Practice Essentials
Myocardial ischemia is a disorder that is usually caused by a critical coronary artery obstruction, which is also known as atherosclerotic coronary artery disease (CAD). CAD is the leading cause of death worldwide, and it is the second most common cause of emergency department visits in the United States. Diagnosing myocardial ischemia prior to a heart attack is important. For approximately one third of patients, CAD is not diagnosed until after a heart attack occurs. Fortunately, treating known CAD has tremendous benefits. Both coronary artery revascularization and medical therapies significantly reduce the morbidity and mortality rates of CAD. Since CAD is common and deadly, but treatable if detected early, early diagnosis of the condition is critical.
When ischemia is severe and prolonged, it causes myocyte death and results in loss of contractile function and tissue infarction. In cases of less severe ischemia, some myocytes remain viable but have depressed contractile function. The disease occurs in both the young and the old, in both women and men, and in both patients with and patients without comorbidities.
The diagnosis of CAD can be difficult to make. Frequently, the disease is diagnosed only after the patient has had a heart attack. The symptoms of CAD range from unstable angina to no symptoms at all. Although guidelines exist for the screening of hyperlipidemia and hypertension, no screening method has been uniformly accepted for CAD.
Several studies have been designed to help the clinician determine a patient’s CAD risk level. [1, 2] With this information, the clinician can decide, with reasonable certainty, which type of workup is indicated. For the low-risk population, exercise treadmill testing alone is frequently sufficient; however, in patients with a moderate-to-high risk for CAD, an imaging study is essential along with the stress test. [3, 4, 5]
The nuclear myocardial scan is the best initial imaging study for the detection of myocardial ischemia; however, in some locales, stress echocardiography is performed more often because it is more readily available and because local clinicians are better trained in echocardiography and are more comfortable with the technology. Nonetheless, the wealth of research into nuclear scanning makes a strong case for its role as the best and preferred test for detecting CAD. Nuclear cardiology studies are used to assess myocardial blood flow, evaluate the heart's pumping function, and visualize the size and location of a myocardial infarction. Among the techniques of nuclear cardiology, myocardial perfusion imaging is the most widely used. [3, 4, 6, 7, 8]
The assessment of myocardial perfusion and function using PET and hybrid positron emission tomography (PET)/CT imaging is becoming more available as the cost of the technology decreases and as positron-emitting radiopharmaceuticals become more available. Cardiac PET has the advantage of higher spatial resolution, temporal resolution, and, in many cases, a decreased radiation exposure to patients. Newer gamma cameras using simultaneous 180° acquisition appear to have the potential of offering similar benefits as PET technology but are able to use the less costly technetium (TC)-99m-based radiopharmaceuticals and thallium-201 (Tl-201). [9, 10, 11, 12, 13, 14, 15]
One potentially important physiologic parameter obtained by these newer technologies is the myocardial perfusion reserve (MPR). In patients with ischemic heart disease who undergo revascularization based on PET viability assessment with fludeoxyglucose F-18 (F-18 FDG), those with a low myocardial perfusion reserve were at an increased risk of adverse cardiac events. [16, 9]
Currently, nuclear myocardial scans include both perfusion and gated wall motion images. [17, 8] Coronary artery blood flow can be assessed, and the scans can also be used to accurately determine the left ventricular ejection fraction, the end-systolic volume of the left ventricle, regional wall motion, and wall thickening. [18] In addition, solid evidence links these findings to clinical outcomes.
In the past, the field of nuclear cardiology encountered large variations in clinician training, quality control, and scan interpretation. In response, the American Society of Nuclear Cardiology (ASNC) was formed by a coalition of cardiologists, radiologists, and nuclear medicine physicians. Along with the American College of Cardiology and the Society of Nuclear Medicine, the ASNC established standard training guidelines for physicians, along with a minimum knoNuclear cardiology studies use noninvasive techniques to assess myocardial blood flow, evaluate the pumping function of the heart as well as visualize the size and location of a heart attack. Among the techniques of nuclear cardiology, myocardial perfusion imaging is the most widely used knowledge base. The Certification Board of Nuclear Cardiology has established rigorous training verification and a knowledge assessment test.
