Practice Essentials
Fibromuscular dysplasia (FMD) is an uncommon angiopathy of uncertain etiology associated with heterogeneous histologic changes that may affect the carotid and vertebral circulation, visceral arteries, and peripheral arteries. Mesenteric artery FMD is rare and presents with abdominal symptoms similar to Crohn disease (CD) and Behcet disease (BD). Surgical pathology may be required to differentiate the disorders. The clinical manifestations reflect the arteries involved and most commonly include hypertension caused by renal-artery stenosis (RAS) or strokes from carotid artery disease. FMD is one of the most important mimics of vasculitis. [1, 2, 3, 4, 5, 6, 7]
The incidence of fibromuscular dysplasia is 0.42-3.4%. Risk factors for FMD include gender, age, and history of smoking, with the disease primarily affecting middle-aged women. The carotid artery is involved in 75% of cases. [8, 9, 10, 11] FMD has been widely recognized as an important predisposing condition for spontaneous coronary artery dissection. [12]
Diagnostic guidelines for initial imaging for fibromuscular dysplasia are available from the American College of Radiology (ACR) and note that CT angiography (CTA) with IV contrast, magnetic resonance angiography with and without IV contrast, and arteriography are usually appropriate for initial imaging, and duplex Doppler ultrasonography and intravascular ultrasonography may be appropriate. [13] Conventional angiography remains the gold standard to diagnose FMD and can also measure the pressure gradient across the stenotic lesions; catheter-based angiography can provide concurrent endovascular therapy. However, Doppler ultrasound is usually the first-line screening test for FMD, revealing turbulence, tortuosity, elevated velocity, and resistive indices. [12, 9, 14, 15]
Angiography is the standard imaging approach for detecting fibromuscular dysplasia/arterial stenoses and aneurysms. The diagnostic and prognostic information available from captopril renography and the increasing availability of magnetic resonance angiography (MRA) have reduced the use of renal arteriography as a diagnostic tool, except in evaluating kidneys with intrarenal branch-artery stenoses and those with complex vascular anatomy, including multiple accessory arteries. CTA with maximum intensity projection (MIP) and quantitative measurement of stenosis is an accurate noninvasive technique for diagnosing fibromuscular dysplasia/stenosis of the visceral arteries, regardless of the etiology. [16, 17]
(See the CT images below.)
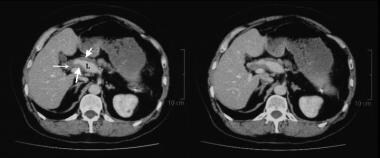 This 52-year-old man presented with pain in the left upper quadrant and was found to have a 3.2-cm aneurysm of the distal splenic artery. During surgery, the aneurysm ruptured, and splenectomy was performed. Histology of the resected splenic artery revealed intimal fibroplasia. Routine 2-year follow-up showed an enlarging aneurysm of the hepatic artery. Contrast-enhanced axial CT images show several narrowings of the common and proper hepatic arteries with intervening aneurysmal dilatation. Note the circumferential, lobulated tissue that is thickening outside the intima. This was assumed to be a manifestation of intimal fibroplasia.
This 52-year-old man presented with pain in the left upper quadrant and was found to have a 3.2-cm aneurysm of the distal splenic artery. During surgery, the aneurysm ruptured, and splenectomy was performed. Histology of the resected splenic artery revealed intimal fibroplasia. Routine 2-year follow-up showed an enlarging aneurysm of the hepatic artery. Contrast-enhanced axial CT images show several narrowings of the common and proper hepatic arteries with intervening aneurysmal dilatation. Note the circumferential, lobulated tissue that is thickening outside the intima. This was assumed to be a manifestation of intimal fibroplasia.
MRA produces excellent contrast-enhanced angiograms without the risk of iodinated compounds and radiation exposure. Unlike contrast-enhanced angiography, MRA has no attendant risk of nephropathy caused by contrast agent or cholesterol-emboli syndrome. MRA provides accurate information about the number of renal arteries, the size of the kidneys, and the presence of anatomic variants. Future developments may shorten MRA imaging times to reduce the problem of claustrophobia while still allowing the test to provide both anatomic and functional information. [18, 19, 20, 21]
(See the magnetic resonance image below.)
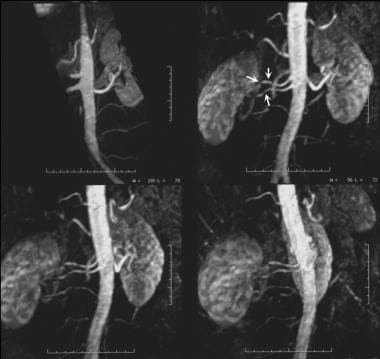 Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
Although FMD is a pathologic diagnosis, a characteristic angiographic change is the string-of-beads appearance (see the images below) caused by areas of relative stenoses or webs alternating with small fusiform or saccular aneurysms of the artery. The string-of-beads sign is typical of medial fibroplasia, which is only 1 of the types of FMD. [22]
 Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
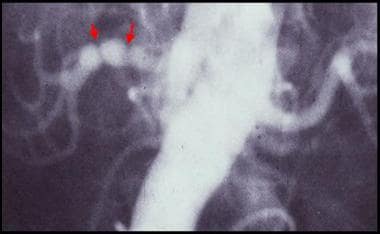 Conventional flush aortogram in a 47-year-old woman with difficult-to-control hypertension shows the characteristic string-of-beads sign of the right renal artery due to medial fibroplasia.
