Practice Essentials
An arteriovenous malformation (AVM) is a tangled cluster of vessels, typically located in the supratentorial portion of the brain, in which arteries connect directly to veins without an intervening capillary bed. The lesion may be compact, containing a core of tightly packed venous loops, or it may be diffuse, with anomalous vessels dispersed among normal brain parenchyma. In 77% of cases, the core, or nidus, of a compact AVM is 2 to 6 cm in diameter. [1, 2]
AVMs are rare, occurring in about 1 in 100,000 people. Anyone can be born with an AVM. AVMs are mainly discovered in younger people from age 20 to 40 years. Risk of symptoms is highest between ages 40 and 50 years. AVMs occur in equal numbers in males and females. [3]
AVMs account for approximately 11% of cerebrovascular malformations; venous angiomas, a more common type of cerebrovascular malformation, account for 64% of cases. AVMs are more likely than other types of vascular malformations to be clinically symptomatic. AVMs typically involve the brain but occasionally are associated with the spinal cord and its dura. [4, 5, 6, 7]
AVMs presenting with epilepsy significantly impact patient quality of life and should be considered very much a seizure disorder. Although hemorrhage prevention is the primary aim of AVM surgery, seizure control should also be at the forefront of therapeutic management. Several hemodynamic and morphologic characteristics of AVMs have been identified as associated with seizure presentation. These include increased AVM flow, presence of a long pial draining vein, venous outflow obstruction, and a frontotemporal location, among other aspects. With the advent of high-throughput image processing and quantification methods, new radiographic attributes of AVM-related epilepsy have been identified. Several treatment approaches are available, including conservative management and interventional modalities such as microsurgery, radiosurgery, and embolization, or a combination thereof. [8]
Because of more frequent use of noninvasive imaging methods, AVMs are gaining attention. Population-based studies have revealed that about 35 to 50% of patients with AVM initially present with hemorrhage. Prior hemorrhage is the most consistent risk factor for future hemorrhage; other risk factors include a deep venous drainage pattern and deep location. Surgical interventions, including surgical excision, intravascular intervention, and stereotactic radiation, have been developed with the aim of eliminating this source of hemorrhage. Among these methods, surgical excision has been considered the gold standard for low-grade intracranial AVM. Gamma knife surgery uses highly focused beams of radiation that slowly shrink, scar, and dissolve an AVM over a few years or that make the AVM easier to remove with surgery. For ruptured AVMs, it is standard to wait several weeks to allow for patient recovery, hematoma liquefaction, and subsidence of inflammatory reactions, except in cases of life-threatening hemorrhage. [3, 9]
Aneurysms associated with brain AVMs represent a hemorrhage risk in addition to that of the AVM nidus. In high-risk or unresectable cases, targeted treatment of an aneurysm causing hemorrhage may effectively decrease future risk of hemorrhage. Ruptured intraventricular aneurysms associated with brain AVMs can be treated surgically to reduce risk of rebleeding in patients in whom the aneurysm is not accessible to endovascular treatment and in whom the AVM nidus may not be safely resected. [10]
AVM represents complex communication of an artery and a vein in which oxygenated blood is forced away from the intended tissue. The incidence of its occurrence in the face and neck is rare and, when present, the most common sign is gingival bleeding. AVM is both a diagnostic and a therapeutic challenge for dentists. The classification and oral manifestations of AVM must be known and understood. Practitioners should be aware of the radiographic appearance of AVM. Recommended precautions should be taken when restorative and endodontic procedures are carried out in a patient with AVM. Antibiotic prophylaxis may be considered before endodontic treatment. Multidisciplinary treatment planning may be required. [11]
Imaging modalities
Because of more frequent use of noninvasive imaging methods, AVMs are gaining attention. [9] Imaging studies performed in patients with suspected AVM include ultrasonography, magnetic resonance imaging (MRI), computed tomography (CT), and angiography. These studies are good for depicting AVMs and are relatively noninvasive, requiring only injection of contrast material into a small vein. Overall, AVMs are best imaged via MRI, which can uniquely show these lesions as a tangle of vascular channels that appear as flow voids.
Nonenhanced CT is superior for visualizing the small foci of calcification often associated with AVMs and may delineate hyperattenuating serpiginous vessels that constitute the nidus. Nonenhanced CT scanning is valuable for showing the extent of acute hemorrhage and hydrocephalus. Contrast-enhanced CT shows enhancement of typical vascular channels.