Because CAD is common and deadly but highly treatable, early diagnosis is critical. Nuclear imaging plays an important role in the diagnosis of myocardial ischemia as well as several other cardiovascular diseases. Quality control forms the backbone of nuclear myocardial imaging, and as a result, nuclear cardiology has developed into a medical specialty. With rigorous training standards and knowledge assessment, the Certification Board of Nuclear Cardiology has established a unique specialty in medicine dedicated to the use of nuclear scanning for the detection of cardiovascular disease. [19]
New hardware and software designs have been developed to optimize image quality with reduced radiation exposure. For example, improved software incorporates iterative reconstruction, resolution recovery, and noise compensation to maintain or improve myocardial perfusion single-photon emission computed tomography (SPECT) image quality with conventional sodium iodide cameras. Temporal correlation among the gated perfusion frames and higher resolution SPECT acquisitions hold promise to further improve image quality and diagnostic accuracy. Novel collimator designs, such as multipinhole or locally focusing collimators arranged in geometries that are optimized for cardiac imaging, enhance photon-detection sensitivity. [20, 21, 22, 23, 24, 8]
Determining the Pretest Probability of Myocardial Ischemia
Nonanginal, atypical, and typical chest pain
The value of nuclear myocardial scanning is its predictive accuracy. Most notable is the fact that normal findings are predictive of a benign outcome: normal scan results are associated with an annual rate of severe cardiac events (myocardial infarction or cardiac death) of less than 1%. By contrast, the value of positive scan findings is dependent on the pretest probability of disease. Because the accuracy of any test that is not 100% sensitive and 100% accurate depends on pretest probability, determining this probability is important for increasing the test’s clinical value.
In their landmark CAD risk analysis article, Diamond and Forrester described the relationship between clinical symptoms and angiographically significant CAD. [25] The authors described 3 types of chest pain: nonanginal, atypical, and typical. The benefit of their categorization is the ease of its use and its powerful risk stratification. Disease is categorized on the basis of 3 symptoms, which are assessed with these questions: (1) Is the pain retrosternal? (2) Is the pain precipitated by stress? (3) Is the pain relieved by rest or nitroglycerin? Patients who answer yes to all 3 questions are determined to have typical chest pain. Patients who answer yes to 2 of the questions have atypical chest pain, and patients who answer yes to only 1 question have nonanginal chest pain.
Diamond and Forrester’s findings showed a large difference in the rates of angiographically significant CAD according to chest pain category. For example, a man in his 30s with nonanginal chest pain has a relatively low risk of CAD (approximately 5%); however, if the pain is typical, the risk is higher (70%) (see the image below).
 The risk of coronary artery disease (CAD) can quickly be stratified by determining whether the patient's pain is nonanginal, atypical, or typical. For men in their 30s with nonanginal chest pain, the pretest probability of disease is approximately 5%; however, men in their 30s with typical chest pain have a 70% likelihood of disease (a 14-fold increase).
The risk of coronary artery disease (CAD) can quickly be stratified by determining whether the patient's pain is nonanginal, atypical, or typical. For men in their 30s with nonanginal chest pain, the pretest probability of disease is approximately 5%; however, men in their 30s with typical chest pain have a 70% likelihood of disease (a 14-fold increase).
Although the absolute values are different for women, the same principle applies. Women with nonanginal chest pain have a lower risk of CAD than women with typical chest pain. For instance, a woman in her 50s with nonanginal chest pain has a relatively low risk of CAD (10%), whereas a woman in her 50s with anginal chest pain has a higher risk (80%) (see the image below).
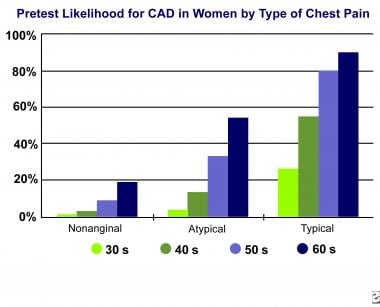 In women, the risk of CAD can quickly be stratified on the basis of the type of chest pain. For example, a woman in her 50s with nonanginal chest pain has a pretest probability of disease of approximately 10%, whereas a woman in her 50s with typical chest pain has a pretest likelihood of approximately 80%; this rate is an 8-fold increase.
In women, the risk of CAD can quickly be stratified on the basis of the type of chest pain. For example, a woman in her 50s with nonanginal chest pain has a pretest probability of disease of approximately 10%, whereas a woman in her 50s with typical chest pain has a pretest likelihood of approximately 80%; this rate is an 8-fold increase.
