Background
Herpes simplex viruses (HSVs) are linear double-stranded DNA viruses that cause acute skin infections and present as grouped vesicles on an erythematous base. Rarely, these viruses can cause serious illness and can affect pregnancy, leading to significant harm to the fetus. Most infections are recurrent and tend to reappear at or near the same location. Herpes labialis is the most common infection caused by HSV type 1 (HSV-1), whereas genital herpes is usually caused by HSV type 2 (HSV-2). Other clinical manifestations of HSV infection are less common.
Pathophysiology
Intimate contact between a susceptible person (without antibodies against the virus) and an individual who is actively shedding the virus or with body fluids containing the virus is required for herpes simplex virus (HSV) infection to occur. Viral shedding occurs during the primary infection, during subsequent recurrences, and during periods of asymptomatic viral shedding. Contact during these periods must involve mucous membranes or open or abraded skin. Following exposure, the primary infection of HSV is established at the site of contact. Here, the viral envelope is fused with the cellular membranes of the skin and mucous membranes and HSV DNA is incorporated into the nucleus. [1] HSV glycoprotein C protects the viral envelope and aids in viral entry. [2] Recognition of HSV DNA by Toll-like receptors results in the activation of the innate and adaptive immune systems and production of interferon gene products. [1] Viral suppression of host cell immune responses and subsequent evasion of the immune system is accomplished via a complex interplay between HSV virion protein products and the immune system. Specifically, the HSV protein, virion host shutoff (VHS), is produced in the early stages of infection to suppress host cell responses. [1] Additionally, glycoprotein C binds to complement C3b, inhibiting complement-mediated immunity and plays a role in inhibiting antibody neutralization. [2] HSV then invades and replicates in neurons as well as in epidermal and dermal cells. Virions travel from the initial site of infection on the skin or mucosa to the sensory dorsal root ganglion, where latency is established. The latent state is established by silencing of the HSV genome in neurons, and reactivation can occur during periods of stress.
Viral replication in the sensory ganglia leads to recurrent clinical outbreaks. These outbreaks can be induced by various stimuli, such as trauma, ultraviolet (UV) radiation, extremes in temperature, stress, immunosuppression, surgical and laser procedures, or hormonal fluctuations. HSV-specific CD8+ T cells appear to play an important role in controlling recurrent infections and reactivation. [3] CD8+ T cells are primed and recruited by innate and adaptive immune responses during the primary infection. The chemokine receptors, CXCR3 and CCR10, have been found to be up-regulated in HSV-specific CD8+ T cells and may contribute to homing mechanisms and control of inflammation during periods of reactivation. [3]
HSV-1 reactivation occurs most efficiently from trigeminal ganglia (affecting the face and the oropharyngeal and ocular mucosae), while HSV-2 has a more efficient reactivation in the lumbosacral ganglia (affecting the hips, buttocks, genitalia, and lower extremities). The clinical difference in site-specific reactivation between HSV-1 and HSV-2 appears to be due, in part, to each virus establishing latent infection in different populations of ganglionic neurons. [4]
Etiology
Herpes simplex virus (HSV) type 1 and HSV-2 are the causative agents of herpes genitalis, herpes labialis, herpes gladiatorum, herpetic whitlow, herpetic keratoconjunctivitis, eczema herpeticum, herpes folliculitis, lumbosacral herpes, disseminated herpes, neonatal herpes, and herpes encephalitis. [5] They have also been linked to some cases of erythema multiforme. In pediatric patients, HSV infection is associated with 18% of cases of erythema multiforme. [6] A febrile illness, exposure to UV light, trauma, upper respiratory infection, or emotional stress may trigger recurrent herpes labialis due to HSV-1.
The patient's geographical location, socioeconomic status, and age influence the frequency of HSV-1 infections. The highest prevalence of antibodies to HSV-2 occurs in female prostitutes, male homosexuals, and HIV-positive individuals.
