Practice Essentials
Leprosy is a chronic granulomatous disease principally affecting the skin and peripheral nervous system. Leprosy is caused by infection with Mycobacterium leprae. Although much improved in the last 25 years, knowledge of the pathogenesis, course, treatment, and prevention of leprosy continues to evolve. The skin lesions and deformities were historically responsible for the stigma attached to leprosy. However, even with proper multidrug therapy (MDT), the extensive sensory and motor damage can result in the deformities and disabilities associated with leprosy. See the image below.
Other articles on leprosy include Leprosy Neuropathy and Leprosy.
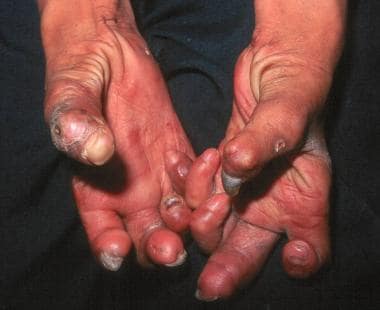 Hands with Z-thumbs, clawing, contractures, and shortening of fingers due to repetitive injury and healing. Ho Chi Minh City, Vietnam. (Courtesy of D. Scott Smith, MD)
Hands with Z-thumbs, clawing, contractures, and shortening of fingers due to repetitive injury and healing. Ho Chi Minh City, Vietnam. (Courtesy of D. Scott Smith, MD)
Signs and symptoms
See Presentation for a full discussion.
Assess for physical signs of leprosy in 3 general areas: cutaneous lesions, neuropathies, and eyes.
For cutaneous lesions, assess the number and distribution of skin lesions. A hypopigmented macule with a raised border is often the first cutaneous lesion. Plaques are also common. Lesions may or may not be hypoesthetic. Lesions on the buttocks often indicate borderline disease. Involvement of the oral mucosa has been reported in borderline tuberculoid leprosy. [1] Necrotizing erythema nodosum has been reported in a case of leprosy relapse. [2]
Regarding neuropathies, assess for areas of hypoesthesia (light touch, pinprick, temperature and anhidrosis), especially peripheral nerve trunks and cutaneous nerves. The most common nerve affected is the posterior tibial nerve. Others commonly damaged are the ulnar, median, lateral popliteal, and facial nerves. Besides sensory loss, patients may have associated tenderness and motor loss. Nerve palpation, monofilament testing, and voluntary muscle testing are the most useful clinical tests for detecting nerve damage. [3]
Clinical grading of nerve thickness, tenderness, and pain should be recorded to track changes over time and with therapy. [4] Eye damage is most often seen with facial lesions. Lagophthalmos (inability to close the eye), a late finding in persons with lepromatous leprosy, results from involvement of the zygomatic and temporal branches of the facial nerve (cranial nerve [CN] VII). Involvement of the ophthalmic branch of the trigeminal nerve (CN V) can result in reduced corneal reflex, leaving dry eyes and reduced blinking.
Diagnostics
See Workup for a full discussion.
The presence of an inflamed nerve in a skin biopsy specimen is considered the criterion standard for diagnosis.
The skin biopsy sample should be examined for morphologic features and for the presence of acid-fast bacilli. Biopsy is useful for determining the morphologic index, which is used in the evaluation and treatment of patients. The morphologic index is the number of viable bacilli per 100 bacilli in the leprous tissue. The bacterial index of granuloma (BIG) does not differentiate between viable and nonviable bacilli. [5]
In pure neural leprosy, a biopsy of skin near the affected nerve is recommended before attempting a nerve biopsy, as it is less invasive. If a nerve biopsy is deemed necessary, sensory nerves should be sampled. The sural nerve is often a logical place to start. [6]
Management
See Treatment for a full discussion.