Magnetic resonance angiography (MRA) or CT angiography (CTA) may be ordered to obtain a very detailed picture of the blood vessels. These tests are done with the patient under general anesthesia and can be used for diagnosis and treatment of an AVM. [12] Aneurysms sometimes may be found on AVM feeding arteries by MRA, CTA, or catheter angiography. [13, 14, 15, 16, 17, 18, 19, 20]
Plain radiography is not among the modern modalities used for imaging cerebral AVMs. Nevertheless, abnormally dilated vascular channels can be seen on plain skull images. Abnormal intracranial calcifications associated with AVMs can also be detected; these are suggestive of an AVM. These findings should prompt the clinician to order cross-sectional imaging. [14, 21, 22, 23, 24] The degree of confidence in radiography is poor because impressions on the calvarium can be seen normally. Plain films of the skull are not considered diagnostic for detection of AVMs in the central nervous system (CNS).
Isotopic cerebral blood flow studies have largely been supplanted by modern ultrasonography, CT scanning, MRI, and digital subtraction angiography of the brain for evaluation of AVM. [17] [14]
(See the images below.)
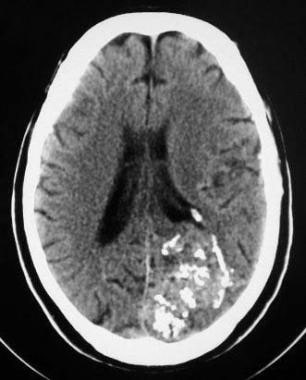 CT scan of the head showing a left occipital arteriovenous malformation (AVM), with multiple calcified phleboliths and numerous hyperattenuating vascular channels.
CT scan of the head showing a left occipital arteriovenous malformation (AVM), with multiple calcified phleboliths and numerous hyperattenuating vascular channels.
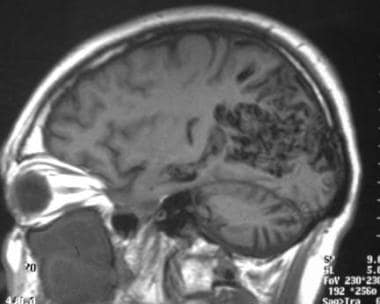 Sagittal T1-weighted MRI showing a large occipital arteriovenous malformation (AVM) with parasagittal flow voids.
Sagittal T1-weighted MRI showing a large occipital arteriovenous malformation (AVM) with parasagittal flow voids.
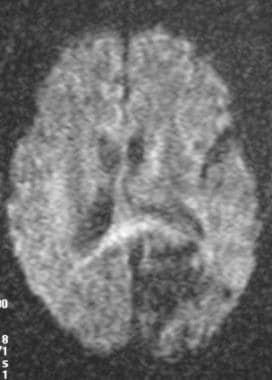 Diffusion-weighted MRI showing lack of signal intensity associated with an arteriovenous malformation (AVM).
Diffusion-weighted MRI showing lack of signal intensity associated with an arteriovenous malformation (AVM).
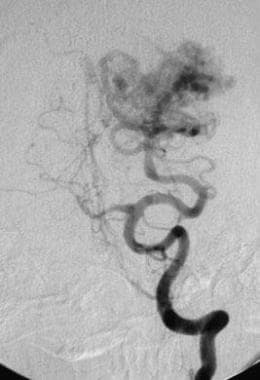
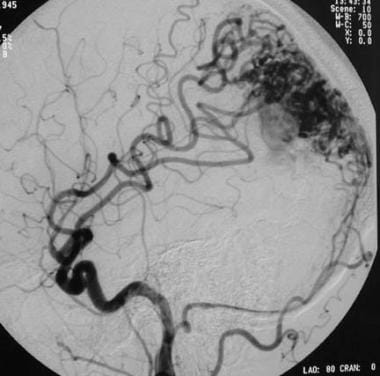 Lateral left carotid angiogram showing a mixed pial-dural arteriovenous malformation (AVM). Arterial and occipital arterial feeders extend to the nidus via distal branches of the middle cerebral artery.
Lateral left carotid angiogram showing a mixed pial-dural arteriovenous malformation (AVM). Arterial and occipital arterial feeders extend to the nidus via distal branches of the middle cerebral artery.
