Practice Essentials
Uterine leiomyomas, better known as uterine fibroids, are benign smooth muscle tumors of the uterus.The most common tumor found in the female reproductive system, uterine fibroids are seen in 50-60% of women (rising to 70% by 50 years of age) and, in 30% of cases, cause morbidity due to abnormal uterine bleeding and pelvic pressure. African-American women have a greater chance of being affected by uterine fibroids, particularly at an earlier age than either white or Asian women. [1] Uterine fibroids can occur at any time between menarche and menopause but are most common in women 35-49 years of age. They typically resolve after menopause.
Before performing uterine fibroid embolization (UFE), it is important to document the presence of fibroids, usually either by pelvic ultrasound or by MRI. MRI is preferred because of its greater ability to demonstrate individual fibroids and denote their size, location, and number within the uterus. [1, 2, 3, 4, 5] It has been estimated that 13,000-14,000 UFE procedures are performed annually in the United States. [6]
In light of the success of embolization in reducing solid tumors and diminishing associated symptoms, uterine fibroid embolization (UFE) is performed to reduce uterine as well as fibroid volume and their associated symptoms. [7, 8, 9, 10, 11]
(Scans of uterine fibroids are shown in the images below.)
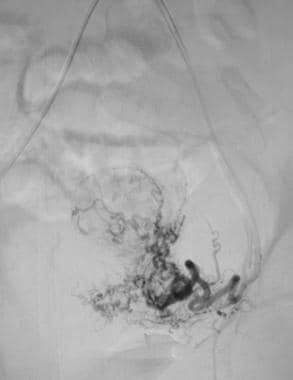
 Nonselective pelvic angiogram demonstrating the tortuous and dilated uterine arteries supplying a hypervascular uterine fibroid.
Nonselective pelvic angiogram demonstrating the tortuous and dilated uterine arteries supplying a hypervascular uterine fibroid.
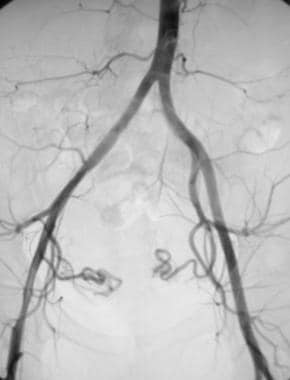
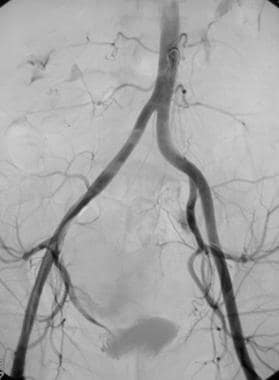 Nonselective angiogram of the pelvis after embolization demonstrating occlusion of both the right and left uterine arteries.
Nonselective angiogram of the pelvis after embolization demonstrating occlusion of both the right and left uterine arteries.
Uterine fibroids can be classified by their location, as follows:
-
Submucosal fibroids are the least common; because they are in the submucosa and near the endometrial cavity, they are associated with heavy and prolonged menstrual periods and an increased miscarriage rate; submucosal fibroids may be pedunculated and may prolapse into the cervix.
-
Intramural fibroids grow within the uterine wall; their growth may be associated with mass-related symptoms such as abdominal distention due to mass or urinary frequency due to bladder compression.
-
Subserosal fibroids develop in the outer portion of the uterus; they may be pedunculated, can potentially grow into the abdomen or in the ligaments of the uterus, and are associated with bladder compression and abdominal distention.
Most patients are asymptomatic. Fibroids typically are discovered incidentally during routine gynecologic examination. Presenting symptoms most commonly include the following:
-
Abnormal uterine bleeding
-
Pelvic pain, possibly resulting from intramural degeneration, torsion of a pedunculated fibroid, or uterine contractions
-
Pelvic pressure
-
Abdominal distention
-
Genitourinary dysfunction, which may manifest as the following: increased urinary frequency resulting from bladder compression, and flank pain resulting from ureteral compression and hydronephrosis
-
Infertility
-
Rarely, lower extremity edema, constipation, or intestinal obstruction
Management of uterine fibroids should be tailored to the size and location of fibroids; the patient's age, symptoms, desire to preserve fertility, and access to therapy; and the physician's experience. [1, 2, 3, 4, 5]
For patient education information, see the Women's Health Center, as well as Fibroids and Amenorrhea.