The management of leprosy includes early pharmacotherapy and physical, social, and psychological rehabilitation. The goals of pharmacotherapy are to stop the infection, reduce morbidity, prevent complications, and eradicate the disease. Since 1981, multidrug therapy (MDT) has been advocated by the World Health Organization (WHO) [7] and the United States government. MDT prevents dapsone resistance, quickly reduces contagiousness, and reduces relapses, reactions, and disabilities. If a patient has been previously treated with dapsone monotherapy, re-treatment with a modified MDT regimen of rifampin, clofazimine, and dapsone can reduce or delay the risk of relapse. [8]
The length of treatment ranges from 6 months to 2 years. Patients are considered noninfectious within 1-2 weeks of treatment (usually after the first dose). These drugs are conveniently packaged in monthly calendar blister packs. Monitor for drug resistance and adverse reactions to medications.
Background
The earliest description of leprosy comes from India around 600 BCE. Leprosy was then described in the Far East around 400 BCE. In the fourth century, leprosy was imported into Europe, where its incidence peaked in the 13th century. Leprosy has now nearly disappeared from Europe. Affected immigrants spread leprosy to North America.
Armauer Hansen discovered M leprae in Norway in 1873. M leprae was the first bacillus to be associated with human disease. Despite this discovery, leprosy was not initially thought to be an infectious disease.
In 2008, the discovery of a new cause of leprosy, Mycobacterium lepromatosis, was announced. Genetically, M leprae and M lepromatosis are very similar, but M lepromatosis causes the diffuse form of lepromatous leprosy found in Mexico and the Caribbean. [9]
Leprosy is included among the Neglected Tropical Diseases as designated by the World Health Organization. [10] In 2016, the WHO released a 5-year global leprosy strategy, running through 2020, to "strengthen government ownership, coordination, and partnership; stop leprosy and its complications, and to stop discrimination and promote inclusion." [11]
Pathophysiology
Leprosy is not a highly infectious disease. The principal means of transmission is by aerosol spread from infected nasal secretions to exposed nasal and oral mucosa. Leprosy is not generally spread by means of direct contact through intact skin, although the most vulnerable are close contacts of patients with untreated multibacillary disease.
However, in 2011, a unique strain of M leprae was genotyped in both humans and wild armadillos infected in the southern United States, suggesting a direct means of transmission. Several people had distinct contact with armadillos, including hunting, cooking, or eating armadillos. [12]
The incubation period for leprosy is 6 months to 40 years or longer. The mean incubation period is 4 years for tuberculoid leprosy and 10 years for lepromatous leprosy.
The areas most commonly affected by leprosy are the superficial peripheral nerves, skin, mucous membranes of the upper respiratory tract, anterior chamber of the eyes, and the testes. These areas tend to be cool parts of the body. Tissue damage depends on the degree to which cell-mediated immunity is expressed, the type and extent of bacillary spread and multiplication, the appearance of tissue-damaging immunologic complications (ie, lepra reactions), and the development of nerve damage and its sequelae.
M leprae is an obligate intracellular, acid-fast, gram-positive bacillus with an affinity for macrophages and Schwann cells. For Schwann cells in particular, the mycobacteria bind to the G domain of the alpha-chain of laminin 2 (found only in peripheral nerves) in the basal lamina, causing demyelination. Their slow replication within the Schwann cells eventually stimulates a cell-mediated immune response, which creates a chronic inflammatory reaction. As a result, swelling occurs in the perineurium, leading to ischemia, fibrosis, and axonal death. In vivo, M leprae has also been demonstrated to reprogram Schwann cells to de-differentiate into mesenchymal stem cells, which may explain the spread of bacteria to other non-neural tissues. [13]
With the completion of the genomic sequence of M leprae, one important discovery is that although it depends on its host for metabolism, the microorganism retains genes for the formation of a mycobacterial cell wall. Components of the cell wall stimulate a host immunoglobulin M antibody and cell-mediated immune response, while also moderating the bactericidal abilities of macrophages.