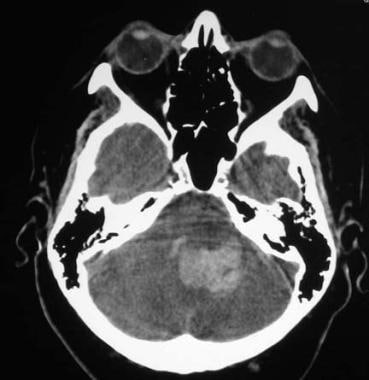 Arteriovenous malformation (AVM) of the brain. CT scan of the posterior fossa showing hemorrhage in the fourth ventricle, with extension to the left cerebellum.
Arteriovenous malformation (AVM) of the brain. CT scan of the posterior fossa showing hemorrhage in the fourth ventricle, with extension to the left cerebellum.
Categorization of AVMs
AVMs are categorized by their blood supply. Pial or parenchymal AVMs are supplied by the internal carotid or vertebral circulation, whereas dural AVMs are supplied by the external carotid circulation, and mixed AVMs are supplied by both.
Pial AVMs, which are almost exclusively congenital, are most common. Dural AVMs are relatively uncommon and are theorized to occur secondary to trauma, surgery, thrombosis of an adjacent venous sinus, or venoocclusive disease. Mixed AVMs usually occur when the lesion is large enough to recruit blood vessels from both internal and external carotid arteries. A pediatric variant of an AVM is the vein of Galen aneurysm, in which an AVM drains to and dilates the great vein of Galen.
Pial AVMs tend to be asymptomatic until the second, third, or fourth decade of life. They most commonly manifest as spontaneous hemorrhage or seizure. Other clinical signs include headache and transient or progressive neurologic deficit. Dural AVMs typically feature pulsatile tinnitus, cranial bruits, headaches, or hemifacial spasm. Infants with a vein of Galen malformation may present with hydrocephalus or severe congestive heart failure.
Saccular aneurysms occur in association with AVMs in 6 to 20% of patients. The preferred site for an AVM-associated aneurysm is a feeding artery. Venous and intranidal aneurysms occur less frequently. When aneurysms and AVMs occur together, they can cause intracranial or subarachnoid hemorrhage; however, intracranial hemorrhage is more likely to stem from an AVM. [25, 26, 27]
Staging of AVMs
Although present at birth, an AVM may be found soon after birth or much later in life, depending on its size and location. AVMs can become apparent after an accident or as a child grows into an adult (during puberty). As the patient's body grows, the AVM grows too. [12]
AVMs grow and change over time. AVMs are often organized by using a scale called the Schöbinger staging system. Not all AVMs pass through every stage [12] :
-
Stage I (quiescence): AVM is "quiet." Skin on top of the AVM may be warm and pink or red.
-
Stage II (expansion): AVM gets larger. A pulse can be felt or heard within the AVM.
-
Stage III (destruction): AVM causes pain, bleeding, or ulcers.
-
Stage IV (decompensation): Heart failure occurs.
Ultrasonography
Ultrasonography is often the first test ordered when there is suspicion that a person might have an AVM. Ultrasonography uses sound waves to make a picture of the blood vessels and tissues under the skin. It can also be used to detect the speed of blood flow, which helps doctors diagnose an AVM. Ultrasonography is a good method for young children because it does not require putting a child to sleep with anesthesia and it is completely painless. [12]
Safety and efficacy in surgical treatment of cerebral AVMs are dictated by thorough understanding of angioarchitectural features, intraoperative identification of feeding vessels, and appreciation of surrounding eloquent areas. Della Pepa and colleagues described the preliminary results of combined application of color Doppler ultrasound (CDUS) and contrast-enhanced ultrasound (CEUS) in a consecutive surgical series of AVM. They adopted these techniques in accordance with a designed protocol to guide safer AVM resection as an adjunct to indocyanine green videoangiography. They performed intraoperative assessment by ultrasonography before, during, and following nidus resection. Investigators reported that the CDUS/CEUS protocol is safe and repeatable and works as real-time imaging, further supporting complete surgical resection of AVMs. [28]
Computed Tomography
Computed tomography (CT) scanning of the brain is used to evaluate acute headache or other acute mental status changes suggestive of acute cerebral hemorrhage. Detection of a lobar hemorrhage can suggest an underlying mass or AVM. Cerebral CT scanning can be used to identify areas of acute hemorrhage, and results can suggest a vascular malformation, particularly with judicious use of contrast material. Furthermore, CT scanning can uniquely show vascular calcifications associated with AVMs. [14, 16]
CT scanning of AVM of the brain can show an isoattenuating-to-hyperattenuating hemispheric mass, as well as an accompanying abnormal vascular supply. In the absence of hemorrhage, nonenhanced CT scanning can show small foci of calcification in as many as 30% of patients. Other possible findings include cystic cavities representing previous hemorrhage and hypoattenuation of surrounding parenchyma representing encephalomalacia, cerebral atrophy, or gliosis.