Preprocedure Evaluation
Imaging
Prior to embolization, it is important to document the presence of fibroids. Viable pregnancy and active pelvic inflammatory disease are two absolute contraindications for the procedure and must be excluded. This can be accomplished using either cross-sectional imaging, preferably MRI, or ultrasound (US). [12] While US may be effective for confirming enlargement of the uterus and demonstrating the presence of fibroids or other pathology that can explain the patient's presenting symptoms, its efficacy depends on the equipment used and the body habitus of the patient. MRI is preferred because of its greater ability to demonstrate individual fibroids and denote their size, location, and number within the uterus. MRI also allows easier determination of the internal characteristics of individual fibroids, as well as their vascularity. [2, 3, 4, 13, 14, 15, 16, 17]
Pelvic MRI can also determine whether coexisting pathology is present within the uterus. For example, menorrhagia and other symptoms commonly associated with uterine fibroids may be similar to those of patients with adenomyosis, a benign uterine disease. Adenomyosis is characterized by the ectopic growth of endometrial glands and stroma into the myometrium and by diffuse enlargement of the uterus. On MRI, a focal adenomyoma may appear as a localized low-signal myometrial mass with poorly defined margins that often contain high-signal foci. Long-term data covering a 10-year period shows successful short- and long-term improvements in symptoms of adenomyosis with uterine artery embolization (UAE) treatment. Improvements have been noted in 75% (387 of 511) of patients. [7]
MRI is also helpful in assessing the results of embolization several months after the procedure. MRI after UAE is recommended by the American College of Radiology (ACR) not only to ensure adequate fibroid infarction but also to exclude underlying leiomyosarcoma. [12]
Technically, the radiologist should obtain measurements of the uterus and of each fibroid in 3 planes, then calculate the volume measurements using the formula for the volume of a sphere (length × width × height × 0.5233).
These measurements provide a baseline for determining the degree of postprocedural reduction in fibroid and uterine volume. After embolization, the signal intensity of the fibroids usually decreases on both T1-weighted and T2-weighted images. Embolization seems to have little effect on the volume or enhancement characteristics of normal myometrium and endometrium.
Other testing
In addition to pelvic MRI, the pre-embolization workup includes evaluation of the patient's renal function (ie, blood urea nitrogen and creatinine levels) and coagulation profile (ie, platelet count, prothrombin time, partial thromboplastin time). All prospective patients should undergo a full gynecologic workup, including a Pap smear every 3 years and/or an endometrial biopsy if a patient has menometrorhagia. [12]
Other preprocedure care
Severe anemia should be corrected and an intrauterine device should be removed before the procedure, as it could act as a source of infection. If the patient is taking a gonadotropin-releasing hormone (GnRH) agonist, there is some debate as to whether it should be stopped for several weeks. because it may make the procedure more difficult due to constriction of the arteries supplying the uterus. Although the evidence to support this is anecdotal, GnRH administration is usually ceased if possible. The newer selective progesterone receptor modulators (eg, ulipristal acetate) may be a better option to control bleeding before treatment. [18]
Technique
Prior to embolization, an intravenous (IV) line and Foley catheter are placed in all patients (see the images below). Prophylactic antibiotics usually are given before the procedure. Other premedications that may be used include corticosteroids, antiemetics, and analgesics. The procedure is carried out under conscious sedation. For an experienced interventional radiologist, UAE is a relatively simple, straightforward procedure usually completed in less than 1 hour. [18]
 Nonselective pelvic angiogram demonstrating the tortuous and dilated uterine arteries supplying a hypervascular uterine fibroid.
