permission from Medtronic, Inc.
Prosthetic Heart Valves. Medtronic Hall mitral valve. Reproduced with permission from Medtronic, Inc.
Bileaflet valves
Bileaflet valves include the St. Jude (St. Jude Medical) (see the following image), the most commonly implanted valve in the United States; CarboMedics valves (Sulzer CarboMedics); ATS Open Pivot valves (ATS Medical); and On-X and Conform-X valves (CryoLife).
 Prosthetic Heart Valves. St. Jude Medical mechanical heart valve. Photograph courtesy of St. Jude Medical, Inc. All rights reserved. St. Jude Medical is a registered trademark of St. Jude Medical, Inc.
Prosthetic Heart Valves. St. Jude Medical mechanical heart valve. Photograph courtesy of St. Jude Medical, Inc. All rights reserved. St. Jude Medical is a registered trademark of St. Jude Medical, Inc.
Bioprosthetic valve
Bioprosthetic (xenograft) valves are made from porcine valves or bovine pericardium. Porcine models include the Carpentier-Edwards valves (Edwards Lifesciences) and Hancock II and Mosaic valves (Medtronic). See the images below.
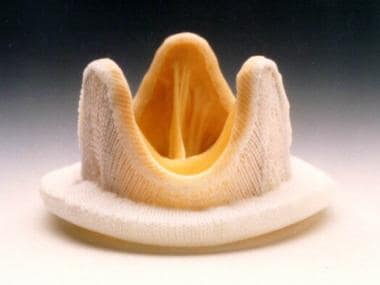 Prosthetic Heart Valves. Carpentier-Edwards Duralex mitral bioprosthesis (porcine). Reproduced with permission from Baxter International, Inc.
Prosthetic Heart Valves. Carpentier-Edwards Duralex mitral bioprosthesis (porcine). Reproduced with permission from Baxter International, Inc.
 Prosthetic Heart Valves. The Hancock M.O. II aortic bioprosthesis (porcine). Reproduced with permission from Medtronic, Inc.
Prosthetic Heart Valves. The Hancock M.O. II aortic bioprosthesis (porcine). Reproduced with permission from Medtronic, Inc.
Pericardial valves include the Perimount series valves (Edwards LifeSciences). Ionescu-Shiley pericardial valves have been discontinued. Stentless porcine valves have also come into use; they offer improved hemodynamics with a decreased transvalvular pressure gradient when compared to older stented models. Stentless models include the Edwards Prima Plus, Medtronic Freestyle, and Toronto SPV valve (St. Jude Medical). [11]
Indications for Heart Valve Surgery
Aortic stenosis
The 2020 American College of Cardiology/American Heart Association (ACC/AHA) recommendations for aortic valve replacement in patients with valvular aortic stenosis (AS) are summarized below. [12] In most adults with symptomatic severe AS, aortic valve replacement (AVR) is the surgical treatment of choice. If concomitant coronary disease is present, AVR and coronary artery bypass graft (CABG) surgery should be performed simultaneously.
Successful AVR produces substantial clinical and hemodynamic improvement in patients with AS, including octogenarians. AVR should be performed in all symptomatic patients with severe AS regardless of left ventricular (LV) function, as survival is better with surgical treatment than with medical treatment.