Conventional flush aortogram in a 47-year-old woman with difficult-to-control hypertension shows the characteristic string-of-beads sign of the right renal artery due to medial fibroplasia.
Local and regional preferences differ regarding the use of CTA vs MRA for cross-sectional imaging. However, as a group, radiologists are using MRA in place of contrast-enhanced angiography as the diagnostic modality of choice. With both CTA and MRA, mild FMD or FMD in the accessory arteries may be missed; therefore, it is still likely that contrast-enhanced angiography is the criterion standard.
Data support the superiority of MRA over duplex Doppler ultrasonography (US) in patients with uremia associated with RAS, though the data do not specifically apply to FMD. [18] As availability increases, MRA will likely become the screening test of choice in the diagnosis of RAS, including FMD.
Beregi et al examined 20 patients with hypertension (mean age, 56 yr) and CTA-proven FMD of the renal artery and concluded that helical CTA, especially the combination of transverse sections and MIP reconstructions, can reliably depict renal artery FMD. They further noted that because some lesions may not be shown, arteriography with pressure measurements is the only technique that can be used to assess the physiologic importance of the dysplasia. [23]
Doppler US can be used to measure the velocity of blood flow. It is a noninvasive technique and has a high sensitivity in expert hands. Color flow Doppler US may demonstrate disorganized flow patterns and a high-velocity flow stream associated with hemodynamically significant stenosis. [24, 25, 26, 27, 28]
Leung et al compared contrast-enhanced MRA with duplex US for the detection of RAS, with catheter angiography as the standard, and they found that contrast-enhanced MRA is a useful technique for diagnosing atherosclerotic renovascular disease. According to the authors, it overcomes the major limitations of duplex renal scanning. However, duplex imaging has the advantage of providing hemodynamic information, and it appears to be best suited for the assessment of suspected FMD. [18]
Gornnik et al have suggested that regardless of the initial site of vascular bed involvement, patients with FMD should undergo imaging of all vessels from the brain to the pelvis at least once, usually with CTA or contrast-enhanced MRA, to identify other areas of FMD and to screen for occult aneurysms and dissections. [10]
Radionuclide renography with technetium-mercaptoacetyltriglycine (MAG3)–captopril has a high sensitivity and specificity and adds a physiologic element to the diagnosis of RAS. In FMD, a positive captopril renographic finding supports intervention, though hypertension is unlikely to be cured. [29, 30]
Hypertensive urography is of historical interest and is no longer used as a screening technique for RAS. Likewise, plain-film radiography has a limited role in patients presenting acutely as a result of mesenteric ischemia, which is a rare complication of FMD.
Renal arteriography can be performed by using conventional or digital subtraction technique. Angiography is particularly indicated when vascular intervention is contemplated. Carbon dioxide has emerged as an alternative angiographic contrast agent for use in combination with digital subtraction angiography (DSA) to avoid the risk of conventional nephrotoxic contrast agents in patients with severe renal insufficiency.
(See the sequence of images in a single patient, below.)
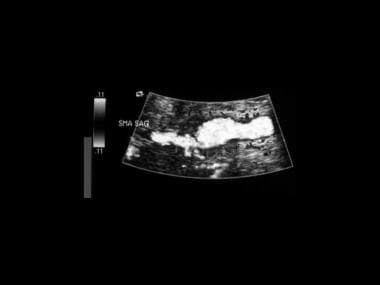 A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
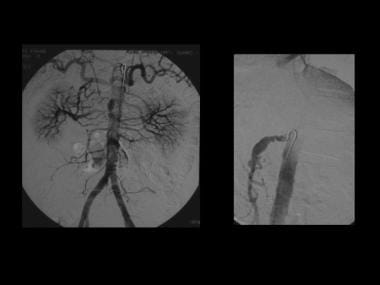 Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
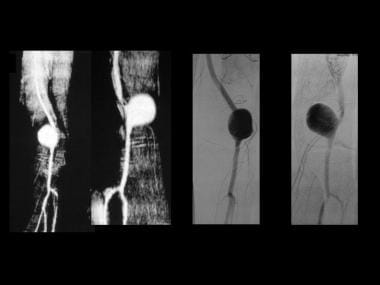 A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
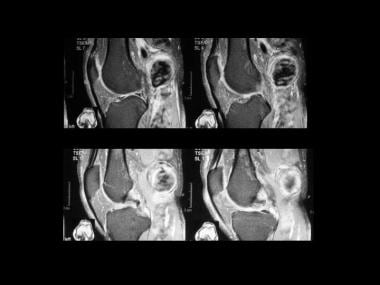 A popliteal artery aneurysm seen on coronal T2-weighted images. The resected popliteal artery aneurysm showed intimal fibroplasia (same patient as in the previous image).
A popliteal artery aneurysm seen on coronal T2-weighted images. The resected popliteal artery aneurysm showed intimal fibroplasia (same patient as in the previous image).
Pathophysiology
Leadbetter and Burkland first reported FMD of a renal artery in 1938 when they removed an ectopic kidney from a 5-year-old boy who presented with sustained hypertension. [31, 32] FMD may involve any layer of a visceral artery, and it may be classified on the basis of the primary involvement of the arterial wall. The classification system includes intimal, medial, or adventitial fibrosis. The medial variety can be subdivided.