Epidemiology
Frequency
United States
Herpes simplex virus (HSV) type 1 infection is typically acquired by early childhood. The seroprevalence of HSV-1 in persons aged 14-49 years in the United States is 53.9%, based on data from the 2005-2010 National Health and Nutrition Examination Survey (NHANES). [7] Only about 30% of these individuals have clinically apparent outbreaks. The seroprevalence of HSV-2 in the United States is 15.7%, and more than 80% of these infections are either asymptomatic or unrecognized. [7, 8] Regardless, these individuals still have episodes of viral shedding and can transmit the virus. The seroprevalence of HSV-1 has declined approximately 7% from prior NHANES data. However, the seroprevalence of HSV-2 has remained stable after increasing between 1976 and 1980 and subsequently declining between 1988 and 2004. [8] Independent predictors of HSV-2 seropositivity include female sex, older age, non-Hispanic black race, and increased lifetime sex partners. [8, 9] Additionally, patients with prior sexually transmitted infections, such as chlamydia, gonorrhea, and syphilis, have higher rates of HSV-2 seroprevalence. [10] Newer data indicate that HSV-1 infections may account for more genital infections than previously thought. A retrospective review of genital HSV infection in college-age students revealed that HSV-1 accounted for 78% of isolates, suggesting a reversal in prior etiologic notions. [11]
International
Serologic evidence of HSV infection varies by country. [12, 13] The seroprevalence of HSV-1 is 78.4% in Germany, whereas, it is 42.7% in the Netherlands. [14, 15] A study of two cities in Italy on the prevalence of HSV-1 and HSV-2 from 2000-2014 showed a significant decline in the study population (14.5% decrease in women; 22% decrease in men) for HSV-1 infection and a decline in HSV-2 infection from 22.4% to 11.5%. [16] Similar findings have been seen in the United States.
Worldwide, the seroprevalence of HSV-1 and HSV-2 is 67% and 11.3%, respectively. [17, 18] As in the United States, HSV-1 prevalence increases with advancing age worldwide. The highest prevalence of HSV-1 and HSV-2 appears to be in Africa. [17, 18]
In the developing world, HSV-2 is becoming a common cause for genital ulcer disease, especially in countries with a high prevalence of HIV infection. [19] International studies show seroprevalence in people co-infected with HIV being close to 90% for HSV-1 and up to 77% for HSV-2. [20]
Race
The seroprevalence of HSV-2 in the United States is approximately three-fold higher in non-Hispanic blacks as compared with non-Hispanic whites. [8] Seroprevalence is highest among older non-Hispanic blacks, affecting approximately 56% of patients aged 30-49 years. [8] In contrast, the seroprevalence of HSV-1 in patients aged 14-19 years has declined from 55.7% (between 1999 and 2004) to 39.6% (between 2005 and 2010) in the non-Hispanic black population, but it has remained stable, at approximately 58%, in the Mexican American population. [7] Additionally, the seroprevalence of HSV-1 in foreign-born patients in the United States is higher than in patients born in the United States [7]
Sex
The frequency of HSV-1 antibodies is slightly higher in females than in males (33.2% vs 27.1%). [7] In a study of more than 600 pregnant women, 63% were seropositive for HSV-1, 22% for HSV-2, and 13% for both, and 28% were seronegative. Nonwhite race and having had four or more sexual partners independently correlated with increased HSV-2 infection. Non-Hispanic white pregnant women had the highest percentage of seronegativity for both HSV-1 and HSV-2. However, this group had the highest risk of having a child with neonatal herpes, indicating their susceptibility for new HSV infection during their third trimester of pregnancy (when a mother is most likely to transmit infection to her neonate). [21]
Socioeconomic factors appear to play a substantial role as well. Seropositivity for HSV-2 has been shown to be as high as 88% in homeless women or women with unstable housing. [22] Associated risk factors in this population were alcohol use, heterosexual orientation, HIV infection, and lower income status. [22]
Age
Overall, the seroprevalence of HSV-1 and HSV-2 increase with increasing age. From 2005-2010, the seroprevalence of HSV-1 among patients aged 14-19 years was 30.1% and 63.6% among patients aged 40-49 years. [7] Similar trends exist for HSV-2 seroprevalence, with 1.2% of persons aged 14-19 years affected and 25.6% of those aged 40-49 years affected. [7] The frequency of HSV-1 infection in children also varies with socioeconomic status. Approximately, one third of children from families of lower socioeconomic status exhibit some evidence of HSV-1 infection by age 5 years. A retrospective review of HSV cases from pediatric dermatology clinics found the average age of presentation to be 6 years and 33% of patients presented at age 2 years or younger. [23] Approximately 40% of these children had a history of atopy and experienced six or more outbreaks per year. [23] Triggers included UV light exposure, cold weather, and systemic corticosteroids for asthma. [23]
Prognosis
For most people, herpes simplex virus (HSV) infections are temporary and resolve without detrimental sequelae; however, recurrence is common. The associated pain, paresthesia, and discomfort, as well as the psychosocial impact, of herpes simplex outbreaks cause significant morbidity to the individuals who are affected. Long-term sequelae (usually CNS) are more common with neonatal HSV infection than with other types of HSV infection. Scarring may occur from severe or superinfected lesions.