Research showed that M leprae also induces lipid droplet accumulation in macrophages and Schwann cells, with an increase in adipophilin/adipose differentiation-related protein (ADRP). ADRP opposes the action of hormone-sensitive lipase (HSL), which degrades lipids. Infected cells from slit-skin smears in lepromatous leprosy correlate with the findings, with expression of ADRP high and HSL low or undetectable. [14] Lipid metabolism, especially ω3 and ω6 polyunsaturated fatty acids, may also play a role. [15]
The strength of the host's immune system influences the clinical form of the disease. Strong cell-mediated immunity (interferon [IFN]-gamma, interleukin [IL]–2) and a weak humoral response results in mild forms of disease, with a few well-defined nerves involved and lower bacterial loads. A strong humoral response (IFN-beta, IL-4, IL-10) but relatively absent cell-mediated immunity results in lepromatous leprosy, with widespread lesions, extensive skin and nerve involvement, and high bacterial loads. Therefore, a spectrum of disease exists such that cell-mediated immunity dominates in mild forms of leprosy and decreases with increasing clinical severity. Meanwhile, humoral immunity is relatively absent in mild disease and increases with the severity of disease. T regulatory cells also appear to suppress the normal immune response.
Toll-like receptors (TLRs) may also play a role in the pathogenesis of leprosy. [16] M leprae activates TLR2 and TLR1, which are found on the surface of Schwann cells, especially with tuberculoid leprosy. Although this cell-mediated immune defense is most active in mild forms of leprosy, it is also likely responsible for the activation of apoptosis genes and, consequently, the hastened onset of nerve damage found in persons with mild disease. Alpha-2 laminin receptors found in the basal lamina of Schwann cells are also a target of entry for M leprae into these cells, while activation of the ErbB2 receptor tyrosine kinase signaling pathway has been identified as a mediator of demyelination in leprosy. [17]
The activation of macrophages and dendritic cells, both antigen-presenting cells, is involved in the host immune response to M leprae. IL-1beta produced by antigen-presenting cells infected by mycobacteria has been shown to impair the maturation and function of dendritic cells. [18] Because bacilli have been found in the endothelium of skin, nervous tissue, and nasal mucosa, endothelial cells are also thought to contribute to the pathogenesis of leprosy. Another pathway exploited by M leprae is the ubiquitin-proteasome pathway, by causing immune cell apoptosis and tumor necrosis factor (TNF)–alpha/IL-10 secretion. [19]
Research continues to explore the pathophysiology of leprosy, with the goal of identifying early markers of disease and new targets for treatment. The most current investigations focus on interferons, [20] the vitamin D – dependent antimicrobial pathway, [21] and NOD2-mediated signaling pathways, [22, 23] as well as the role of T regulatory cells, Th-17/IL-17a/IL-17F cytokines, CD163, and galectin-3. [14]
A sudden increase in T-cell immunity, particularly in the Th1 pattern, is responsible for type I reversal reactions. TLR2 and TLR4 have also been implicated. [14] Type II reactions (erythema nodosum leprosum, ENL) result from activation of TNF-alpha and the deposition of immune complexes in tissues with neutrophilic infiltration and from complement activation in organs. Activated memory T cells are also increased in untreated ENL. [24] One study found that cyclooxygenase 2 was expressed in microvessels, nerve bundles, and isolated nerve fibers in the dermis and subcutis during reversal reactions. [25] Another found high levels of TNF, IFN-γ, IL-1β, and IL-17A Th-17 and low levels of IL-10 and TGF-β in ENL that were reversed after a 24-week course of prednisolone. [26] Other cytokines, cortisol levels, CXC ligand 10, and matrix metalloproteinases may also have a role in both type I and II reactions. [14, 27, 28]
Etiology
Leprosy is caused by M leprae, an obligate intracellular, acid-fast, gram-positive bacillus. Humans are the primary reservoir of M leprae. Animal reservoirs of leprosy have been found in three species: 9-banded armadillos, chimpanzees, and mangabey monkeys.
Most persons are immune to leprosy. Subclinical disease is common in endemic areas, and the infection progresses to clinical disease in only a select few.