Risk stratification
This principle makes creating a rough risk stratification system easy and straightforward: in all patients with chest pain who undergo myocardial perfusion scanning, the pain should be categorized as nonanginal, atypical, or typical. The definitions of these categories must be rigorously followed. All too often, clinicians loosely use a description of atypical chest pain to mean a nebulous categorization of intermediate risk according to the clinician's gut feeling. This approach is unacceptable because these terms have been precisely defined and, when used correctly, carry significant meaning regarding the pretest likelihood of CAD. [19, 26, 27, 28, 29, 30, 31, 11]
Categorization of pretest CAD probability should include not only the patient's symptoms but also the clinical risk factors and the results of the stress test. Data from the Framingham Heart Study have enabled a more precise risk stratification according to age, cholesterol levels, blood pressure, presence of diabetes, and smoking history. [32] The value of exercise treadmill testing in risk stratification is optimized by using the Duke Treadmill Score. [33, 34] Several online risk calculators are available. An especially good site is maintained by the Stanford University Cardiology Department.
Using the clinical symptoms criteria developed by Diamond and Forrester for risk stratification can show that in patients with an intermediate-to-high pretest likelihood of disease, an exercise treadmill test alone is insufficient for excluding CAD. [25] As seen in the image below, of 1000 American men older than 60 years who have nonanginal chest pain, the exercise stress test causes clinicians to miss CAD in almost one third.
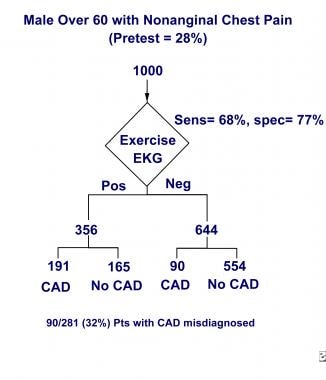 An exercise stress test is inadequate for excluding CAD when the pretest probability of disease is intermediate to high. Of 1000 American men older than 60 years who have nonanginal chest pain, the exercise stress test causes clinicians to miss almost one third of CAD cases.
An exercise stress test is inadequate for excluding CAD when the pretest probability of disease is intermediate to high. Of 1000 American men older than 60 years who have nonanginal chest pain, the exercise stress test causes clinicians to miss almost one third of CAD cases.
Adding nuclear cardiac imaging to the stress electrocardiography (ECG) greatly increases the clinical value of the test; as seen in the image below, of 1000 American men older than 60 years who have nonanginal chest pain, CAD is misdiagnosed with nuclear scanning in only 25 of the 281 patients with CAD. Additionally, of these 25 patients misidentified as not having CAD, the annual rate of myocardial infarction or cardiac death is still less than 1% per year.
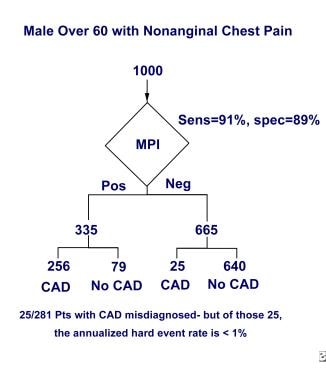 Adding nuclear cardiac imaging to the stress ECG test greatly increases the clinical value of the test. Of 1000 American men older than 60 years who have nonanginal chest pain, CAD is misdiagnosed in 25 of the 281 men who have the disease; however, of these 25 patients, the annual rate of severe cardiac events (myocardial infarction or cardiac death) is still less than 1% per year.
Adding nuclear cardiac imaging to the stress ECG test greatly increases the clinical value of the test. Of 1000 American men older than 60 years who have nonanginal chest pain, CAD is misdiagnosed in 25 of the 281 men who have the disease; however, of these 25 patients, the annual rate of severe cardiac events (myocardial infarction or cardiac death) is still less than 1% per year.
Because determining the pretest likelihood of disease greatly affects the final risk categorization, nuclear myocardial scanning reports are most useful when they include notes regarding the type of pain the patient is experiencing and the results from the stress test and clinical risk factors. Usually included are the patient's type of chest pain, whether ECG and/or clinical evidence of ischemia was noted on the stress test, and a list of the important risk factors. Including these notes as part of the nuclear myocardial scanning report takes little time and makes the report more useful to the clinician who ordered the test.