In 1967, McCormack et al histologically classified FMD on the basis of the primary site of involvement of the arterial wall. [33] Their classification of fibrosing lesions of renal arteries included the categories of intimal fibroplasia, medial fibrosis with microaneurysms, subadventitial fibroplasia, and fibromuscular hyperplasia. They coined the term chain of beads to describe radiographic changes in medial fibroplasia of the renal artery. The term has subsequently been modified to string of beads. [22] Medial fibroplasia is the most common type of FMD and represents 80-95% of cases. The string-of-beads sign is classically seen in medial fibroplasia.
Subadventitial fibroplasia can have a similar radiographic appearance. However, in this variant, the size of the aneurysms does not exceed the diameter of the renal artery. Medial fibroplasias may appear as a single stenosis of a visceral artery, but it is most often seen as multiple stenoses with intervening outpouchings that form a chain. This is radiographically depicted as the string-of-beads sign.
On histologic evaluation, medial fibroplasias can be subdivided into 2 types: a peripheral form and a diffuse form. The peripheral form generally affects the outer media, replacing the smooth muscle with fibrous-appearing tissue. The diffuse form affects the media more extensively than the other form, with replacement of the media with fibrous tissue and medial thinning. The media can be completely absent in some areas, giving rise to aneurysmal dilatation. Although FMD was initially described in the renal arteries, many other visceral arteries are now known to be involved, and multiple visceral artery aneurysms have been reported. [34, 35]
Complete obstruction of the renal artery (20%) leading to total renal infarction has been reported. [36] Studying potential arterial donors with angiographic evidence of FMD, Cragg et al found that 26% developed hypertension, compared with 6% of age- and sex-matched control subjects. [37]
Hepatic and superior mesenteric involvement occurs infrequently, and sporadic cases of severe intestinal ischemia and HAA rupture have been reported. FMD is a rare cause of abdominal aortic aneurysm. [38]
Angiographic findings in systemic necrotizing vasculitis include 4 basic arterial anomalies: saccular microaneurysms (62%), arterial thrombosis (81%), arterial stenosis (81%), and luminal irregularities (90%). Alterations in the renal vascular flow are also observed in accordance with changes in the cortical medullary differentiation, heterogeneous nephrogram, and prolonged washout. Microaneurysms may regress after immunosuppressive therapy.
The clinical features, presenting symptoms, and vascular events were reviewed for the first 447 patients enrolled in a national FMD registry from 9 US sites. In this registry, FMD occurred primarily in middle-aged women. Cerebrovascular FMD occurred as frequently as renal FMD. Although a significant proportion of FMD patients may present with a serious vascular event, many present with nonspecific symptoms and a subsequent delay in diagnosis. [4]
The European/International Fibromuscular Dysplasia Registry enrolled 1022 patients with FMD from 22 countries for clinical phenotypes and predictors of diseases. All patients had the string-of-beads or focal stenosis in at least one vascular bed based on imaging studies. The patients were predominantly women (82%) and white (88%). Age at diagnosis was 46 ± 16 years (12% ≥65 yr), 86% were hypertensive, 72% had multifocal FMD, and 57% had multivessel FMD. Independent predictors of multivessel FMD were age at diagnosis, stroke, multifocal subtype, presence of aneurysm or dissection, and family history of FMD. Predictors of aneurysms were multivessel and multifocal FMD. Predictors of dissection were age at diagnosis, male gender, stroke, and multivessel FMD. [39]
Limitations of techniques
With CTA or MRA, the physician may overlook mild cases of FMD. Most false-negative and false-positive results for RAS arise from accessory renal arteries. MRI is expensive, and its availability is limited.
Measurements of the size of RAS on angiography (an important clinical consideration) may be imprecise and do not permit an assessment of the cross-sectional area or, most importantly, the flow through the stenotic segment. Some of these limitations may be overcome by using pressure measurements across the stenosis and by using intravenous US (IVUS). However IVUS has limited availability.
The various histologic types of FMD are difficult to distinguish on angiograms; this difficulty has an important clinical bearing from a prognostic point of view.
Doppler US is operator dependent, time consuming, and cumbersome. Several factors, including anatomic, technical, patient-related, and pathologic factors, can affect Doppler US.
The false-positive and false-negative rates of hypertensive urography are too high for it to be used as a screening test for RAS. Acceptance of radionuclide renography as a primary screening tool for RAS has been hindered by the lack of standardized protocols.
Radiography
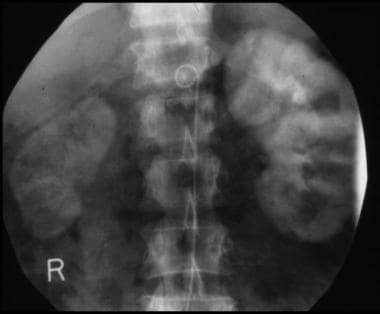
Plain radiographs have a role only when patients present acutely with bowel ischemia as a result of severe stenosis of the mesenteric arteries in association with thrombosis (see the images below). The radiographic findings include bowel distention, mucosal edema (shown as thumb printing), small-bowel pseudo-obstruction, gasless abdomen, pneumatosis, mesenteric and portal venous gas, and ascites.
 Conventional flush aortogram in a 47-year-old woman with difficult-to-control hypertension shows the characteristic string-of-beads sign of the right renal artery due to medial fibroplasia.
Conventional flush aortogram in a 47-year-old woman with difficult-to-control hypertension shows the characteristic string-of-beads sign of the right renal artery due to medial fibroplasia.
