Background
LEOPARD syndrome is a complex dysmorphogenetic disorder of variable penetrance and expressivity. Gorlin et al introduced the acronym LEOPARD as the name of the syndrome in 1969 to recall the main features of the disorder, as follows:
-
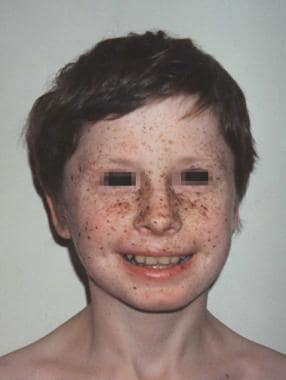
Lentigines (multiple) as shown below
-
Electrocardiographic conduction abnormalities
-
Ocular hypertelorism
-
Pulmonary stenosis
-
Abnormalities of genitalia
-
Retardation of growth
-
Deafness
Not all of the findings are present in any given patient. [1] Zeisler and Becker first described the syndrome in 1936 in a 24-year-old woman with progressive generalized lentigines, hypertelorism, pectus carinatum, and prognathism. The first familial cases were reported in twins by Rosen and subsequently in 8 persons from a large 3-generation pedigree reported by Pipkin. Subsequent communications added new findings in isolated patients or families. Moynahan first documented the association of the syndrome with cardiac abnormalities and short stature in 1962. LEOPARD syndrome, also known as Noonan syndrome with multiple lentigines, is a rare autosomal dominant disorder most often caused by missense mutations in the PTPN11 gene, which encodes the protein tyrosine phosphatase SHP2. [2, 3]
Pathophysiology
The term RASopathies includes disorders with mutations in the genes that code for the proteins of the RAS/MAPK pathway, such as neurofibromatosis type 1, Noonan syndrome, Legius syndrome, LEOPARD syndrome, Costello syndrome, and cardiofaciocutaneous syndrome. [4] Molecular studies have proven that LEOPARD syndrome and Noonan syndrome are allelic disorders caused by different missense mutations in PTPN11, a gene encoding the protein tyrosine phosphatase SHP-2 located at band 12q24.1. [5] SHP2 mutations seem to facilitate melanin synthesis in melanocytes. [6, 7] Variation in SHP2 catalytic activity may have important clinical implications. [8]
In 2005, Ogata and Yoshida documented that PTPN11 mutations can be identified in approximately 40% of Noonan syndrome patients and in greater than 80% of LEOPARD syndrome patients. [9] Because the vast majority of mutations reside in and around the broad intramolecular interaction surface between the N-SH2 and PTP domains of the PTPN11 protein, they have been suggested to affect the intramolecular N-SH2/PTP binding in the absence of a phosphopeptide, leading to excessive phosphatase activities.
In 2006, Hanna et al found that Noonan syndrome mutations enhance SHP-2 catalytic activity, whereas the activity of representative LS mutants is undetectable when assayed using a standard PTP substrate. [10] The results are also supported by studies by Kontaridis et al. [11] They revealed that whereas Noonan syndrome is caused by gain-of-function PTPN11 mutations, LEOPARD syndrome mutants are catalytically defective and act as dominant negative mutations that interfere with growth factor/Erk-mitogen-activated protein kinase–mediated signaling. LEOPARD syndrome may be caused by heterozygous missense mutation of Tyr 279 Cys in the PTPN11 gene. [12] In one Bosnian family, five patients had the same recurrent mutation Y279C in the PTPN11 gene, but had different phenotypes and a variable expression of multiple lentigines. [13]
In 2006, Tartaglia et al reported that germline mutations in the PTPN11 gene cause LEOPARD and Noonan syndromes, whereas somatic mutations in the same gene contribute to leukemogenesis. [14] To date, 2 patients with LEOPARD syndrome and myelomonocytic or acute lymphoblastic leukemias have been reported. [15, 16]
Importantly, however, not all patients with LEOPARD syndrome demonstrate linkage to 12q24.1.
Reported in 2005, Kalidas et al performed mutation screening and linkage analysis of PTPN11 in 3 families, each of which had a history of LEOPARD syndrome for 3 generations. [17] One family was found to carry a novel mutation (Q510P; 176876.0022). No variations in sequence were observed in the other 2 families, and negative lod scores excluded linkage to the PTPN11 locus, showing that LEOPARD syndrome is genetically heterogeneous.
Writzl et al reported a family with molecularly proven (p.Thr468Met in PTPN11) LEOPARD syndrome in a father and his adult son. [18] The father had multiple lentigines equally dispersed over his body as depicted below, whereas his son was devoid of lentigines on the left part of the thorax, back, and left arm. In addition, the son was found to have a mosaic karyotype in lymphocytes (47, XXY/46XY). On skin biopsy, mainly 47,XXY karyotype was present in the pigmented skin and 46,XY karyotype in the unpigmented areas. The authors considered various pathogenetic mechanisms: revertant mosaicism, silencing of a second PTPN11 mutation, genes located on a sex chromosome influencing the phenotype, and epigenetic influences.
See the image below.
Etiology
Familial cases suggest an autosomal dominant mode of inheritance with variable expressivity. [19] Speculation exists on the more severe course of the disease in males, which may partially explain the slight preponderance of men in the large collected series of Voron et al in 1976. [20]
Epidemiology
Frequency
No epidemiologic data are available. The syndrome seems to be rare both in the United States and internationally.
Race
LEOPARD syndrome has no clear racial predilection.
Sex
In a large collected series of 77 patients, a slight preponderance of men has been documented (47 men, 30 women).
Age
Lentigines may be present at birth or develop during childhood. They become more numerous and darker with age. Other skin lesions, such as nevocellular nevi and malignant melanomas, reported sporadically in the LEOPARD syndrome, may undergo depigmentation.
Prognosis
Prognosis is determined mainly by cardiac complications. Most patients with LEOPARD syndrome lead a normal life.
Cardiac pathologic findings (eg, obstructive cardiomyopathy, cardiac dysrhythmias) may be a cause of death in selected patients. The prevalence of cardiac lesions was 66.7% in a study from the Republic of China. [21]
A 19-year-old woman who died as a result of respiratory insufficiency secondary to thoracic deformities and a congenital heart defect has been reported.
-
Multiple lentigines on the face of a child with LEOPARD syndrome.
-
Lentigines on the sclerae in a child with LEOPARD syndrome.
-
Area of disordered pigmentation on the trunk of a patient with LEOPARD syndrome.
-
Onychodystrophy in a child with LEOPARD syndrome.
-
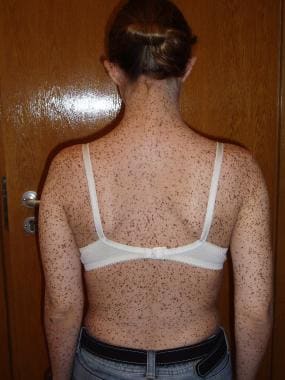
Multiple, small lentigines evenly distributed over the trunk of an adult female with LEOPARD syndrome.