Indications for Gated Myocardial Perfusion Single-Photon Emission CT
The indications for gated myocardial perfusion single-photon emission computed tomography (SPECT) are based on its prognostic value, cost, and feasibility in virtually all patients. The prognostic value is exceptional. Although gated myocardial perfusion SPECT is more expensive than stress echocardiography, it has a better negative predictive value. This advantage makes the overall value of the tests comparable because fewer additional tests are required. The cost is also significantly less than the cost of angiography. [35, 27, 36, 37, 10, 11, 12, 13, 17, 38]
A meta-analysis of 34 studies with a combined 25,574 individuals reported an annual cardiac event rate of 1.40 and a population event risk of 2.72 following a negative SPECT result. This study provides further evidence that negative results convey an excellent prognosis and support the decision to forgo further testing. [39]
Another important advantage of nuclear scanning is that it can be performed in all patients. Many patients are excluded from treadmill testing because of osteoarthritis, poor conditioning, and amputation (among other reasons). Stress echocardiography frequently provides poor results because of the inability to obtain a good window in patients with large chest walls.
Scanning is performed for 3 reasons: (1) to aid in the diagnosis of CAD, (2) to stratify the risk in patients with known CAD, and (3) to evaluate the patient's response to therapy for CAD.
To diagnose CAD, the first step is to establish the pretest probability of disease based on patient history, physical examination findings, and laboratory data. Using this information, clinicians can generally place patients into the low-risk, intermediate-risk, or high-risk categories. Some patients with a low likelihood of disease do not need further testing; instead, only counseling on how to reduce their specific risk factors is necessary. In patients with a low-to-intermediate pretest likelihood of disease, the next step is exercise treadmill testing. If the exercise treadmill test result is negative, the patient can be safely treated by medical means, and no further testing is necessary. Patients with a positive result from the exercise stress test are good candidates for nuclear scanning, as are patients with an intermediate-to-high risk of CAD.
Stress myocardial perfusion imaging also can play an important role in the evaluation of patients presenting with acute chest pain. After an initial evaluation and diagnostic workup in the emergency department is negative, myocardial perfusion imaging performed within 48 hours of discharge appears to be effective and safe in confirming or ruling out the diagnosis of coronary artery disease. [40]
In patients with known CAD, nuclear scanning is helpful in risk stratification. With decades of clinical experience, nuclear scanning has consistently offered high clinical and economic value. Perhaps the most important finding in nuclear scanning is a negative one, with no perfusion defects or wall-motion abnormalities. Regardless of findings during catheterization, patients with a negative scan have an extremely low risk of myocardial infarction or cardiac death. [39] Patients from widely different backgrounds in whom scan findings are normal have consistently been shown to have an excellent prognosis. This observation applies whether they are male or female and old or young, whether they have positive or negative treadmill test results, and whether they have anginal or nonanginal chest pain.
In addition, nuclear scanning is helpful for follow-up observation after a coronary artery intervention. Obtaining a scan approximately 4 months after angioplasty, stenting, or bypass surgery helps determine the success of the intervention (eg, if the native artery or bypass graft remains patent). Nuclear scanning can also help determine whether a patient's chest pain results from the sternotomy or from recurrent myocardial ischemia. Because single-vessel CAD is often treated medically, nuclear scanning can also be used to ascertain whether medical treatment is reducing the ischemia.
Although myocardial perfusion SPECT imaging has several other indications, in almost all patients, detecting myocardial ischemia is the critical issue. For example, a nuclear scan after a myocardial infarction can demonstrate whether viable myocardium remains in the affected area. This information can help predict whether bypass surgery, angioplasty, or stenting will be of benefit. A nuclear scan obtained preoperatively (especially before major vascular surgery) also aids in determining risk, primarily based on the presence or absence of myocardial ischemia. Newer metabolic imaging procedures involving positron emission tomography (PET) can help determine whether the region of the heart affected by a myocardial infarction remains viable. [41] Newer fatty acid imaging serves a similar role.