Biopsies of nasal and oral mucosa of individuals who remain untreated for years have demonstrated M leprae positivity, [29, 30] suggesting respiratory secretions are the main cause of infection. However, transmission is not completely understood.
Exposure to insect vectors and infected soil has also been suspected as a possible mode of transmission.
In endemic countries, household contacts of patients are at increased risk for contracting leprosy. The relative risk is 8-10 times for lepromatous leprosy and 2-4 times for tuberculoid leprosy. In nonendemic countries, household contacts rarely acquire the disease.
HIV infection is not a risk factor for acquiring leprosy, nor does it increase the clinical symptoms or virulence of leprosy. However, latent cases of leprosy infections may emerge as part of the immune reconstitution inflammatory syndrome after starting highly active antiretroviral therapy. [31, 32]
One report describes 2 cases of leprosy developing after treatment with infliximab. [33] Both patients developed type I reversal reactions after stopping the TNF-alpha inhibitor. Another patient developed a type I reversal reaction after stopping adalimumab therapy, despite no prior diagnosis of leprosy. [34]
Several cases of tattoo inoculation leprosy have been reported, most in India. [35]
Leprosy has been reported in conjunction with visceral leishmaniasis (kala-azar).
Several reports have described leprosy developing in solid organ transplant recipients (especially kidney) and after bone marrow transplantation. It is not clear about the susceptibility of patients due to general immunosuppressive conditions (as with HIV infection); however, most affected transplant recipients developed multibacillary disease. [36, 37]
The following genes have been associated with leprosy; hence, susceptibility or resistance to leprosy may be at least partially inheritable [23] :
With the first genome-wide association study (GWAS), the following loci have markers with the strongest associations:
-
HLA-DR-DQ: HLA-DR2 and HLA-DR3 (tuberculoid disease), as well as HLA-DQ1 (lepromatous leprosy); HLA-DRB1*04 is associated with resistance, and HLA-DRB1*10 is associated with susceptibility to leprosy in Brazilian and Vietnamese patients. [38] RIPK2, TNFSFIS
-
LACC1, CCDC122, and NOD2
-
Additionally, there are numerous studies looking into the role of other HLA, KIR, MICA and cytokine genes in contracting leprosy. [39]
-
Genetic variants have been found in the shared promoter region of the PARK2 (parkin) and PACRG genes expressed on monocytes.
-
Polymorphisms in the gene promoter regions of TNF (multibacillary leprosy) and IL-10 (-819T allele) are noted in leprosy susceptibility.
-
Mutations in TLR1 and TLR2 may be involved in susceptibility and/or resistance to other infectious diseases.
-
Polymorphisms in the NRAMP1 gene appear on macrophages in multibacillary disease in African patients.
-
TaqI polymorphism (tt genotype) at exon 9 of the vitamin D receptor gene is noted. [42]
-
IFGR1 gene promoter polymorphisms found in one family demonstrated an autosomal recessive susceptibility to leprosy. [43]
-
Genetic markers that may identify those more susceptible to T1R and T2R include polymorphisms in vitamin D receptor, IL-6, complement component C4b, TLR1 and TLR2, and natural resistance-associated macrophage protein 1 (NRAMP1). [44]
Epidemiology
Frequency
United States
Approximately 6500 patients with leprosy live in the United States, about 50% of which require active medical management. Approximately 95% of these patients acquired their disease in developing countries. In the United States, 200-300 cases of leprosy are reported each year. States with large immigrant populations (eg, California, New York, Florida) have the largest number of new cases of leprosy. Small endemic foci of leprosy exist in Texas, Louisiana, and Hawaii.
