Practice Essentials
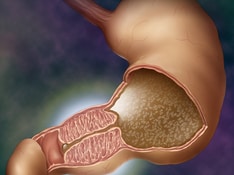
Pyloric stenosis, also known as infantile hypertrophic pyloric stenosis (IHPS), is the most common cause of intestinal obstruction in infancy. IHPS occurs secondary to hypertrophy and hyperplasia of the muscular layers of the pylorus, causing a functional gastric outlet obstruction. See the image below.
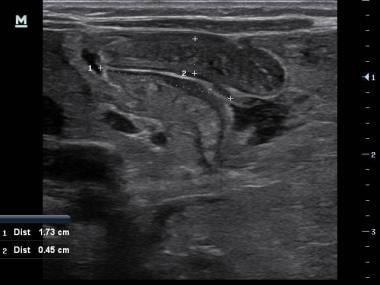 Point-of-care ultrasound performed by a pediatric emergency physician accurately identifying the pyloric wall thickness and length that meets criteria for pyloric stenosis diagnosis.
Point-of-care ultrasound performed by a pediatric emergency physician accurately identifying the pyloric wall thickness and length that meets criteria for pyloric stenosis diagnosis.
In 1717, Blair first reported autopsy findings of pyloric stenosis. Although the description of the signs and symptoms of infantile hypertrophic pyloric stenosis can be found in the 17th century, the clinical picture and pathology were not accurately described until 1887 by the Danish pediatrician, Hirschsprung. Prior to 1912, early successful surgical procedures included gastroenterostomy, pyloroplasty, and forcible dilatation via gastrostomy. In 1912, Ramstedt observed an uneventful recovery in a patient following pyloroplasty, where sutures used in reapproximating the seromuscular layer had disrupted. Following this observation, he began leaving the split muscle layer unsutured in all subsequent repairs. The Ramstedt pyloromyotomy remains the standard procedure for pyloric stenosis today.
According to Pandya and Heiss, current recommendations include ultrasonography for diagnosis, preoperative corrections of electrolytes, and use of surgical techniques. [1]
Signs and symptoms
Classically, the infant with pyloric stenosis has nonbilious vomiting or regurgitation, which may become projectile. As the obstruction becomes more severe, the infant begins to show signs of dehydration and malnutrition, such as poor weight gain, weight loss, marasmus, decreased urinary output, lethargy, and shock.
In as many as 60-80% of the infants with IHPS, a firm, nontender, and mobile hard pylorus that is 1-2 cm in diameter, described as an "olive," may be present in the right upper quadrant at the lateral edge of the rectus abdominis muscle.
See Presentation for more detail.
Diagnosis
Laboratory studies
Electrolyte, pH, blood urea nitrogen (BUN), and creatinine levels should be obtained at the same time as intravenous access in patients with pyloric stenosis. Hypochloremic, hypokalemic metabolic alkalosis is the classic electrolyte and acid-base imbalance of pyloric stenosis.
Imaging studies
Ultrasonography is the imaging modality of choice when evaluating a child for IHPS.
See Workup for more detail.
Management
The definitive treatment for IHPS is corrective surgery.
Nonsurgical treatment for IHPS with atropine sulfate, either intravenous or oral, is an alternative in the rare case that general anesthesia or surgery is contraindicated.
See Treatment and Medication for more detail.
Pathophysiology
Marked hypertrophy and hyperplasia of the 2 (circular and longitudinal) muscular layers of the pylorus occurs, leading to narrowing of the gastric antrum. The pyloric canal becomes lengthened, and the whole pylorus becomes thickened. The mucosa is usually edematous and thickened. In advanced cases, the stomach becomes markedly dilated in response to near-complete obstruction.
The causes of infantile hypertrophic pyloric stenosis are multifactorial. [2] Both environmental factors and hereditary factors are believed to be contributory. Possible etiologic factors include deficiency of nitric oxide synthase containing neurons, abnormal myenteric plexus innervation, infantile hypergastrinemia, exposure to macrolide antibiotics, lack of exposure to vasoactive intestinal peptide in breast milk, and hypersensitivity to motilin.