Myocardial Perfusion Imaging
Procedure
The procedure for nuclear myocardial scanning almost always involves a stress test, a resting scan, and a poststress scan. Patient preparation is the first step of the procedure; preparation includes ensuring that the patient has not had any caffeine for at least 12 hours prior to imaging, in case a dipyridamole stress test is necessary. Usually, fasting for 4 hours before imaging is recommended. [20, 34, 37, 39, 6, 42]
If the purpose of the test is for diagnosing possible CAD, all medications should be withheld before the test. [43] On the other hand, if the purpose of the test is to assess response to therapy, most patients should continue all cardiac medications as usual. Patients with diabetes who require insulin should be monitored on a case-by-case basis. Before the stress test, assessment of the patient's cardiovascular history and physical examination should be performed; baseline vital signs should be evaluated, and a 12-lead ECG should be performed.
In addition to careful history-taking and physical examination, all patients undergoing stress testing must provide written informed consent for the procedure, because it is associated with a complication rate of 1-5 cardiac events per 10,000 patients tested. Approximately 4 out of 10,000 patients tested have a myocardial infarction, and 1 out of 10,000 patients tested die of cardiac causes during the test. Therefore, emergency cardiac equipment and medications must be immediately available.
Complication rates may be slightly less when the patient is undergoing pharmacologic stress instead of exercise stress. Complication rates also appear to be declining slowly as advancements are made in the treatment of acute myocardial infarction. Nonetheless, the risk is definite and real. Although greater regulatory emphasis may be placed on the handling of radionuclides, the risk of the procedure comes from stress testing, not radioactivity.
Several imaging procedures are available. A common protocol is the same-day single-radiopharmaceutical protocol performed by using 8 mCi (370 MBq) of technetium-99m (99mTc) sestamibi for the rest study. This step is followed by the stress test, during which the patient receives an injection of 32 mCi of 99mTc sestamibi at peak exertion. The poststress study is performed by using ECG gating. SPECT imaging is performed for both studies by using a 180° collection. A 64 × 64 matrix with 32 stops is used. The Society of Nuclear Medicine Procedure Guidelines list the procedural protocols in detail.
Radiopharmaceuticals
Radiopharmaceuticals in use include thallium-201, palmitate, and rubidium-82. Iodinated fatty acids based on iodine-123 include iodohexadecanoic acid and iodophenylpentadecanoic acid. These are newer agents used to image myocardial energy metabolism. [25, 29, 44, 12, 13, 14] . Technetium-99m-sestamibi and Tc-99m-tetrofosmin are commercial radiopharmaceuticals for MPI by SPECT. Basic pathophysiology of ischemic heart disease has been improved by the application of F-18 fluorodeoxyglucose (FDG) and F-18 sodium fluoride (NaF). [8]
Image evaluation
The overall quality of the study must be initially assessed by looking for artifacts or other sources of error. Reviewing raw cine data at the computer monitor is important to assess for patient motion, breast or diaphragmatic attenuation, and other sources of artifacts (eg, gating abnormalities), as well as to evaluate pulmonary and noncardiac uptake. These raw data are reviewed for every patient study.
Reconstructed perfusion images are then evaluated. The images are displayed in a standardized format as recommended by the ASNC. On top are the short-axis poststress images. The next line has the short-axis rest images. The next 2 lines display the vertical long-axis stress/rest images, and the final 2 lines contain the horizontal stress/rest images. Again, the study is interpreted by viewing the images as displayed on the computer monitor, which allows for manipulation of the images and greater resolution. Images are usually reviewed in both a continuous color format, such as gray scale or hot body, and in a color scale, such as spectrum or hot metal.
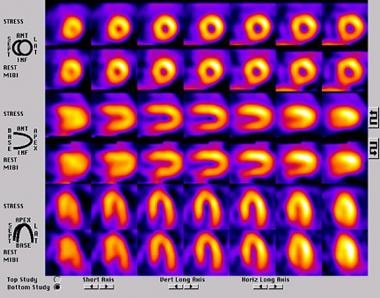
Generally, the author views all studies in 3 formats (ie, gray scale, spectrum, and hot metal). Viewing the images in a continuous color format is important because several of the other color scales can artificially create image defects. If a defect is present on the images when viewed with a continuous color format, the other color scales can help visually quantify the significance of the defects. Normal findings on perfusion studies result in almost identical stress/rest images with no defects (see the image below), whereas in patients with infarcted myocardium, the findings demonstrate fixed defects.