ACC/AHA recommendations for AVR in AS are as follows [12] :
-
Symptomatic patients (by history or on exercise) with severe high-gradient AS (class I)
-
Patients with asymptomatic severe AS and LV ejection fraction (LVEF) below 50% (class I)
-
Patients with severe AS undergoing other cardiac surgery (class I)
-
Asymptomatic patients with very severe AS and low surgical risk or decreased exercise tolerance or hypotension on exercise (class IIa)
-
Symptomatic patients with low-flow/low-gradient severe AS with reduced LVEF and results of a low-dose dobutamine stress study showing aortic velocity ≥4 m/s or a mean pressure gradient ≥40 mmHg with a valve area ≤1.0 cm2 (class IIa)
-
Patients with moderate AS undergoing CABG surgery or surgery on the aorta or other heart valves (class IIa)
-
Asymptomatic patients with severe AS and rapid progression with low surgical risk (class IIb)
The class recommendations indicated above are defined as follows [12] :
-
Class I: Conditions for which there is evidence and/or general agreement that the procedure or treatment is beneficial, useful, and effective
-
Class II: Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment
-
Class IIa: Weight of evidence/opinion is in favor of usefulness/efficacy
-
Class IIb: Usefulness/efficacy is less well established by evidence/opinion
-
Class III: Conditions for which there is evidence and/or general agreement that the procedure/treatment is not useful/effective and in some cases may be harmful
Choice of intervention AS include the following [12] :
-
Symptomatic patients with severe AS with low to intermediate surgical risk: Surgical AVR (SAVR) is preferred (class I)
-
Symptomatic patients with severe AS and high surgical risk, SAVR or transcatheter aortic valve replacement (TAVR) is offered on a case-by-case basis with evaluation of patient-specific risk and preferences (class I)
-
Symptomatic patients with severe AS and prohibitive risk for SAVR should undergo TAVR (class I)
-
TAVR is a reasonable alternative for symptomatic patients with severe AS and intermediate surgical risk (class IIa)
-
Percutaneous balloon aortic valvuloplasty (BAV) can be used as a bridge to AVR in patients with hemodynamic instability due to severe AS (class IIb)
Aortic regurgitation
Under the 2020 ACC/AHA guidelines, aortic valve surgery is recommended for patients with chronic, severe aortic regurgitation (AR) when the patient is symptomatic. [12] It is also recommended in the asymptomatic patient with chronic, severe AR who has a resting EF of 50% or less or LV dilatation. Additional circumstances in which aortic valve surgery may be reasonable are listed below. [12] Surgical treatment of AR usually requires replacement of the diseased valve with a prosthetic valve, although valve-sparing repair is increasingly possible with advances in surgical technique and technology.
ACC/AHA recommendations for AVR in AR are as follows [12] :
-
Symptomatic patients with severe AR, irrespective of LV systolic function (class I)
-
Asymptomatic patients with chronic severe AR and LV systolic dysfunction (EF < 50%) at rest (class I)
-
Patients with chronic severe AR while undergoing CABG or surgery on the aorta or other heart valves (class I)
-
Asymptomatic patients with severe AR with normal LV systolic function (EF >50%) but with severe LV dilatation (end-diastolic dimension [EDD] >65 mm or end-systolic dimension [ESD] >50 mm) (class IIa)
-
Patients with moderate AR while undergoing surgery on the ascending aorta (class IIa)
-
Patients with moderate AR while undergoing CABG (class IIa)
-
Asymptomatic patients with severe AR and normal LV systolic function at rest (EF >50%) when the degree of LV dilatation exceeds an EDD of 70 mm or ESD of 50 mm, when there is evidence of progressive LV dilatation, declining exercise tolerance, or abnormal hemodynamic responses to exercise (class IIb)
-
Asymptomatic patients with mild, moderate, or severe AR and normal LV systolic function at rest (EF >50%) when degree of dilatation is not moderate or severe (EDD < 70 mm, ESD < 50 mm) (class III)
Mitral stenosis
Valve replacement for mitral stenosis (MS) may be considered in patients who are candidates for surgical therapy when the valve is not suitable for valvotomy (either surgical or percutaneous). The recommendations for surgery in patients with mitral stenosis, according to the 2020 ACC/AHA guidelines, are described below. [12]
1. Mitral valve surgery (repair if possible) is indicated in patients with symptomatic (New York Heart Association [NYHA] functional class III–IV) severe MS (mitral valve area [MVA] ≤1.5 cm2) under any of the following circumstances (class I):
-
Percutaneous mitral balloon valvotomy is unavailable
-
Percutaneous mitral balloon valvotomy is contraindicated because of left atrial thrombus despite anticoagulation or because concomitant moderate to severe mitral regurgitation (MR) is present
-
The valve morphology is not favorable for percutaneous mitral balloon valvotomy based on a Wilkins score in a patient with acceptable operative risk
2. Patients with severe MS (MVA ≤1.5 cm2) undergoing other cardiac surgery (class I)
3. Severely symptomatic patients (NYHA III/IV) with severe MS (MVA ≤1.5 cm2) with other operative indications (class IIa)
4. Patients with moderate MS (MVA of 1.6-2.0 cm2) undergoing other cardiac surgery (class IIb)
5. Patients with severe MS (MVA ≤1.5 cm2) with recurrent embolic events while on adequate anticoagulation are candidates for mitral valve surgery and excision of the left atrial appendage (class IIb)
Mitral regurgitation
Chronic primary MR
Although more technically demanding, mitral valve repair is recommended over mitral valve replacement (MVR) in most patients with severe, chronic mitral regurgitation (MR) who require surgery. Such patients should be referred to surgical centers experienced with mitral valve repair. If mitral valve repair is not feasible, MVR with preservation of the chordal apparatus is preferred, as this preserves LV function and enhances postoperative survival. [12] Note the following ACC/AHA recommendations for surgery in patients with MR [12] :
-
Mitral valve surgery is recommended in symptomatic severe MR, irrespective of LV systolic function (class Ib).