Mortality/morbidity
Severe complications may be associated with herpes simplex. This is especially true in females who are pregnant and in individuals with immunosuppression, who may develop disseminated infection and encephalitis.
Complications of HSV-1 infections vary based on the site of involvement and whether the patient is immunocompromised. HSV-1 infections of the oral mucosa result in gingivostomatitis in children and pharyngitis in adults. Reactivation of infections of the oral mucosa present as herpes labialis and are less severe than the primary infection. HSV-1 can also infect any cutaneous surface. Patients with Darier disease, atopic dermatitis, or mycosis fungoides may develop life-threatening eczema herpeticum (Kaposi varicelliform eruption). Organ transplant recipients and patients with HIV/AIDS may develop herpetic lesions that exhibit an unusual morphology. Additionally, HSV DNA has been associated with erythema multiforme in approximately 43% of cases. [24]
Ocular and neurologic sequelae of HSV-1 infection include conjunctivitis, blepharitis, keratitis, acute retinal necrosis, Bell palsy, and encephalitis. Herpes simplex keratitis is characterized by ocular pain and is a common infectious cause of unilateral vision loss. [25] Clinical manifestations of HSV-1 encephalitis include fever, headache, nausea, vomiting, seizures, confusion, and focal deficits. [26] Approximately 15% of hospitalized patients die from HSV encephalitis, and survivors have long-term disability affecting cognitive function. [26]
The most common complication of primary HSV-2 genital infection is bacterial superinfection. Extragenital complications such as urinary retention, aseptic meningitis, and proctitis can occur in patients with HSV-2. Urinary retention and aseptic meningitis occur more frequently in women. Proctitis due to HSV-2 occurs more commonly in men who have sex with men and accounts for approximately 15% of cases. [27] Additionally, HSV-1 and HSV-2 are more common in men with proctitis and HIV infection than in those without HIV infection. [28]
Individuals co-infected with HSV and HIV and who have herpetic mucosal lesions are more likely to transmit HIV during sexual contact. In studies, despite being compliant with highly active antiretroviral therapy (HAART) for treatment of HIV-1 infection, 30-50% of women co-infected with HSV-2 and HIV-1 were shown to have genital HIV-1 shedding at least once in a 3-month period. Studies also suggest that co-infection with HSV-2 may accelerate HIV disease progression by elevating the HIV viral load. A 2008 study by Baeten et al found that HSV suppressive therapy decreased genital and plasma HIV levels in women with HSV-2/HIV co-infection. [29]
Another serious consequence of HSV infection is transmission of the virus to a neonate by a mother who is infected. [30] Asymptomatic maternal shedding occurs approximately 7% of the time and is responsible for most neonatal HSV infections. The risk of HSV transmission to the newborn is as high as 30-50% from a mother who acquired a new HSV infection near the time of delivery. Among women who have acquired HSV infection before their third trimester of pregnancy, the risk of transmission is less than 1%. HSV infections in neonates are most commonly due to HSV-2 and most are acquired peripartum from exposure to lesions (or shedding virus) in the birth canal, although in utero and postpartum transmission can rarely occur. Transmission is estimated to occur at a rate of 1 case in 3500-5000 deliveries in the United States. Neonatal infection can cause long-term sequelae and even death.
Reported in 2019, HSV-1 has been implicated in the pathogenesis of Alzheimer disease. HSV-1 has been shown to cause intracellular accumulation of amyloid-β protein (Aβ), which is involved in the pathogenesis of Alzheimer disease. [31] Additionally, patients that are apolipoprotein E carriers who had IgM or high-IgG titers against HSV-1 have an increased risk of Alzheimer disease. [32]
Patient Education
Patients who are immunocompetent should be reassured that the disease does not cause serious harm. However, physical and psychological concerns must be validated, and appropriate counseling is warranted.
For patient education resources, visit the Sexual Health Center, Oral Health Center, and Skin Conditions and Beauty Center. Also, see the patient education articles Genital Herpes, Genital Herpes in Woman, Oral Herpes, and Eczema.
-
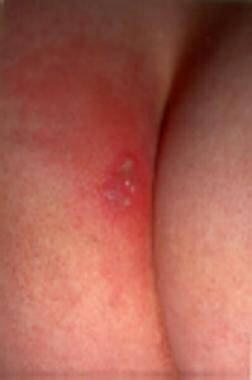
Characteristic cluster of vesicles on an erythematous base. Photo courtesy of Dr. John Reeves.