The degree of confidence for CT is moderate. An additional study such as MRI or catheter angiography may be necessary to confirm the presence of an AVM.
(See the image below.)
 CT scan of the head showing a left occipital arteriovenous malformation (AVM), with multiple calcified phleboliths and numerous hyperattenuating vascular channels.
CT scan of the head showing a left occipital arteriovenous malformation (AVM), with multiple calcified phleboliths and numerous hyperattenuating vascular channels.
Contrast-enhanced CT scanning can reveal serpiginous vascular enhancement that is uniquely typical of an AVM. Occasionally, CT scans show edema, mass effect, or ischemic changes that may be associated with AVM, and further contrast-enhanced imaging may identify small AVMs missed by plain CT examination.
In the hyperacute stage of hemorrhage, a pial AVM appears as a hyperattenuating parenchymal lesion on nonenhanced CT scans because CT attenuation values and blood hemoglobin concentrations are directly proportionate. Attenuation is increased in the acute stage as a result of clot formation and the concomitant increase in hemoglobin concentration. The hyperattenuating region may be surrounded by a rim of hypoattenuation caused by extruded serum and edema.
Because attenuation of a hematoma decreases over time, the ruptured hemorrhagic component of an AVM evolves through a stage of isoattenuation that progresses to normal brain parenchyma. Nonenhanced lesions viewed during the isoattenuating phase may appear almost normal or may shine through, appearing minimally abnormal. If intravenous contrast material is administered during this stage, vascular enhancement may be noted, as well as nonspecific or ringlike areas of enhancement.
AVM in the chronic stage of intracerebral hemorrhage appears as a hypoattenuating area relative to normal brain tissue. In general, AVM enhancement that is not contiguous with the site of hemorrhage points to an associated aneurysm or venous varix.
Dural AVMs can be visualized by CT scanning.
In an emergency setting, CT scanning can show a presenting cerebral or extra-axial hemorrhage. CT scans may reveal secondary signs that infer the presence of a dural AVM (ie, abnormal enlarged dural sinuses or draining cerebral veins). Typically, these are best appreciated on contrast imaging. Unfortunately, the dural malformation nidus typically is poorly demonstrated on CT scans alone. [14]
False-positive CT results may occur, with lesions showing enhancement or calcification. Tumor neovascularity occasionally mimics an AVM, particularly that of a neovascular glioblastoma multiforme. In addition, a wide variety of CNS abnormalities are associated with CNS calcifications, which can lead to false-positive results.
False-negative results may be found if an AVM is isoattenuating relative to regional parenchyma. Some lesions may be detectable only if iodinated contrast is administered. Furthermore, an AVM may be overlooked if it is compressed by an adjacent parenchymal hemorrhage. Finally, vascular AVM may be misconstrued as cerebral hemorrhage because of the presence of large hyperattenuating vessels. Contrast-enhanced CT scanning or supplemental MRI or MRA can help clarify diagnosis in difficult cases.
Limitations of CT scanning
CT scanning is an excellent tool for detecting cerebral hemorrhage, but it can miss an underlying AVM. AVMs are typically isoattenuating relative to normal parenchyma and therefore can be overlooked, particularly if a contrast agent is not administered. In an emergency setting, administration of an iodinated contrast agent is typically deferred in favor of patient stabilization. Contrast-enhanced CT scanning also poses an inherent risk of radiation, and, because of CT costs, MRI may be a better screening examination for AVM in the general population. Contrast-enhanced CT scanning is performed to detect cerebral AVM when MRI is contraindicated or otherwise is not feasible. [14]
On CT scans, an AVM that appears as a noncalcified mass or a calcified and hyperattenuating focal mass must be distinguished from other calcified masses such as tuberous sclerosis, colloid cysts, neoplasms, and aneurysms.