-
Mitral valve surgery is indicated in asymptomatic patients with chronic severe primary MR and an LVEF < 60% and an LVESD ≥ 40 mm (class Ib).
-
In patients with chronic severe primary MR limited to the posterior leaflet, mitral valve repair is preferred over MVR (class I).
-
In patients with chronic severe primary MR involving the anterior leaflet or both leaflets when a successful and durable repair can be accomplished, mitral valve repair is preferred over MVR (class I).
-
In patients with chronic severe primary MR undergoing other cardiac surgery, mitral valve repair or MVR is indicated (class I).
Chronic severe secondary MR
In patients with chronic severe secondary MR undergoing CABG or AVR, mitral vlave surgery is reasonable (class IIa).
In severely symptomatic patients (NYHA III-IV) despite being on goal-directed medical therapy who have chronic severe secondary MR, mitral valve surgery can be considered (class IIb).
Tricuspid regurgitation
Valve replacement is required in both tricuspid regurgitation (TR) and tricuspid stenosis (TS) if the anatomic characteristics of the leaflet are not amenable to repair. Note the following:
-
In patients with severe TR undergoing left-sided valve surgery, tricuspid valve (TV) surgery is indicated (class I).
-
In symptomatic patients with severe primary TR unresponsive to medical therapy, TV surgery can be beneficial (class IIa).
-
In asymptomatic or minimally symptomatic patients with severe primary TR and progressive right ventricular systolic dysfunction and/or dilatation, TV surgery can considered (class IIb).
Tricuspid stenosis
TV surgery is indicated in the following (all class I) [12] :
-
Patients with severe TS undergoing left-sided valve surgery
-
Patients with isolated, symptomatic severe TS
Clinical Implementation of Bioprosthetic Valves
Factors involved in decision making for the type of valve prosthesis (mechanical or bioprosthetic) to use include the following:
-
Age: Mechanical valves, given their longer durability, are indicated in younger patients (age < 50 y). In older patients (age >65 y), bioprosthetic valves are favored due to the higher risk of anticoagulation complications in this population.
-
Risk of thrombogenicity: Mechanical valves have a high risk of thrombogenicity and patients who receive these valves require lifelong anticoagulation, thus making such valves unsuitable for anyone with contraindications to anticoagulation. Bioprosthetic valves are used in clinical scenarios where warfarin is contraindicated or if the patient is at a higher risk of bleeding.
-
Compliance with regular internation normalized ratio (INR) monitoring: Consistent INR monitoring is required in patients receiving warfarin therapy for a mechanical valve.
-
Other indications for long-term anticoagulation: Consider using mechanical valves in patients with a high risk of thromboembolism who many benefit from anticoagulation (eg, atrial fibrillation).
-
Specific anatomic considerations: For example, a valve-in-valve procedure is relatively contraindicated in individuals with a small aortic root size.
-
Patient preference: Religious values, lifestyle choices, inconvenience of long-term anticoagulation, and/or the risk of reintervention may influence a patient's preference of the valve type.
ACC/AHA recommendations for selection of a prosthetic aortic valve include the following [12] :
-
A mechanical prosthesis is recommended for aortic valve replacement (AVR) in patients with a mechanical valve in the mitral or tricuspid position (class I).