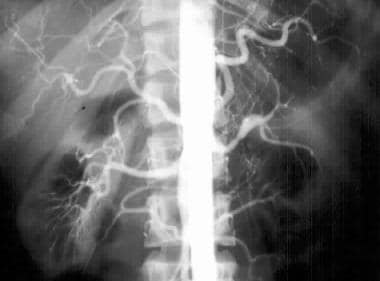 Conventional flush aortogram in a 43-year-old woman with difficult-to-control hypertension shows focal stenosis of the left mid renal artery; this finding may represent intimal fibroplasia.
Conventional flush aortogram in a 43-year-old woman with difficult-to-control hypertension shows focal stenosis of the left mid renal artery; this finding may represent intimal fibroplasia.
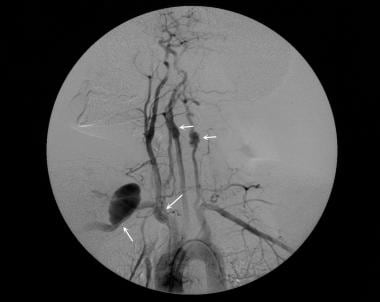
 Fibromuscular dysplasia in a 36-year-old woman with difficult-to-control hypertension. Delayed nephrogram phase of a flush aortogram shows a substantially reduced right kidney.
Fibromuscular dysplasia in a 36-year-old woman with difficult-to-control hypertension. Delayed nephrogram phase of a flush aortogram shows a substantially reduced right kidney.
 This 32-year-old man had fibromuscular dysplasia involving many arteries, including 1 renal, the carotid, the vertebral, right brachiocephalic, the right subclavian, and several intracranial arteries. He had severe renovascular hypertension and initially presented with a pulsatile mass in the right supraclavicular fossa. His hypertension responded to renal angioplasty. The aneurysm in the right subclavian artery was resected. Histologic examination of the resected specimen confirmed medial fibroplasia. The extent of the patient's intracranial disease precluded further surgery.
This 32-year-old man had fibromuscular dysplasia involving many arteries, including 1 renal, the carotid, the vertebral, right brachiocephalic, the right subclavian, and several intracranial arteries. He had severe renovascular hypertension and initially presented with a pulsatile mass in the right supraclavicular fossa. His hypertension responded to renal angioplasty. The aneurysm in the right subclavian artery was resected. Histologic examination of the resected specimen confirmed medial fibroplasia. The extent of the patient's intracranial disease precluded further surgery.
For the evaluation of renal artery stenosis (RAS), hypertensive urography is performed by rapidly administering an intravenous bolus into a peripheral vein and taking sequential 1-minute radiographs for 5 minutes. Additional delayed radiographs may be obtained to assess the rest of the urinary tract.
Findings that suggest renovascular hypertension caused by RAS include the following:
-
Small kidneys
-
Delayed excretion caused by decreased glomerular filtration
-
Increased density of contrast medium on the involved side caused by increased water reabsorption
-
Delayed washout of contrast material
-
Lack of distention of the renal collecting system
-
Extrinsic impression on the renal pelvis and or upper ureter from collateral vessels.
Computed Tomography
CT angiography (CTA), maximum intensity projection (MIP), multiplanar reconstruction (MPR), and quantitative measurement of stenosis are accurate, noninvasive techniques in the diagnosis of visceral artery stenosis. The advent of spiral and multisection CT scanning has made CTA feasible. Continuous scanning through an area of interest during a single breath hold provides sufficient data to reconstruct 3-dimensional (3D) images (see the mages below). [16, 23]
 This 52-year-old man presented with pain in the left upper quadrant and was found to have a 3.2-cm aneurysm of the distal splenic artery. During surgery, the aneurysm ruptured, and splenectomy was performed. Histology of the resected splenic artery revealed intimal fibroplasia. Routine 2-year follow-up showed an enlarging aneurysm of the hepatic artery. Contrast-enhanced axial CT images show several narrowings of the common and proper hepatic arteries with intervening aneurysmal dilatation. Note the circumferential, lobulated tissue that is thickening outside the intima. This was assumed to be a manifestation of intimal fibroplasia.
This 52-year-old man presented with pain in the left upper quadrant and was found to have a 3.2-cm aneurysm of the distal splenic artery. During surgery, the aneurysm ruptured, and splenectomy was performed. Histology of the resected splenic artery revealed intimal fibroplasia. Routine 2-year follow-up showed an enlarging aneurysm of the hepatic artery. Contrast-enhanced axial CT images show several narrowings of the common and proper hepatic arteries with intervening aneurysmal dilatation. Note the circumferential, lobulated tissue that is thickening outside the intima. This was assumed to be a manifestation of intimal fibroplasia.
Many scanning protocols are available. The general consensus is that both a timed bolus and a rapid injection rate improve image quality. No positive oral contrast material should be used because it severely degrades image quality. Immediately before the procedure, the authors' patients ingest water, and glucagon is then given intravenously to diminish bowel movement and maximize bowel distention. Some centers do not find this protocol essential.
The 3 most common techniques used for 3D reconstruction are MIP, shaded surface display (SSD), and volume rendering (VR). MIP is often the single most useful technique for 3D reconstructions. Axial CT with sagittal and coronal reconstruction may elegantly depict visceral artery aneurysms associated with arterial-wall thickening, as seen in fibromuscular dysplasia (FMD).