Possible causes (in addition to vascular malformation) for parenchymal hematomas seen on CT scans include trauma; coagulopathy; hypertension; other vascular pathologies such as aneurysm, amyloid angiopathy, or vasculitis; vascular occlusion, as from a venous infarct or an embolic stroke with reperfusion hemorrhage; infection; and neoplasm.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) can help identify and characterize AVMs of the CNS, including brain and spinal cord, without the use of radiation or invasive techniques. MRI is the examination of choice for patients with chronic headaches, seizure disorders of unknown etiology, and pulsatile tinnitus (among other conditions).
MRI typically follows CT scanning in the acute setting of neurologic illness when an underlying vascular lesion such as AVM is suggested. MRI scans can reveal areas of parenchymal AVM involvement, showing both dilated feeding arteries and enlarged draining veins.
Magnetic resonance angiography (MRA) and venography can further supplement conventional MRI in demonstrating, in a near angiographic fashion, the anatomy and microarchitecture of an AVM. [29] MRI is the study of choice for detecting vascular malformations of the spinal cord and the spinal dura. [13, 14, 30, 31] On MRI, a typical unruptured AVM appears as a tightly packed or loose tangle of vessels. [18, 20]
The degree of confidence in MRI is high. MRI scans of vascular malformations of the brain are unique and typically are diagnostic of cerebral or spinal AVM, with a high degree of confidence. MRI findings may prompt catheter angiography for confirmation and preoperative or postoperative AVM treatment.
(See the image below.)
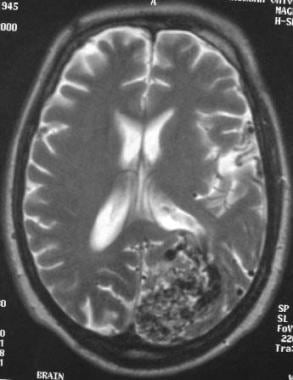 Arteriovenous malformation (AVM) of the brain. Axial T2-weighted MRI showing numerous flow voids corresponding to CT findings (not shown). Note the mass effect on the lateral ventricle despite the lack of a mass or hemorrhage.
Arteriovenous malformation (AVM) of the brain. Axial T2-weighted MRI showing numerous flow voids corresponding to CT findings (not shown). Note the mass effect on the lateral ventricle despite the lack of a mass or hemorrhage.
Rapid blood flow through enlarged arteries causes a signal or flow void on routine spin-echo T1- and T2-weighted images. This finding is uniquely characteristic of AVM.
MRI scans can show lesion size and usually the primary supply of the AVM and its venous drainage. MRI can reveal associated aneurysms on arterial feeders, as well as associated sequelae such as mass effect, edema, or ischemic changes.
Vascular steal in brain or spinal cord adjacent to the lesion may be visualized as a region of abnormally reduced signal intensity on T1-weighted images and increased signal intensity on T2-weighted, proton density–weighted, and short-tau inversion recovery (STIR) images.
MRI is particularly well suited to document AVM rupture. The appearance of the lesion depends on the stage of the hematoma.
Acute hemorrhage appears isointense on T1-weighted images and hypointense on T2-weighted images because of the presence of deoxyhemoglobin in extravasated but unlysed erythrocytes. Subacute intraparenchymal hemorrhage appears hyperintense on both T1- and T2-weighted imaging, which is consistent with the presence of methemoglobin. Chronic hematoma is characterized by a central hyperintense core surrounded by a ring of hypointensity resulting from hemosiderin deposits in macrophages in surrounding brain. Hemosiderin is mildly hypointense on T1-weighted images and markedly hypointense on T2-weighted images.
MRI is an excellent preoperative planning tool for delineating the relationship between an AVM nidus and critical brain structures. In particular, the relationship between hemispheric AVMs and eloquent brain regions can be clarified, particularly with functional MRI. Associated aneurysms may be seen within a hematoma as a flow void. Unfortunately, the sensitivity of MRI for detecting aneurysms smaller than 1 to 2 cm is low. [14]
Postoperative MRI
Postoperative MRI is useful for studying effects of surgery on adjacent brain; however, complete obliteration of the nidus is best documented with conventional angiography because MRI may fail to depict small amounts of residual nidus or persistent AV shunting. MRI can show the extent of nidal, arterial, or venous thrombosis following embolization. T2-weighted imaging is particularly useful for detecting embolic complications. [14]
Limitations of MRI
MRI is excellent for showing the AVM nidus and abnormal flow voids typical of an AVM; however, in acute cerebral hemorrhage, flow of compressed AVMs may no longer be visible and may be overlooked. This may lead to the need for serial MRI studies to search for an underlying cause of cerebral hemorrhage not shown on a single MRI study. MRI can cause underestimation of the number of feeding arteries, and associated aneurysms might be missed. Furthermore, MRI can have relatively poor sensitivity in detecting dural malformations. Gadolinium-based contrast material may be needed to reveal abnormal vascular channels. [14]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). This disease has occurred in patients with moderate to end-stage renal disease after they were given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Dark areas on T2-weighted MRI can be caused by rapid blood flow, as from an AVM, aneurysm, or neoplasm; dense calcification, as from an AVM, infection, or neoplasm; or a variety of other causes not associated with vascular malformations. These include the presence of air, minerals or metals, hemorrhage, and mucinous or dense proteinaceous material.