-
A bioprosthesis is recommended for AVR in patients of any age who will not take warfarin or who have major medical contraindications to warfarin therapy (class I).
-
A bioprosthesis is reasonable for AVR in patients aged 70 years or older who do not have risk factors for thromboembolism (class IIa).
-
AV re-replacement with a homograft is reasonable for patients with active prosthetic valve endocarditis (class IIa).
-
A bioprosthesis might be considered for AVR in a woman of childbearing age (class IIb).
-
A mechanical prosthesis is reasonable in younger patients (age < 50 y) with no contraindications to anticoagulation therapy (class IIa).
ACC/AHA recommendations for selection of a prosthetic mitral valve include the following [12] :
-
A mechanical prosthesis is recommended for mitral valve replacement (MVR) in patients with a mechanical valve in the mitral or tricuspid position (class I).
-
A mechanical prosthesis is reasonable for MVR in patients younger than 50 years who have long-standing atrial fibrillation (class IIa).
-
A bioprosthesis is reasonable for MVR in patients aged 65 years or older (class IIa).
Clinical Trial Evidence for Bioprosthetic Valves
In a Veterans Affairs (VA) study comparing bioprosthetic valves with mechanical valves, all-cause mortality at 15 years after aortic valve replacement (AVR) was lower in patients who received a mechanical valve than in those who received a bioprosthetic valve (66% vs 79%, respectively). [13] In the study, 575 patients at 13 VA medical centers undergoing single AVR (n = 394) or single mitral valve replacement (MVR) (n = 181) were randomized to receive a Hancock porcine valve or a Bjork-Shiley spherical disc valve. Long-term survival and valve-related complications were compared. No significant difference in all-cause mortality was seen between the two MVR valve type groups. [13]
There was a higher reoperation rate after AVR with the bioprosthetic valve than with the mechanical valve (29 ± 5% vs 10 ± 3%). [13] Valve-related deaths after AVR accounted for 41% of all deaths in the bioprosthetic group and 37% in the mechanical valve group; valve-related deaths after MVR were 57% of all deaths in the bioprosthetic group and 44% in the mechanical valve group. Primary valve failure was significantly greater with bioprosthetic valves for AVR (bioprosthetic vs mechanical, 23 ± 5% vs 0 ± 0%) and for MVR (44 ± 8% vs 5 ± 4%). [13]
Almost all the primary valve failures were in patients younger than 65 years (18 of 20 patients in the AVR group; 20 of 21 patients in the MVR group). [13] Bleeding occurred more frequently in patients with a mechanical valve than in those with a bioprosthesis (AVR, 51 ± 4% vs 30 ± 4%; MVR, 53 ± 7% vs 31 ± 6%). However, no statistically significant differences were seen between the two valve groups for systemic embolism, infective endocarditis, or valve thrombosis. [13]
Similar results were seen in an Edinburgh heart valve trial, in which 533 patients (AVR, n = 211; MVR, n = 261; double valve replacement, n = 61) were randomized to receive a Bjork-Shiley 60° spherical tilting disc valve (n = 267) or a porcine bioprosthesis (Hancock, n = 107; Carpentier-Edwards, n = 159). [14] Long-term survival at 20 years were not significantly different between the two valve groups (mechanical 25.0 ± 2.7%, porcine 22.6 ± 2.7%), but major bleeding was more common in the Bjork-Shiley group than in the bioprosthesis group (40.7 ± 5.4% vs 27.9 ± 8.4%, respectively). No significant differences were seen in major embolism or endocarditis. [14]
Pathophysiology
Valve failure
Primary valve failure, although rare, is an important complication in patients with prosthetic heart valves, and it is a major cause of morbidity and mortality. As noted earlier, bioprostheses are less thrombogenic than mechanical valves, but this advantage is balanced by their diminished durability when compared with mechanical valves. Although 30-35% of bioprostheses will fail within 10-15 years, it can be anticipated that most mechanical valves will remain functional for 20-30 years.