Mesenteric occlusion is a rare complication of FMD. In an acutely ill patient, CTA may show either an arterial mesenteric occlusion or high-grade stenosis. Scrutinizing the axial CT images may show thickening of the bowel wall, pneumatosis, portal venous gas, or mesenteric hemorrhage.
Degree of confidence
The diagnostic accuracy of CT and gadolinium-enhanced 3D MRA have generally both been good, with sensitivities and specificities of 90-100%. The sensitivity and specificity of CTA in the diagnosis of RAS of 50% or more are approximately 90% and 97%, respectively. With RAS stenosis of 75% or more, the sensitivity of CTA is even higher. Because most of the false-negative and false-positive results arise from accessory renal arteries, the accuracy in detecting RAS of the main renal artery with CTA may be as good as that of angiography. Most published data apply to atherosclerotic RAS. The accuracy of detection of FMD remains unknown. [19, 23, 40]
Accessory renal arteries are reliably identified by using CTA. RAS of either the mainstem artery or its intrarenal branches is detected with a high degree of accuracy.
An advantage of CTA is that the lumen of the vessel and the pathologic vessel wall are both visualized. This can be an advantage in determining the choice of interventional therapy, such as stenting, angioplasty, or surgery, though it applies more to atheromatous disease than to FMD. [41]
Visualization of small intrarenal vessels is difficult, and accurate assessment of peripheral stenoses beyond the renal hilum may be problematic. However, once again, this feature applies more to atheromatous disease than to FMD. [19]
One distinct drawback of CTA is the 120-150 mL of iodinated contrast used per scan. This amount of contrast agent increases the risk of nephropathy. In the setting of a kidney that is already compromised by arterial stenosis, nephropathy may cause further function compromise. Olbricht et al have shown that in patients with preexisting impaired renal function, CTA may be unreliable in the assessment of RAS, though their study was not specific for FMD. [16]
In patients with chronic symptoms that are possibly related to mesenteric angina, a normal celiac axis and superior mesenteric artery (SMA) virtually exclude mesenteric ischemia. However, in patients with moderate arterial stenosis, MRA may be more precise than CTA.
False positives/negatives
Andreoni et al expressed concern that CTA or MRA may cause mild cases of digital subtraction angiography (DSA)-detectable FMD to be overlooked. Most of the false-negative and false-positive results for RAS arise from accessory renal arteries. [42]
Verswijvel et al have reported magnetic susceptibility artifacts due to titanium surgical clips that mimicked FMD of the renal artery in a kidney transplant. [43]
Magnetic Resonance Imaging
Magnetic resonance angiography (MRA) produces excellent contrast-enhanced angiograms without the risk of iodinated compounds and without radiation exposure. MRA provides accurate information about the number of renal arteries, the size of the kidneys, and the presence of anatomic variants (see the images below).
 Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
 A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
 Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
 A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
 A popliteal artery aneurysm seen on coronal T2-weighted images. The resected popliteal artery aneurysm showed intimal fibroplasia (same patient as in the previous image).
A popliteal artery aneurysm seen on coronal T2-weighted images. The resected popliteal artery aneurysm showed intimal fibroplasia (same patient as in the previous image).
Comprehensive examination includes both 3D dynamic gadolinium-enhanced and 3D phase-contrast MRA techniques, which allow for evaluation of the renal arteries and other visceral arteries. The 3D phase-contrast technique is flow based and is subject to dephasing in the presence of clinically significant arterial stenosis. 3D gadolinium-enhanced MRA produces excellent contrast-enhanced angiograms without the risk of iodinated compounds and without radiation exposure. Local and regional preferences differ regarding the use of CTA or MRA for cross-sectional imaging. However, as a group, MRA is replacing contrast-enhanced angiography as the diagnostic modality of choice. [18, 19]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Degree of confidence
Gadolinium-enhanced MRA has high sensitivity for detecting stenosis in the main and accessory renal arteries. At present, MRA provides anatomic information regarding a vascular stenosis, but it provides little information about the functional significance of a stenosis.
Andreoni et al expressed concern that CTA or MRA may cause mild cases of DSA-detectable FMD to be overlooked. [42] Although false-negative studies in RAS are rare, stenoses tend to be overestimated, and a false-positive diagnosis may result. Phase-contrast MRA, a type of MRA based on accumulated phase differences, can be performed to somewhat compensate for this tendency. Both CTA and MRA may cause mild FMD or FMD in the accessory arteries to be missed. Therefore, contrast-enhanced angiography likely remains the criterion standard.
Ultrasonography
Data about the use of ultrasonography, specifically in fibromuscular dysplasia (FMD), are limited. Most studies have been dedicated to the more common atheromatous stenoses of the visceral arteries (see the image below). In a study by Gowda et al, both color flow duplex imaging and IVUS depicted blood-flow and endoluminal abnormalities of renal-artery FMD. Balloon angioplasty eliminated or improved IVUS findings and produced substantial, sustained reductions in blood pressure, an effect that was independent of baseline arteriographic appearance. This finding calls into question the legitimacy of arteriography as the diagnostic criterion standard. [44] (See the image below.)(See the image below.)