Angiography
Catheter angiography remains the criterion standard for characterization and delineation of brain and spinal AVMs. Angiography is a dynamic real-time study that shows not only the presence or absence of an AVM, but vascular transit time as well. Diagnostic angiography can uniquely delineate size and number of feeding arteries, and it can define the pial, dural, or mixed origin of an AVM.
Angiography can be used to measure the size of an AVM and to judge the compactness of the nidus. Furthermore, angiography can be used to evaluate the venous drainage pattern (superficial, deep, or mixed). Angiography frequently depicts associated risk factors for hemorrhage, including aneurysms and venous stenosis. Planning an angiography is a vital step in both interventional neuroradiologic and neurosurgical evaluation of patients with AVM. [14]
Angiography can reveal features that are believed to correlate with increased risk of hemorrhage. These include the presence of associated intranidal, remote, or pedicular aneurysms; central or deep venous drainage; stenosis of a draining vein; and a periventricular or intraventricular location. [4, 5, 6, 7, 26, 14]
This study should include both internal carotid arteries and both vertebral arteries, along with sequential evaluation of arterial, capillary, and venous phases. External carotid arteries should be evaluated for dural contributions. The goal of this study should be to identify number and locations of feeding arteries, angiographic location and size of the nidus, shunt type of the lesion (eg, high flow vs low flow), and pattern of venous drainage (eg, superficial, deep, or mixed).
The degree of confidence for angiography is high. The presence of abnormal CNS vascularity is usually best assessed by catheter angiography. Catheter angiography is a useful adjunct to cross-sectional imaging for overall assessment of CNS AVMs. Each test provides complementary information.
(See the images below.)
 Lateral left carotid angiogram showing a mixed pial-dural arteriovenous malformation (AVM). Arterial and occipital arterial feeders extend to the nidus via distal branches of the middle cerebral artery.
Lateral left carotid angiogram showing a mixed pial-dural arteriovenous malformation (AVM). Arterial and occipital arterial feeders extend to the nidus via distal branches of the middle cerebral artery.
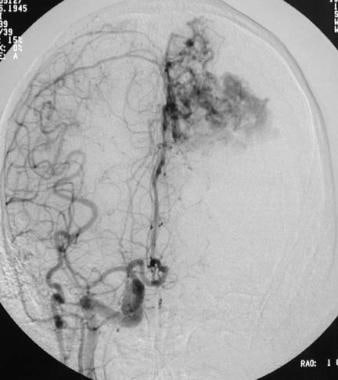 Arteriovenous malformation (AVM) of the brain. Anteroposterior right carotid angiogram showing left anterior cerebral artery supply secondary to vascular steal. Note that the left anterior cerebral artery does not opacify with an ipsilateral carotid injection of contrast material (see also the previous image).
Arteriovenous malformation (AVM) of the brain. Anteroposterior right carotid angiogram showing left anterior cerebral artery supply secondary to vascular steal. Note that the left anterior cerebral artery does not opacify with an ipsilateral carotid injection of contrast material (see also the previous image).
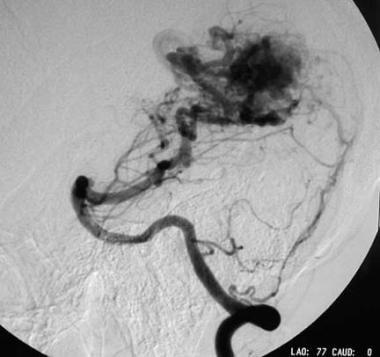 Arteriovenous malformation (AVM) of the brain. Lateral left vertebral angiogram showing a huge left posterior cerebral artery feeder to the nidus.
Arteriovenous malformation (AVM) of the brain. Lateral left vertebral angiogram showing a huge left posterior cerebral artery feeder to the nidus.