A meta-analysis that investigated perinatal factors associated with hypertrophic pyloric stenosis onset and reported that first-born (OR 1.19, 95% CI: 1.07-1.33), cesarean section delivery (OR 1.63, 95% CI: 1.53-1.73), preterm birth (OR 1.37, 95% CI: 1.12-1.67), and bottle-feeding (OR 2.46, 95% CI: 1.76-3.43) were associated with the hypertrophic pyloric stenosis onset, with bottle-feeding as the most significant risk factor. [3, 4]
A cohort study found that treatment of young infants with macrolide antibiotics was strongly associated with infantile hypertrophic pyloric stenosis (IHPS). [5] A meta-analysis of 9 studies reaffirmed a significant association of postnatal exposure of erythromycin and the development of pyloric stenosis. This association is strongest if the exposure occurred in the first 2 weeks of life, although persists to a lesser degree in children between 2 and 6 weeks of age. [6, 7, 8] Maternal use of macrolides during the first 2 weeks after birth was also associated with an increased risk of IHPS. [5]
A study by Cohen Elias et al found that maternal diabetes is associated with the development of IHPS in infants. [9] Another study showed that maternal smoking is a risk factor for IHPS. [10]
Nitric oxide has been demonstrated as a major inhibitory nonadrenergic, noncholinergic neurotransmitter in the GI tract, causing relaxation of smooth muscle of the myenteric plexus upon its release. Impairment of this neuronal nitric oxide synthase (nNOS) synthesis has been implicated in infantile hypertrophic pyloric stenosis, in addition to achalasia, diabetic gastroparesis, and Hirschsprung disease.
Another study reported the possibility that low serum lipids could be a risk factor for IHPS. Further studies are needed to determine the significance of these findings. [11]
Rogers has suggested, that persisting duodenal hyperacidity, due to a high parietal cell mass (PCM) and loss of gastrin control, produces pyloric stenosis from repeated pyloric contraction in response to hyperacidity. [12]
No specific pattern of inheritance exists, although there is likely a genetic component to IHPS development. It is more common in first-born White males of northern European ancestry and occurs more frequently in monozygotic than dizygotic twins. Children of affected parents are also affected at a higher rate (as high as 7%).
A nationwide study of nearly 2 million Danish children born between 1977 and 2008 shows strong evidence for familial aggregation and heritability of pyloric stenosis. Results of the study found a heritability rate of 87% in affected families, lending to the idea that familial aggregation may be explained by shared genes that affect responses to postnatal factors in causing pyloric stenosis. [13]
The etiology of infantile hypertrophic pyloric stenosis remains unknown and is probably multifactorial (genetic and environmental factors).
Epidemiology
United States statistics
The incidence of infantile hypertrophic pyloric stenosis is 2-4 per 1000 live births.
Race-, sex-, and age-related demographics
Infantile hypertrophic pyloric stenosis is more common in Whites than in Hispanics, Blacks, or Asians. The incidence is 2.4 per 1000 live births in Whites, 1.8 in Hispanics, 0.7 in Blacks, and 0.6 in Asians. It is also less common among children of mixed race parents.
Infantile hypertrophic pyloric stenosis has a male-to-female predominance of 4-5:1, with 30% of patients with infantile hypertrophic pyloric stenosis being first-born males.
The usual age of presentation is approximately 2-6 weeks of life. Approximately 95% of infantile hypertrophic pyloric stenosis cases are diagnosed in those aged 3-12 weeks. Infantile hypertrophic pyloric stenosis is rare in premature infants. In addition, premature infants have a delayed diagnosis secondary to low birth weight and atypical presentation.
Prognosis
Surgery is curative with minimal mortality. [14] The prognosis is very good, with complete recovery and catch-up growth if detected in a timely fashion.
Morbidity/mortality
Death from infantile hypertrophic pyloric stenosis is rare and unexpected. The reported mortality rate is very low and usually results from delays in diagnosis with eventual dehydration and shock.
Complications
In the patient who presents with vomiting and has a missed/delayed diagnosis of pyloric stenosis, there is risk of significant dehydration leading to hypovolemic shock.
-
Point-of-care ultrasound performed by a pediatric emergency physician accurately identifying the pyloric wall thickness and length that meets criteria for pyloric stenosis diagnosis.
-
The ‘antral nipple sign’ demonstrated by the arrow, the ‘X’ indicates the ‘shoulder sign’
-
The ‘donut’ sign demonstrated by the arrow.
-
Lateral view from an upper GI study demonstrates the double-track sign.