 A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
The diagnosis of renal artery stenosis (RAS) is based on systolic and diastolic velocity changes throughout the length of the renal artery. Renal artery flow patterns can be classified into 4 categories: normal, diameter-reducing stenosis less than 60%, diameter-reducing stenosis more than 60%, and occlusion. [24, 25, 26, 45]
The peak systolic velocity in normal renal arteries is 120 cm/s ± 12. With an average peak systolic aortic velocity of 60 m/s ± 15, both velocities decrease with age. The kidneys offer a low-resistance vascular bed; therefore, the Doppler spectral waveform from the normal kidney is that of a constant, forward, diastolic flow. In renal parenchymal disease, vascular resistance is increased; this results in a decrease in the diastolic flow component and an increase in the pulsatility of the Doppler spectral waveform. Parenchymal diastolic flow velocities of less than 20% of the peak systolic velocities are consistent with renal parenchymal disease.
In RAS of whatever cause, the peak systolic velocity increases more than 150 cm/s for angles less than 60° or 180 cm/s for angles greater than 70°. Poststenotic spectral broadening may be present with or without flow reversal. Flow may be absent during diastole in stenosis of more than 50%. A ratio of the peak systolic renal artery velocity to the aortic peak systolic velocity of 3.5 or more is said to be predictive of more than 60% stenosis.
Certain indirect Doppler US signs have been described for RAS. One such sign is a tardus-pavus pulse, [45] which is demonstrated as a gradual slope of Doppler waveform during systole (pulse time increase >0.07-0.12 s) and as an attenuated Doppler waveform amplitude (peak systolic velocity < 20-30 cm/s). The acceleration index is determined by dividing the slope of the systolic upstroke (in kilohertz per second) by the carrier Doppler frequency, and the acceleration time is the interval between the onset of systole and the initial peak. The acceleration index in RAS is greater than 3 m/s2. The resistive index in RAS is usually less than 0.56. The early systolic peak may be absent in RAS. [45]
Color flow Doppler images may demonstrate disorganized flow patterns and a high-velocity flow stream associated with hemodynamically significant stenosis. A false-negative diagnosis may occur with an accessory renal artery, whereas a false-positive diagnosis may be made when coarctation of the aorta is present.
SMA and celiac-axis stenosis
Color flow Doppler imaging is effective in demonstrating flow disturbance associated with tortuosity and stenosis at the origin of the celiac axis. Doppler spectral waveforms in a normal fasting celiac axis demonstrate a forward flow with an average peak systolic velocity of 123 cm/s ± 9 (age range, 48-79 yr) associated with a significant increase in the systolic or diastolic flow velocity after a meal. This increase is also reflected in the hepatic and splenic arteries. The average postprandial systolic velocity 30 minutes after a meal of 355 kcal is 132 cm/s ± 7. In the presence of 60% celiac-axis stenosis, the peak velocity is increased to 167-208 cm/s ± 9. With this degree of stenosis, color Doppler imaging demonstrates a high-velocity jet at the stenotic site associated with poststenotic turbulent flow.
The potential for collateralization between the celiac axis, the superior mesenteric artery (SMA), and the inferior mesenteric artery (IMA) is remarkable. As a result, the reading of peak systolic pressure in the celiac axis may be lower or higher than that expected when concomitant SMA occlusion is present. This feature may result in the overestimation or underestimation of ischemic disease.
The fasting SMA demonstrates a low diastolic flow; however, after a meal, both the peak systolic and end-diastolic velocities show a tremendous increase in the absence of arterial stenosis. The normal fasting SMA velocities are 128 cm/s ± 16 (age range, 23-42 yr). After a meal, the peak systolic velocity increases to 162 cm/s ± 11 with an end-diastolic velocity in the range of 48 cm/s ± 7 within 15 minutes after a meal. The peak systolic velocity almost doubles within 45 minutes after a meal. With significant SMA stenosis, the peak systolic velocity exceeds 270 cm/s, with a concomitant increase in diastolic flow. Color flow Doppler imaging shows a jet through the stenotic segment with turbulent flow downstream for some distance.
By using color and pulsed Doppler evaluation of visceral artery aneurysms, turbulent arterial flow can be shown within the anechoic masses related to major visceral arteries. Diagnosing these aneurysms is essential because the mortality rate is as high as 82% with spontaneous rupture.
Degree of confidence
Duplex US of the abdominal visceral arteries is perhaps the most technically demanding US procedure. This study has operator- and patient-related limitations, such as the operator's expertise and optimization of the equipment and the patient's habitus and preparation.
Doppler US has several limitations in the diagnosis of RAS. These include patient-related factors (eg, bowel gas, obesity, respiratory renal movements, poor compliance), anatomic factors (eg, multiple renal arteries [16-28%], variation of renal veins used as imaging landmarks, horseshoe kidneys, crossed ectopia), technical factors (eg, false-positive results due to suboptimal angles, variation in operator experience, incomplete examination [because complete renal evaluation is cumbersome], need to visualize the entire length of artery, transmitted cardiac and/or aortic pulsation that may obscure renal waveforms, different emphasis on variable parameters), and pathologic factors (eg, false tracings recorded from large collateral vessels and reconstituted main renal artery, variable effects of RAS of different causes, including atheroma, fibromuscular hyperplasia, vasculitis, arteriovenous fistula, retroperitoneal fibrosis, and neurofibromatosis).
Orificial stenoses and FMD may particularly difficult to interpret.
Color flow Doppler imaging in RAS of whatever cause may demonstrate disorganized flow patterns and a high-velocity flow stream associated with hemodynamically significant stenosis. [24, 25, 26, 46]
US is a sensitive yet noninvasive technique that may provide useful information in mesenteric ischemia by demonstrating absent or barely visible color-flow arterial signals and thickened bowel wall loops. Doppler techniques are particularly useful for investigating chronic mesenteric ischemia.