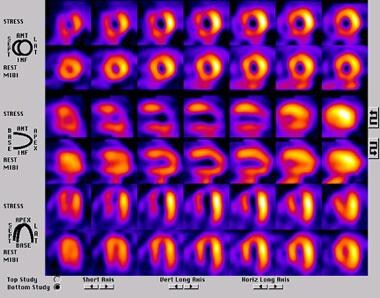
Findings in patients with myocardial ischemia demonstrate reversible defects (ie, defects on the stress images without a similar defect on the rest images) (see the image below).
Gated SPECT
Procedure
As previously noted, gated images are obtained after the stress test is performed. With the use of a single-headed camera, a 180° acquisition is usually obtained with 32 stops. ECG gating is used, even when the patient has a cardiac arrhythmia, such as atrial fibrillation. After image acquisition, processing generates cine images that show wall motion and wall thickening. In addition, ventricular volumes and polar maps are generated. [27, 28, 29, 30, 36, 45, 10, 17]
Image evaluation
Myocardial wall motion is assessed at the computer monitor. Although the computer calculates an ejection fraction, visually verifying whether the calculated value is reasonable is essential. Occasionally, the calculated ejection fraction value is affected by overlapping liver activity or poor contour tracking, which can make computer-generated values inaccurate. Usually, wall motion is assessed by viewing the inner myocardial wall. A mesh grid is also displayed, which statically shows the end-diastolic image of the myocardial wall. This helps determine wall motion as normal, hypokinetic, akinetic, or dyskinetic (see the video below). Wall motion must be viewed from at least 2 angles to visualize all surfaces. Views from the anterior and lateral angles are common.
In addition to viewing the wall motion from a 3-dimensional perspective, wall thickening can be visualized by viewing the gated short, vertical long, and horizontal long axes. Polar maps can be helpful in quantifying abnormalities in perfusion, regional ejection fraction, motion, and wall thickening (see the image below); however, abnormalities shown on polar maps must be verified visually.
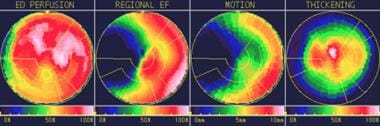 Polar maps can help identify abnormal findings on nuclear cardiac images. The polar map shows fairly good end-diastolic perfusion, a decreased regional ejection fraction and motion by the septum, and fairly normal thickening.
Polar maps can help identify abnormal findings on nuclear cardiac images. The polar map shows fairly good end-diastolic perfusion, a decreased regional ejection fraction and motion by the septum, and fairly normal thickening.
On nuclear myocardial scans, a left ventricular ejection fraction greater than 50% is normal. End-systolic volumes greater than 70 mL are considered abnormal, as are end-diastolic volumes greater than 120 mL.
Reporting Guidelines
Some variation exists in reporting guidelines for nuclear myocardial scans; however, several common principles are applied. First, pertinent patient history is included in the report. Scanning findings address 3 main sets of images: the raw data, the reconstructed sections, and the gated images. The conclusion should address the presence or absence of reversible defects (suggesting myocardial ischemia) [46] and fixed defects (suggesting myocardial infarction), as well as left ventricular function. Finally, the specific question asked by the clinician must be addressed in the report.
For example, if the clinician orders the test to assist in the diagnosis of CAD, the report should include the note "low risk of CAD," "high risk of CAD," or a similar comment. If the clinician orders the test to determine whether a stent is patent, the report should state whether the scan suggests that the stent is patent or occluded.
A general guideline to reporting nuclear myocardial scans follows; it is based mostly on the approach taken by Germano and Berman and the guidelines established by the Society of Nuclear Medicine. [30] The general data are as follows:
-
Scan data include the type of scan, examination date, and location of the procedure.
-
Patient identification includes the patient's name, date of birth, and age.
-
The patient's history includes symptoms (if chest pain is reported, specify the type of chest pain according to criteria by Diamond and Forrester), relevant history (including the results of previous scans or catheterization reports), medications (state if the medications were withheld for the test or not), CAD risk factors, and the resting ECG; also included is information about the indication for the study and the clinical question asked by the ordering clinician.
-
The report of technique includes the protocol, the radiopharmaceutical used, and the doses.