Nonselective pelvic angiogram demonstrating the tortuous and dilated uterine arteries supplying a hypervascular uterine fibroid.
 Nonselective angiogram of the pelvis after embolization demonstrating occlusion of both the right and left uterine arteries.
Nonselective angiogram of the pelvis after embolization demonstrating occlusion of both the right and left uterine arteries.
Uterine fibroid embolization (UFE) typically is performed as follows:
A sheath is placed into either the right or left common femoral artery.
Under fluoroscopic guidance, a flush catheter is advanced into the abdominal aorta.
A pelvic angiogram is performed to identify the right and left uterine arteries; ovarian, lumbar, or other collateral parasitic supplies to a large myomatous uterus may be seen; the uterine arteries, which typically arise from the anterior division of the internal iliac artery, are responsible for most of the uterine blood supply; a selective arteriogram of the uterine artery reveals the terminal branches, known as the spiral arteries, which dilate and uncoil in response to changes in hormonal levels and endometrial thickness; in the presence of fibroids, arteriography of the uterine arteries shows these vessels as dilated, tortuous, and laterally displaced; one can confirm the hypervascular nature of the fibroid by noting the extensive network of intrinsic arteries within the fibroid.
Once identified, the uterine arteries should be selectively catheterized.
An embolic agent, such as polyvinyl alcohol (PVA) or Embospheres, is injected directly into the uterine arteries to induce thrombosis; both uterine arteries should be embolized because the spiral arteries arising from them anastomose across the midline; unilateral embolization caused one of the earliest reported cases of a UFE treatment failure; in some patients, a 4 Fr or 5 Fr catheter may be occlusive within one or both uterine arteries; if this occurs, a coaxial microcatheter may be used to administer the embolic agent; if a microcatheter is used, some difficulty may be encountered if 500-710-micron PVA is used as the embolic agent; diluting the agent appropriately along with frequent catheter flushing may minimize the risk of catheter occlusion in this setting.
After embolization of both uterine arteries, a complete angiogram should be performed to confirm that arterial occlusion has occurred; it will also allow a search for collateral vessels (eg, ovarian arteries, vaginal arteries) that may represent a significant source of collateral blood supply to the fibroids
Embolic agents
There are several embolic products that can be used for this procedure. Polyvinyl alcohol (PVA) is the most commonly used embolic agent for UFE. The biocompatibility of PVA was established before its first medical use in 1949 as a filling material following pneumonectomy. [19] Newer agents include Embospheres and Embozene. [18] Some interventional radiologists have advocated for the use of Gelfoam, a temporary embolic agent frequently used for postpartum hemorrhage control. [20, 37]
The desired level of occlusion (ie, proximal, distal) determines the particle size selection for each embolization procedure. Generally, using small particles results in a more distal occlusion, increasing the risk for end-organ infarction. However, the tendency of PVA particles to clump can make the effective size larger than that of individual particles, which may account for the proximal occlusion often seen during PVA embolization. Most centers use particles measuring 350-500 or 500-710 micrometers in diameter.
Histologically, PVA particles adhere to the vessel wall, causing slow flow within that vessel. The result is intraluminal thrombus formation, inflammatory reaction, foreign-body reaction, and focal angionecrosis of the vessel wall. The foreign-body reaction induced by PVA is reported to persist up to 28 months after embolization.
PVA is not biodegradable and thus is considered by many to be a "permanent" embolic agent; however, the reported duration of PVA-induced vascular occlusion has varied. Persistent occlusion will occur with organization of the thrombus, disappearance of the inflammatory infiltrate, and ingrowth of connective tissue into the particles, resulting in fibrosis. Luminal recanalization has been reported and may be caused by angioneogenesis and capillary regrowth either because of vascular proliferation inside the organized thrombus or because of resorption of the thrombus found between clumps of PVA.