False positives/negatives
With RAS, a false-negative diagnosis may occur with accessory renal artery, whereas a false-positive diagnosis may be due to coarctation of aorta.
There are several limitations of Doppler analysis of celiac-axis and SMA ischemia. First, the extensive potential for collateralization in splanchnic vessels may make assessment of a single vessel stenosis difficult. Second, the risk of error is increased when the angle of insonation used is greater than 60°. Third, careful placement of the sample volume is crucial. Unless the SMA is examined throughout, visualization of the vessel may result in a false-negative finding.
Nuclear Imaging
Radionuclide renography can be performed in the diagnosis of renal artery stenosis (RAS) regardless of the cause. Several radiopharmaceuticals are available, including diethylenetriamine pentaacetic acid (DTPA) and 131-iodine–labeled orthoiodohippurate (OIH), to serve this purpose. Technetium-labeled mertiatide (MAG3) has also been used. Since the introduction of captopril renography, various modifications have been made. Some centers use only 1 agent—either DTPA or MAG3. [29, 30, 47]
A positive ACE-inhibitor radionuclide scan indicates that renovascular hypertension is present; it also implies the existence of hemodynamically significant RAS (>60-75% of the lumen).
Preliminary data suggest that aspirin renography may be as sensitive as captopril renography for RAS. Data about the use of aspirin renography in FMD are not available. Given that aspirin, compared with captopril, reduces renal blood flow and thus tubular tracer delivery in poststenotic kidneys, aspirin renography is expected to be more useful than other techniques, particularly if tubular tracers are used. [48, 49, 50]
Degree of confidence
Standard renography with OIH has low specificity and sensitivity in the diagnosis of RAS, including stenosis from FMD.
For RAS greater than 50%, Tc-MAG3-captopril renography has a sensitivity of 90%, a specificity of 91%, a positive predictive value of 70%, and a negative predictive value of 97%.
Data from Imanishi et al suggest that the sensitivity of iodine-123 OIH or MAG3 renography for the diagnosis of unilateral renovascular hypertension increases when aspirin is given. [48] Although Maini et al found that aspirin renography is superior to captopril renography, [49] van de Ven et al concluded that, for the identification of RAS, the usefulness of aspirin renography equals but does not surpass that of captopril renography. [29, 30, 50]
In fibromuscular dysplasia, a result on positive captopril renography supports intervention, though hypertension is unlikely to be cured. However, the value of captopril renography for patients with significantly impaired renal function is not clear, and little evidence suggests that a positive test can be used to predict a favorable outcome from intervention in terms of preservation of renal function.
The sensitivity and specificity of studies of the captopril renin test are 75-100% and 60-95%, respectively. A major limitation is the need to discontinue antihypertensive therapy, including ACE inhibitors, beta blockers, and diuretics, because they can affect the baseline plasma renin activity. The sensitivity of the test is also low, and its predictive value is less than that of a captopril renogram.
Estepa et al examined 20 children (aged 5 days to 15 years) with hypertension whose diagnoses were confirmed on aortography. [51] Captopril renograms and Doppler sonograms were initially obtained in only 8 children and suggested the diagnosis of RAS (7 with FMD).
False positives/negatives
With standard radionuclide (OIH) renography, false-positive results can occur from conditions that cause unilateral reduction in blood flow. These include chronic pyelonephritis, renal outlet obstruction, renal vein thrombosis, compression of the renal hilum, perirenal abscess, perirenal hematoma, and ptosis of the kidney.
Patients with renovascular hypertension may have false-negative captopril renograms after the long-term administration of captopril despite adequately maintained blood pressure. Therefore, when possible, ACE inhibitors should be discontinued before captopril renography is performed. Overhydration may result in false-negative findings, and underhydration may result in a false-positive finding. Bilateral RAS may be difficult to diagnose. Poor renal function most often results in a nondiagnostic examination. An asymmetric, small kidney with poor function is often unresponsive to captopril renography.
Angiography
Conventional angiography remains the criterion standard for the detection of renal artery stenosis (RAS), though it is being challenged by CTA and MRA. The hemodynamic significance of RAS may be assessed by evaluating the severity of the stenosis and the presence of collateral circulation to the kidney. (See the images below.)
 A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
 Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
 A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
Indications for renal artery interventions are determined on the basis of clinical, anatomic, and physiologic criteria. Anatomic criteria are stenosis greater than 60% of the diameter, poststenotic dilatation, collateral circulation, and diminished renal size. Physiologic criteria are abnormal radionuclide scan, lateralizing renal vein renin, duplex US findings, and transstenotic pressure gradient (during angiography, >10% peak systolic). When the stenosis is 70%, it is most likely hemodynamically significant, and no pressure measurement is needed. For 50-70% stenosis, the pressure gradient should be determined before intervention.
Flush aortography is usually performed in the anteroposterior and slight left anterior oblique projections to best visualize the origins of the renal arteries. If the main renal arteries are normal, selective renal arteriography should be performed in the anteroposterior and contralateral oblique projections by using the magnification technique to best define renal branch stenoses.
Intimal fibroplasia affects the main renal artery and major segmental arterial branches. It is often bilateral and characterized by narrow annular radiolucent bands associated with poststenotic fusiform dilatation.