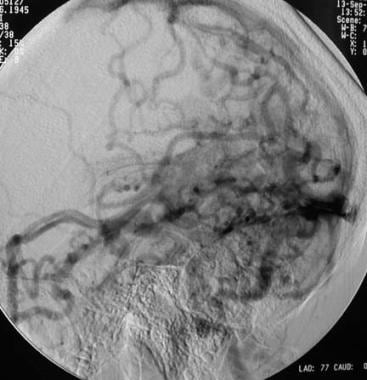 Arteriovenous malformation (AVM) of the brain. Venous phase of a vertebral angiogram showing numerous superficial and deep draining veins.
Arteriovenous malformation (AVM) of the brain. Venous phase of a vertebral angiogram showing numerous superficial and deep draining veins.
On conventional angiography, patent pial AVMs show enlarged cerebral or spinal arteries and veins, rapid AV shunting, and early draining veins.
Dural malformations typically show slower flow or AV shunting and are supplied by dural vessels such as meningeal branches or occipital arteries of external carotid arteries or meningeal branches of internal carotid or vertebral arteries.
Catheter angiography usually can be used to map all malformation feeders (pial, dural, or mixed) and to accurately assess the size of the nidus.
The Spetzler-Martin 5-point grading scale is commonly used to predict surgical outcomes for patients with brain AVMs. This scheme is typically applied to the angiographic data described above. In brief, grade I lesions are small, superficial, and located in noneloquent areas of brain, whereas grade V AVMs are large, deep, and found in functionally critical locations. Inoperable lesions are assigned to grade VI. [14, 17, 32, 33]
Angiographic false-positive findings are unusual but can occur in the presence of an early draining vein. This vein can be seen in a variety of disorders (most typically, in stroke). Abnormal neovascularity and abnormal venous drainage can also be seen in CNS neoplasms, particularly vascular glioblastomas and hemangioblastomas.
False-negative results can occur after acute hemorrhage, when an AVM may become angiographically occult or compressed by an adjacent hematoma. Partially or totally thrombosed lesions may show less-pronounced or absent AV shunting or may appear largely normal, with a vascular-shift mass effect of stagnant flow; this can lead to a false-negative result. [34]
Magnetic resonance angiography
Magnetic resonance angiography (MRA) is a noninvasive alternative to conventional angiography. Certain lesions hidden on conventional angiograms may be identified only on MRI scans because of their ability to depict hemosiderin deposits or other evidence of blood breakdown. Blood-breakdown products appear in a time-dependent manner after intracranial hemorrhage.
MRA offers several advantages over conventional angiography. For example, because of its ability to image all vessels in a given volume nonselectively, an AVM with multiple feeding arteries can be imaged noninvasively in a single study. In addition, 2-dimensional (2D) and 3-dimensional (3D) phase-contrast MRA can be used to examine the direction, rate, and quantity of blood flow. Another advantage of MRA is that it allows clinicians to retrospectively examine images in any plane.
3D time-of-flight (TOF) angiography may be used to image fast-flow components of AVMs. Flip angles of approximately 15º and repetition time (TR) of 40 ms are usually adequate for saturating stationary background tissues while allowing visualization of fully magnetized inflowing blood. Slower-flowing components of the AVM tend to be visualized poorly without the use of an MRI contrast agent, because vessels become more saturated as they course through the imaging volume. This is not entirely undesirable, as it allows an unobstructed view of feeding arteries and the nidus through effective suppression of overlying venous structures.
The arterial supply may be identified by means of 3D TOF, phase-contrast slab, or 3D phase-contrast acquisitions. Visualization of vessels with angiomatous change may require phase-contrast slab angiograms encoded for low flow velocities, such as velocity encoding (V enc) = 20 cm/s. Otherwise, imaging with V enc of 80 to 100 cm/s typically reveals the arterial supply. Complex flow in the AVM nidus is best seen on 3D TOF acquisitions, with small voxel size, partial echo sampling, and short echo time (TE). [13, 14, 15, 17]
Vessel-selective 4D MRA (VS-4D-MRA) has been found to be useful in detecting feeding arteries and in estimating details of the nidus structure in intracranial AVM. [29]
False-positive results may be noted when other types of CNS vascular malformation are encountered; these include cavernous angiomas, venous angiomas, and capillary telangiectasias. Lesions are associated with lower risk of rupture; they can mimic the appearance of an AVM, but they lack characteristic AV shunting. Nevertheless, false-positive findings may prompt catheter angiography for clarification. MRI scans can show abnormally enlarged arteries (atriomegaly)—a finding that suggests an underlying malformation when none is present.