Bioprosthetic valve failure can be secondary to structural degeneration due to thickening, calcification, valve dehiscence/tearing/disruption, or it may develop from nonstructural causes such as patient-prosthesis mismatch, prosthesis malposition, paravalvular regurgitation, thrombosis, and pannus formation. It results in either obstruction or incompetence of the underlying valve, causing hemodynamic changes.
Due to the excellent durability of mechanical valves, structural valve deterioration is extremely rare; primary valve failure in patients who receive these valves may be secondary to suture line dehiscence, thrombus formation, or breakage or separation of the valve components. Acute valvular regurgitation or embolization of the valve fragments may also result.
When the mitral valve acutely fails, rapid left atrial (LA) volume overload causes increased LA pressure. Pulmonary venous congestion and, ultimately, pulmonary edema occur. Cardiac output is decreased because a portion of the left ventricular (LV) output is being regurgitated into the LA. The compensatory mechanism of increased sympathetic tone increases the heart rate and the systemic vascular resistance (SVR). This may worsen the situation by decreasing diastolic filling time and impeding LV outflow, thereby increasing the regurgitation.
Acute failure of a prosthetic aortic valve causes a rapidly progressive LV volume overload. Increased LV diastolic pressure results in pulmonary congestion and edema. The cardiac output is reduced substantially. The compensatory mechanism of an increased heart rate and a positive inotropic state, mediated by increased sympathetic tone, partly helps to maintain output. However, this is hampered by an increase in SVR, which impedes forward flow. Increased systolic wall tension causes a rise in myocardial oxygen consumption. Myocardial ischemia in acute aortic regurgitation may occur, even in the absence of coronary artery disease.
Biological prosthetic valves often degenerate slowly over time, become calcified, or suffer from thrombus formation. These events result in the slow progressive failure of the valve. The clinical presentation is usually that of gradually worsening congestive heart failure, with increasing dyspnea. Alternatively, patients may present with unstable angina or systemic embolization, or they may be entirely asymptomatic.
The first transcatheter aortic valve implantation (TAVI) device for use in the United States was approved in November 2011. Subsequently, not enough time has passed yet to gather data concerning longevity and use. Vascular complications and strokes related to the procedure are falling with improved delivery techniques and equipment. Complications related to the conduction system requiring permanent pacemaker implantation occur in 14% of patients. This risk is increased with the use of the CoreValve prosthesis. [15]
Prosthetic valve endocarditis
Even with antibiotic prophylaxis, the yearly incidence of prosthetic valve endocarditis (PVE) is around 0.5%. PVE occurring within 1 year of implantation (early PVE) is usually due to perioperative contamination or hematogenous spread. [16] PVE occurring after 1 year (late PVE) is usually caused by hematogenous spread.
The pathologic hallmark of PVE in mechanical valves is ring abscesses. Ring abscesses may lead to valve dehiscence and perivalvular leakage. Local extension results in the formation of myocardial abscesses. Further extension to the conduction system often results in a new atrioventricular block. Valve stenosis and purulent pericarditis occur less frequently.
Bioprosthetic valve PVE usually causes leaflet tears or perforations. Valve stenosis is more common with bioprosthetic valves than with mechanical valves. Ring abscess, purulent pericarditis, and myocardial abscesses are much less frequent in bioprosthetic valve PVE.
Finally, glomerulonephritis, mycotic aneurysms, systemic embolization, and metastatic abscesses also may complicate PVE.
Prosthetic valve thrombosis
Prosthetic valve thrombosis is a major complication of mechanical heart valves although the newer generation valves have lower thrombogenic risks. Bioprosthetic heart valve thrombosis although rare, if present, is usually seen within 3 months of placement of the valve and requires anticoagulation for that time period (time period required for appropriate endothelization). The pathophysiology involves increased thrombogenicity of the valve material, interaction between the prosthesis material and the suture zone, localized regions of turbulent flow, and location of the valve itself. For example, tricuspid mechanical prostheses are known to be more thrombogenic than others. Low cardiac output can favor thrombosis and other factors such as subtherapeutic anticoagulation; other risk factors of thrombosis may contribute as well. Sometimes, overgrowth of granulation tissue, called pannus formation, can mimic prosthetic valve thrombosis; it is thought to be secondary to increased levels of cytokines, resulting in excess fibrosis and scar tissue formation at the periannular tissue. [2, 3, 4]
Etiology
Prosthetic valve endocarditis (PVE) has been divided into two subcategories. These reflect differences in clinical features, microbial patterns, and mortality. Early PVE occurs within the first year of valve insertion, whereas late PVE occurs after the first year.