-
Results of the stress test include the type of stress test used, the clinical response, the ECG response, and the blood pressure response; treadmill stress test results also include the peak heart rate achieved, the associated percentage of the maximum predicted heart rate, and the duration of the exercise.
-
Raw data include an evaluation of the quality of the study, degree of artifacts, pulmonary uptake, and any abnormal noncardiac uptake.
-
Section reports include reconstructed data showing the short, vertical long, and horizontal long axes; if the data show abnormalities, ventricular sizes, uptake by the right ventricle, and the presence or absence of transient ischemic dilation should be noted; be sure to include some degree of quantification (for example, defects may involve the basal inferior wall or may be larger, running from the apex to the base. Stating simply that "an inferior wall defect is present" does not provide the clinician with information concerning how much myocardium is involved; similarly, the severity of the defect and the amount, if any, of redistribution should be noted. Berman recommends using the summed stress score and a summed difference score to help standardize readings between different laboratories. [3] This scale is automated on many nuclear medicine systems, but it can also be calculated visually.).
-
Gated images include the left ventricular ejection fraction. If this fraction is not calculated automatically, estimate the ejection fraction. End-systolic volume should also be included. Provide a qualitative interpretation of wall motion and thickening.
-
Conclusions should include the following: (1) Note whether any reversible defects are present. If they are, describe the extent and severity of the defect and how much redistribution occurs. (2) Note whether any fixed defects are present. Comment on the size and severity of the defects. (3) Answer the clinical question asked by the ordering clinician (for example, if the patient was referred for imaging to assist in the possible diagnosis of CAD, directly address the posttest likelihood of CAD; if the patient was referred for follow-up imaging after stent placement, state whether the scanning results suggest patency or occlusion. Directly answering the clinical question is essential to maximize the value of the study.).
-
Other comments can address any unusual features of the scan, such as a focus of abnormal pulmonary uptake.
Quality Control
Quality control begins with proper equipment selection. Although single-head cameras are used widely and are probably the most popular system, dual-head cameras separated by 90° are gaining popularity. A circular or elliptical orbit may be used. Step-and-shoot acquisition is the most common method of imaging, although continuous acquisition cameras may provide greater sensitivity.
A procedure manual containing a step-by-step procedure for each examination should be on hand. The manual also must contain quality-control procedures for all instruments and radiation safety measures. The procedures manual should be updated and reviewed regularly.
Daily quality-control procedures for most cameras include energy peaking and a low-count intrinsic flood (3-10 million counts). An intrinsic resolution is performed weekly. An extrinsic high-count flood (30 million counts) and center-of-rotation analysis are performed at least monthly. Weekly center-of-rotation analysis is required on many cameras. These quality-control measures are only rough guidelines. Following the manufacturer's recommendations for each specific camera is important.
One must look for several sources of error on each patient study. Interstitial infiltration of the radiopharmaceutical can alter uptake and clearance kinetics. Patient motion artifacts and diaphragmatic creep can create artificial defects. Frequently, minor motion artifacts can be corrected by reprocessing the data.
Suboptimal stress decreases the sensitivity of the test; therefore, in the final report, stating the actual heart rate achieved as a percentage of the age-predicted and gender-predicted maximum heart rate is important. Study processing needs to be standardized.
Raw data need to be reviewed in a rotational cinematic format prior to the examination of reconstructed tomographic sections. The purpose of this is to identify attenuation artifacts (eg, breast shadows) or overlapping artifacts (eg, liver or bowel activity), motion, abnormal noncardiac uptake, increased lung uptake, or anything else unusual.
Summary and Conclusion
Myocardial ischemia is a major cause of morbidity and mortality worldwide. Because the condition is often treatable, the early diagnosis of CAD is of great importance.
To determine the proper workup for patients with chest pain or other signs indicative of myocardial ischemia, an understanding of risk stratification is necessary. A rapid way to stratify patients with chest pain is to classify their pain as nonanginal, atypical, or typical on the basis of the Diamond and Forrester criteria. Patients with a low risk of myocardial ischemia may simply be treated with medical therapies or an exercise treadmill test. Patients with an intermediate-to-high risk need an imaging study in addition to the stress test. Nuclear myocardial scanning is usually the preferred imaging study because of its proven and powerful risk-stratification capability. In patients with normal findings on nuclear myocardial scans, the risk of myocardial infarction or cardiac death is less than 1% per year for as long as 5 years.