Medial fibroplasias also involve the main renal artery and larger branches, often bilaterally; it is characterized by the classic string-of-beads sign with alternating areas of stenoses and intervening aneurysmal dilatation. A single focal stenosis may occur.
Subadventitial fibroplasia usually affects the distal main renal artery and is characterized by long irregular stenosis. There may be associated beading, but usually there is no aneurysm formation. The diameter of beads is not wider than the normal diameter of artery.
Celiac, hepatic, splenic, left gastric, SMA, and IMA stenoses caused by FMD are rare, but all of these have been reported. The angiographic appearances are similar to those seen in RAS. When stenosis occurs at these sites, the collateral circulation is more obvious on angiograms, as a result of rich potential pathways between the celiac axis and the SMA and IMA.
Renal vein renin measurements
Ischemic kidneys release high levels of renin in their veins. Renal venous sampling for measuring renin levels to compare the venous drainage from each kidney can be used to predict the degree of renal ischemia and the potential success of revascularization. A 1.5-fold increase in renin level in the ischemic kidney constitutes a positive result; in such cases, use of revascularization to treat elevated blood pressure will likely be successful.
Renin secretion in the contralateral kidney is suppressed, as evidenced by the similar levels of renin measured in the renal artery, infrarenal inferior vena cava and renal vein. In approximately 10% of healthy patients, the ratio is above 1.5; in fewer than 20% is the ratio below 1.1. The accuracy of the measurements may be improved with the prior administration of an ACE inhibitor, which increases renin secretion on the affected side.
False-negative and false-positive results are frequent. Although more than 90% of patients with unilateral RAS and increased renin levels in the affected renal vein have a positive response to angioplasty or surgery, approximately 50% of patients with nondiagnostic findings also benefit from revascularization. As a result, most clinicians rely on the clinical index of suspicion rather than renal vein renin measurements to estimate the physiologic significance of RAS. However, renal renin vein measurements may still be useful in patients with bilateral RAS, in whom measurements may determine the side that contributes most to the hypertension.
Degree of confidence
Renal angiography remains the criterion standard for the diagnosis of RAS. Angiography is an essential step when renovascular intervention is contemplated. Angiography only provides anatomic information; it does not allow assessment of blood flow through the stenosis.
Angiography may lead to an underestimation of the severity of renal artery obstruction, which is better assessed by means of pressure measurements. On occasion, carbon dioxide angiography cannot be used to image a particular segment of a renal artery because of superimposed bowel gas. Artifact caused by moving structures such as peristalsis on digital subtraction angiography (DSA) may be mistaken for RAS.
-
Distribution of various types of fibromuscular dysplasia.
-
Three-dimensional gadolinium-enhanced magnetic resonance angiograms (MRAs) show medial fibroplasia, which appears as classic string-of-beads sign. This sign is due to multiple stenoses with intervening outpouchings that form a chain. In this case, the lesions involve the main right renal artery and the right accessory renal artery in a 37-year-old man with difficult-to-control hypertension.
-
Conventional flush aortogram in a 47-year-old woman with difficult-to-control hypertension shows the characteristic string-of-beads sign of the right renal artery due to medial fibroplasia.
-
Conventional flush aortogram in a 43-year-old woman with difficult-to-control hypertension shows focal stenosis of the left mid renal artery; this finding may represent intimal fibroplasia.
-
Fibromuscular dysplasia in a 36-year-old woman with difficult-to-control hypertension. Delayed nephrogram phase of a flush aortogram shows a substantially reduced right kidney.
-
This 32-year-old man had fibromuscular dysplasia involving many arteries, including 1 renal, the carotid, the vertebral, right brachiocephalic, the right subclavian, and several intracranial arteries. He had severe renovascular hypertension and initially presented with a pulsatile mass in the right supraclavicular fossa. His hypertension responded to renal angioplasty. The aneurysm in the right subclavian artery was resected. Histologic examination of the resected specimen confirmed medial fibroplasia. The extent of the patient's intracranial disease precluded further surgery.
-
This 52-year-old man presented with pain in the left upper quadrant and was found to have a 3.2-cm aneurysm of the distal splenic artery. During surgery, the aneurysm ruptured, and splenectomy was performed. Histology of the resected splenic artery revealed intimal fibroplasia. Routine 2-year follow-up showed an enlarging aneurysm of the hepatic artery. Contrast-enhanced axial CT images show several narrowings of the common and proper hepatic arteries with intervening aneurysmal dilatation. Note the circumferential, lobulated tissue that is thickening outside the intima. This was assumed to be a manifestation of intimal fibroplasia.
-
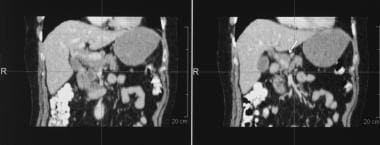
Coronal reconstructions of CT images in the same patient as in the previous image.
-
A 28-year-old man presented with episodic, postprandial abdominal pain, hypertension, ischemic changes in the right toes, and a pulsatile swelling behind the knee. In this ultrasonogram, the superior mesenteric artery has a beaded appearance.
-
Angiogram of the same patient as in the previous image confirms that the superior mesenteric artery has a beaded appearance.
-
A popliteal artery aneurysm depicted by MRA and angiography (same patient as in the previous image).
-
A popliteal artery aneurysm seen on coronal T2-weighted images. The resected popliteal artery aneurysm showed intimal fibroplasia (same patient as in the previous image).