Early PVE is usually the result of perioperative contamination. Causative organisms include Staphylococcus epidermidis (25-30%), Staphylococcus aureus (15-20%), gram-negative aerobes (20%), fungi (10-12%), streptococci (5-10%), and diphtheroids (8-10%).
Late PVE is usually the result of transient bacteremia from dental or genitourinary sources, gastrointestinal manipulation, or intravenous drug abuse. The causative organisms are similar to those that cause native valve endocarditis, includinf Streptococcus viridans (25-30%), S epidermidis (23-38%), S aureus (10-12%), gram-negative bacilli (10-12%), group D streptococci (10-12%), fungi (5-8%), and diphtheroids (4-5%). An increase in cases of PVE due to methicillin-resistant S aureus (MRSA) has been observed.
Multiple negative blood culture results are unusual with common pathogens, but they are seen more commonly with infections by the Haemophilus aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae (HACEK) group; Serratia and Rickettsia species; as well as Aspergillus, Histoplasma, and Candida species.
Rarely, Brucella can cause PVE. [17]
Epidemiology
Prosthetic valve thrombosis is more common in mechanical valves. With proper anticoagulation, the rate of thrombosis in all valves is within the range of 0.1-5.7% per patient-year. Caged ball valves have the highest rate of thromboembolic complications, whereas bileaflet valves have the lowest. Valve thrombosis is increased with valves in the mitral position and in patients with subtherapeutic anticoagulation; it is also known to be increased in tricuspid mechanical protheses relative to left-side mechanical valves.
Anticoagulant-related hemorrhagic complications of mechanical valves include major hemorrhage in 1-3% of patients per year and minor hemorrhage in 4-8% of patients per year.
Low-grade hemolytic anemia occurs in 70% of prosthetic heart valve recipients, and severe hemolytic anemia occurs in 3%. The incidence is increased with caged ball valves and in those with perivalvular leaks.
Primary valve failure occurs in 3-4% of patients with bioprostheses within 5 years of implantation and in up to 35% of patients within 15 years. Mechanical valves have a much lower incidence of primary failure.
Prosthetic valve endocarditis (PVE) occurs in 2-4% of patients. The incidence is 3% in the first postoperative year, then 0.5% for subsequent years. The incidence is higher when valve surgery is performed in patients with active native valve endocarditis, and it is higher in mitral valves. Mechanical and biological valves are equally susceptible to early PVE, but the incidence of late PVE is higher for bioprostheses. Despite improvements in surgical techniques, no appreciable change in the incidence has been observed. [18]
Age
In children, bioprostheses rapidly calcify and, therefore, undergo rapid degeneration and valve dysfunction. The incidence of bioprosthetic failure is much higher in patients younger than 40 years. The incidence of having any prosthetic valve complication decreases with age.
Prognosis
In a retrospective cohort analysis of 4253 patients who underwent primary isolated aortic-valve replacement, 15-year survival and stroke rates were equivalent with bioprosthetic and mechanical valves. [1, 19] For bioprosthetic valves, the risk of repeat surgery was greater, but the incidence of major bleeding was lower.
In propensity-matched comparisons, actuarial 15-year mortality was 60.6% with the bioprosthetic aortic valve and 62.1% with the mechanical valve. [1, 19] Cumulative 15-year stroke rates were 7.7% and 8.6% in the two groups, respectively. Reoperation was 12.1% in the bioprosthetic valve group at 15 years and 6.9% in the mechanical valve group, whereas major bleeding occurred in 6.6% of bioprosthesis patients and in 13.0% of the mechanical-valve group. [1, 19]
Morbidity/mortality
Acute failure of a prosthetic aortic valve usually leads to sudden or near-sudden death. Prompt recognition and treatment of acute prosthetic mitral valve failure can be lifesaving. [16]
Prosthetic valve endocarditis (PVE) has an overall mortality of 50%. In early PVE, it is 74%, whereas in late PVE, mortality is 43%. With a fungal etiology, mortality is 93%; mortality for staphylococcal infections is 86%. PVE due to Staphylococcus has a 25-40% mortality. [16, 18]
Fatal anticoagulant-induced hemorrhage occurs in 0.5% of patients per year.