International
Overall, the worldwide prevalence of leprosy (defined as the number of people on multidrug therapy at a particular point) has decreased significantly since the introduction of short-course multidrug therapy in 1982. The WHO’s elimination goal of less than 1 case per 10,000 population was reached in the early 2000s. Approximately 95% of affected persons are found in 16 countries, most of them in the tropics and subtropics: Bangladesh, Brazil, China, Democratic Republic of the Congo, Ethiopia, India, Indonesia, Ivory Coast, Madagascar, Myanmar, Nepal, Nigeria, Philippines, South Sudan, Sri Lanka, and the United Republic of Tanzania. [45]
Despite achieving the elimination goal quickly, eradication has proved to be more elusive. Globally, annual new case detection rates for leprosy remain unchanged, and even increased slightly from 2015 to 2016. While this increase is at least partially due to active methods of case-finding and new methods of reporting and data collection, there are still gaps and inconsistent reporting, especially from countries with endemic populations. [46] Clinically, transmission remains an issue. Twenty-two countries, including most of the ones listed above, account for 94-96% of new cases and have been deemed by the WHO as "global priority countries". [11]
Race
Leprosy occurs in persons of all races. African Blacks have a high incidence of the tuberculoid form of leprosy. People with light skin and Chinese individuals tend to contract the lepromatous type of leprosy. Leprosy is endemic in Asia, Africa, the Pacific basin, and Latin America (excluding Chile). Leprosy is more a rural than urban disease.
Sex
In adults, the lepromatous type of leprosy is more common in men than in women after puberty, with a male-to-female ratio of 2:1. In children, the tuberculoid form of leprosy predominates and no sex preference is reported. Women tend to have a delayed presentation, which increases rates of deformity.
Age
Leprosy has a bimodal age distribution, with peaks at ages 10-14 years and 35-44 years. Leprosy is rare in infants. Children appear to be most susceptible to leprosy and tend to have the tuberculoid form.
Prognosis
The prognosis depends on the stage of disease. Borderline tuberculoid leprosy usually involves rapid and severe nerve damage. Reversal reactions are uncommon with lepromatous disease; therefore, lepromatous leprosy is a chronic state with long-term complications. Even with MDT, patients have long-term nerve damage and disability.
The prognosis also depends on the patient's access to therapy, the patient's compliance, and the early initiation of treatment.
Relapse (new disease after adequate MDT is completed) occurs in 0.01-0.14% of patients per year in the first 10 years. Dapsone and/or rifampin resistance should be considered. [47]
Approximately 5-10% of patients have a type I reversal reaction in the first year after completing MDT.
Because of reduced cell-mediated immunity, pregnancy can precipitate a relapse or reaction of the disease, especially type II reactions in pregnant women younger than 40 years. Dapsone is generally thought to be safe in pregnancy; the safety of clofazimine and rifampin are controversial, and thalidomide (used in type II reactions) is contraindicated during pregnancy.
Type I and type II reactions can precipitate a relapse of the disease.
Perineural granulomas have been reported to persist 18 months after MDT and clinical improvement, and they are not considered to be a relapse of the disease. [48]
Overall, children have a good prognosis because multibacillary disease and leprous reactions are uncommon.
Patient Education
Patients first need an explanation of the diagnosis and prognosis. Their fears should be addressed because of the cultural stigma associated with leprosy. Importantly, refute any myths that the patient may have about leprosy. Patients may need psychological counseling because they may have difficulty coming to terms with the disease or may feel rejected by society. The patient should be reassured that within a few days of starting MDT, they are not infectious and can lead a normal life.
Patients need education about how to deal with anesthesia of a hand or foot. They must learn to carefully inspect their extremities for trauma each day. Patients should also be told to wear proper footwear and protective equipment as necessary. Inexpensive canvas shoes with protective insoles are as effective as special orthopedic shoes. Inspecting limbs and eyes for the onset of anesthesia or weakness is also important. Physical therapy and occupational therapy are important tools in rehabilitation.
Patients must learn how to recognize the onset of lepra reactions, and they should be told to seek immediate medical attention if these reactions develop.
Potential deformities can be prevented by educating patients about how to deal with existing nerve damage and by treating any sequelae of this damage.
-
Hands with Z-thumbs, clawing, contractures, and shortening of fingers due to repetitive injury and healing. Ho Chi Minh City, Vietnam. (Courtesy of D. Scott Smith, MD)