Indications for nuclear myocardial scanning include diagnosing CAD, risk stratification, and the evaluation of response to therapy. Patients unable to perform an exercise stress test because of medical conditions, such as severe osteoarthritis, are also excellent candidates for scanning.
Myocardial perfusion imaging has been standardized worldwide, and established protocols must be followed rigorously. Protocols are readily available online from the Society of Nuclear Medicine and the ASNC. [4, 45, 6, 47] SPECT and gated imaging should always be included whenever feasible.
Quality control is essential in nuclear scanning because of the highly technical nature of the studies. This ranges from proper camera selection to standardized report generation. Daily, weekly, and monthly quality control must be performed on the camera, as recommended by the manufacturer. Viewing raw cinematic data and reconstructed sections on the computer monitor by the interpreting clinician is essential, as is customizing the report to answer the specific question asked by the referring clinician.
New technologies, protocols, and quantifying methods have significantly improved the diagnostic performance and prognostic value of nuclear cardiology imaging. Automation, artificial intelligence, and machine learning will increase performance even further. [8] Machine learning algorithms can complement nuclear cardiology analyses packages and reporting software, with data being derived to calculate risk estimates factored in decision support tools (DSTs). [6, 48]
Questions & Answers
Overview
What is the role of a nuclear myocardial scan in the workup of myocardial ischemia?
How is the probability of myocardial ischemia determined prior to a nuclear myocardial scan?
How is myocardial ischemia risk stratified prior to nuclear myocardial scan?
How is nuclear myocardial scanning performed in the workup of myocardial ischemia?
What is the role of radiopharmaceuticals in the imaging of myocardial ischemia?
Which findings on myocardial perfusion imaging are characteristic of myocardial ischemia?
What are the guidelines for reporting nuclear myocardial scan findings?
Which quality control procedures are used in the nuclear imaging for myocardial ischemia?
What is included in the initial evaluation of myocardial ischemia?
-
The risk of coronary artery disease (CAD) can quickly be stratified by determining whether the patient's pain is nonanginal, atypical, or typical. For men in their 30s with nonanginal chest pain, the pretest probability of disease is approximately 5%; however, men in their 30s with typical chest pain have a 70% likelihood of disease (a 14-fold increase).
-
In women, the risk of CAD can quickly be stratified on the basis of the type of chest pain. For example, a woman in her 50s with nonanginal chest pain has a pretest probability of disease of approximately 10%, whereas a woman in her 50s with typical chest pain has a pretest likelihood of approximately 80%; this rate is an 8-fold increase.
-
An exercise stress test is inadequate for excluding CAD when the pretest probability of disease is intermediate to high. Of 1000 American men older than 60 years who have nonanginal chest pain, the exercise stress test causes clinicians to miss almost one third of CAD cases.
-
Adding nuclear cardiac imaging to the stress ECG test greatly increases the clinical value of the test. Of 1000 American men older than 60 years who have nonanginal chest pain, CAD is misdiagnosed in 25 of the 281 men who have the disease; however, of these 25 patients, the annual rate of severe cardiac events (myocardial infarction or cardiac death) is still less than 1% per year.
-
Normal perfusion on a single-photon emission computed tomography (SPECT) perfusion study.
-
A SPECT perfusion study in a patient with a large degree of tracer redistribution affecting the left anterior descending and right coronary artery territories.
-
This study in a patient with normal findings on myocardial perfusion scanning represents the value of gated imaging in nuclear cardiac scans. The gated images show akinesis of the distal anterior wall along with a reduced left ventricular ejection fraction and an elevated end-systolic volume.
-
Polar maps can help identify abnormal findings on nuclear cardiac images. The polar map shows fairly good end-diastolic perfusion, a decreased regional ejection fraction and motion by the septum, and fairly normal thickening.
Tables
What would you like to print?
- Practice Essentials
- Determining the Pretest Probability of Myocardial Ischemia
- Indications for Gated Myocardial Perfusion Single-Photon Emission CT
- Myocardial Perfusion Imaging
- Gated SPECT
- Reporting Guidelines
- Quality Control
- Summary and Conclusion
- Questions & Answers
- Show All
- Media Gallery
- References