Complications
Complications of prosthetic valves include primary valve failure, PVE, prosthetic valve thrombosis, thromboembolism, and mechanical hemolytic anemia. In addition, anticoagulant-related hemorrhage may occur.
Primary valve failure may occur abruptly from the tearing or breakage of components, or from a thrombus suddenly impinging on leaflet mobility. More commonly, valve failure presents gradually from calcifications or thrombus formation. Bioprostheses are less thrombogenic than mechanical valves, but this advantage is balanced by their diminished durability when compared with mechanical valves. Primary valve failure occurs in 3-4% of patients with bioprostheses within 5 years of implantation and in up to 35% of patients within 15 years. In contrast, it is anticipated that most mechanical valves will remain functional for 20-30 years.
When the mitral valve fails acutely, rapid left atrial (LA) volume overload causes increased LA pressure. Pulmonary venous congestion and, ultimately, pulmonary edema ensue. Cardiac output is decreased because a portion of the left ventricular (LV) output is regurgitated into the LA. The compensatory mechanism of increased sympathetic tone increases the heart rate and the systemic vascular resistance (SVR), which may worsen the situation by decreasing diastolic filling time and impeding LV outflow, thereby increasing the regurgitation.
Acute failure of a prosthetic aortic valve causes a rapidly progressive LV volume overload. Increased LV diastolic pressure results in pulmonary congestion and edema. The cardiac output is reduced substantially. The compensatory mechanism of an increased heart rate and a positive inotropic state, mediated by increased sympathetic tone, partly helps to maintain output. However, this is hampered by an increase in SVR, which impedes forward flow. Increased systolic wall tension causes a rise in myocardial oxygen consumption. Myocardial ischemia may follow acute aortic regurgitation, even in the absence of coronary artery disease.
Stenosis or incompetence of prosthetic valves may develop as a result of a tear or perforation of the valve cusp, valvular thrombosis, pannus formation, valve calcification, or stiffening of the leaflets.
PVE occurs in 2-4% of patients. The incidence is 3% in the first postoperative year, then 0.5% for subsequent years, and it is higher in mitral valves. Mechanical and biological valves are equally susceptible. PVE occurring within 60 days of implantation (early PVE) is usually due to perioperative contamination or hematogenous spread. PVE occurring after 60 days (late PVE) is usually caused by hematogenous spread. Glomerulonephritis, congestive heart failure, mycotic aneurysms, and metastatic abscesses may complicate PVE.
Bioprosthetic valve endocarditis usually causes leaflet tears or perforations. Valve stenosis is more common with bioprosthetic valves than with mechanical valves. Ring abscess, purulent pericarditis, and myocardial abscesses are much less frequent in bioprosthetic valve endocarditis.
-
Prosthetic Heart Valves. Medtronic Hall mitral valve. Reproduced with permission from Medtronic, Inc.
-
Prosthetic Heart Valves. The Hancock M.O. II aortic bioprosthesis (porcine). Reproduced with permission from Medtronic, Inc.
-
Prosthetic Heart Valves. Starr-Edwards Silastic ball valve mitral Model 6120. Reproduced with permission from Baxter International, Inc.
-
Prosthetic Heart Valves. Carpentier-Edwards Duralex mitral bioprosthesis (porcine). Reproduced with permission from Baxter International, Inc.
-
Prosthetic Heart Valves. Carpentier-Edwards Perimount pericardial aortic bioprosthesis. Reproduced with permission from Baxter International, Inc.
-
Prosthetic Heart Valves. St. Jude Medical mechanical heart valve. Photograph courtesy of St. Jude Medical, Inc. All rights reserved. St. Jude Medical is a registered trademark of St. Jude Medical, Inc.
-
Prosthetic Heart Valves. Edwards Sapien transcatheter aortic valve.