False-negative MRI findings of CNS AVMs can occasionally occur as the result of a small AVM or an inconspicuous location. AVM may be overlooked or may not be apparent if compressed by an adjacent hematoma. AVM can also be missed if it is indistinguishable from the flow void of an adjacent normal vessel.
Limitations of angiography
Diagnostic angiography is the criterion standard for evaluation of AVMs; however, it is invasive and carries risks related to catheter placement, contrast agents, and injection of these agents. Specific neuroangiographic risks include stroke, arterial dissection, reactions to contrast material, and renal insufficiency and/or failure (among others). Nevertheless, modern cerebral angiography remains a safe and reliable method for AVM analysis, with an overall complication rate less than 1%. Spinal angiography can be tedious and is associated with risk of spinal cord infarction. [14]
-
CT scan of the head showing a left occipital arteriovenous malformation (AVM), with multiple calcified phleboliths and numerous hyperattenuating vascular channels.
-
Arteriovenous malformation (AVM) of the brain. Axial T2-weighted MRI showing numerous flow voids corresponding to CT findings (not shown). Note the mass effect on the lateral ventricle despite the lack of a mass or hemorrhage.
-
Sagittal T1-weighted MRI showing a large occipital arteriovenous malformation (AVM) with parasagittal flow voids.
-
T2-weighted coronal MRI of an arteriovenous malformation (AVM) of the brain.
-
Diffusion-weighted MRI showing lack of signal intensity associated with an arteriovenous malformation (AVM).
-
Lateral left carotid angiogram showing a mixed pial-dural arteriovenous malformation (AVM). Arterial and occipital arterial feeders extend to the nidus via distal branches of the middle cerebral artery.
-
Arteriovenous malformation (AVM) of the brain. Anteroposterior right carotid angiogram showing left anterior cerebral artery supply secondary to vascular steal. Note that the left anterior cerebral artery does not opacify with an ipsilateral carotid injection of contrast material (see also the previous image).
-
Arteriovenous malformation (AVM) of the brain. Lateral left vertebral angiogram showing a huge left posterior cerebral artery feeder to the nidus.
-
Arteriovenous malformation (AVM) of the brain. Anteroposterior left vertebral angiogram.
-
Arteriovenous malformation (AVM) of the brain. Venous phase of a vertebral angiogram showing numerous superficial and deep draining veins.
-
Sagittal T1-weighted MRI showing a small, right parietal arteriovenous malformation (AVM) with superficial venous drainage.
-
Arteriovenous malformation (AVM) of the brain. T2-weighted axial MRI showing flow voids.
-
Arteriovenous malformation (AVM) of the brain. Diagnostic angiogram obtained with carotid injection showing the distal anterior cerebral artery (pericallosal artery) feeder with superficial venous drainage to the superior sagittal sinus.
-
Arteriovenous malformation (AVM) of the brain. Vertebral injection image showing the collateral arterial supply stemming from distal posterior cerebral artery vessels.
-
Arteriovenous malformation (AVM) of the brain. Venous-phase image showing large, superficial draining veins.
-
Arteriovenous malformation (AVM) of the brain. CT scan of the posterior fossa showing hemorrhage in the fourth ventricle, with extension to the left cerebellum.
-
Arteriovenous malformation (AVM) of the brain. Axial T2-weighted MRI showing abnormal vascular flow voids along the left cerebellar tentorium.
-
Arteriovenous malformation (AVM) of the brain. Left carotid angiogram showing feeders to the dural malformation via the posterior division of the middle meningeal artery and the occipital arteries.
-
Arteriovenous malformation (AVM) of the brain. Left vertebral angiogram showing small dural arterial feeders via posterior meningeal arteries, which arise directly from the vertebral artery.
-
Arteriovenous malformation (AVM) of the brain. Venous-phase image showing a venous aneurysm that projects inferiorly from the cerebellar tentorium; this is the presumed cause of the patient's hematoma.
-
Lateral left carotid angiogram in a patient with seizures and an unruptured left parasagittal arteriovenous malformation (AVM).
-
Lateral left carotid angiogram obtained after a successful staged embolization with a liquid adhesive showing lack of arterial venous shunting.