Recovery and Follow-up
Uterine fibroid embolization (UFE) postembolization syndrome is similar to solid tumor postembolization syndrome. It affects most patients undergoing UFE and lasts approximately 2-7 days. Signs and symptoms may include the following:
-
Pelvic pain and cramping
-
Nausea and vomiting
-
Low-grade fever
-
General malaise
Most patients experience these features to some degree; however, the variation in severity has prompted the development of different strategies to increase patient comfort during recovery. Regimens involve oral, IV, epidural, and/or patient-controlled analgesia in both an inpatient and outpatient setting. Reports suggest that most centers admit patients for 1-2 days to aggressively manage these constitutional symptoms with the patient discharged on oral analgesia. [18]
At the author's institution, the outpatient protocol includes the following medication regimen:
-
Opioid analgesics (eg, oxycodone [OxyContin], acetaminophen and oxycodone [Percocet], meperidine, hydrocodone with acetaminophen): Currently, the authors administer OxyContin at 10-20 mg orally every 12 hours, with 1-2 tabs of Percocet 5/235 orally every 6 hours for pain not relieved with OxyContin.
-
Nonsteroidal anti-inflammatory medications (eg, ketorolac, celecoxib [Celebrex], ibuprofen): Currently, the authors administer celecoxib at 100 mg orally every 12 hours for the first 7 days after embolization, followed by ibuprofen at 200 mg every 4-6 hours, as needed.
-
Antinausea medications (eg, prochlorperazine): Supply the patient with 25-mg suppositories for relief of nausea associated with embolization and the medications provided above for pain relief.
Patients are offered the opportunity to recover at home following the embolization procedure. In the author's experience, most patients are best managed at home when the following conditions are met:
-
Patients are provided with the medication regimen detailed above
-
Patients are ambulated as soon as possible (to prevent potential pulmonary embolus)
-
Patients are given adequate education
-
Patients receive diligent follow-up care
-
The care provider is readily available to the patient
-
Family or friends can provide home support
Once discharged, recommended patient activity is as follows:
-
On the day of the procedure, the patient should observe bedrest with only limited activity
-
The next day, the patient may engage in limited activity around her home
-
After 2 days, normal activity is permitted; at this point, individual tolerance for activity and reliance on pain medication are the best indicators of the patient's capabilities
-
After 6-10 days, most of the author's patients are able to return to work
The patient should receive follow-up imaging (MRI preferred) at 4-6 weeks post procedure and again at 6 months. Expected findings are of completely infarcted fibroids with a typical uterine volume reduction of 50–70%. [18]
Outcomes
Goodwin et al were the first to publish experience with UFE in the United States. They subsequently conducted a prospective, multicenter study of the short- and long-term outcomes of UFE, the Fibroid Registry for Outcomes Data (FIBROID) for Uterine Embolization. In the FIBROID registry, mean symptom scores in 1,278 patients showed normalization of health-related quality-of-life; 9.79% of patients subsequently underwent hysterectomy, 2.82% underwent myomectomy, and 1.83% underwent repeat UFE. [21]
Compared with hysterectomy and myomectomy, uterine artery embolization has a significantly decreased length of hospitalization (mean of 3 fewer days), decreased time to normal activities (mean of 14 days), and a decreased likelihood of blood transfusion (OR = 0.07; 95% CI, 0.01 to 0.52). [22] Long-term studies show a reoperation rate of 20 to 33% within 18 months to 5 years. [5]
The Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids conducted a randomized trial comparing UFE in 106 patients with surgery (hysterectomy or myomectomy) in 51 patients. At 1-year follow-up, quality of life was not significantly different between the groups. The UFE patients had experienced more rapid recovery (shorter hospital stays and faster return to regular activities), but 9% required repeat UFE or hysterectomy because of inadequate symptom control. [8]
Although UAE is highly effective for treating symptoms (reduction in bleeding and fibroid size), the risk of reoperation is 15-20% after successful embolization and up to 50% in cases of incomplete infarction. [1]
In the United Kingdom, the multicenter retrospective HOPEFUL study compared the outcome of treatment for symptomatic fibroids in 459 women who underwent hysterectomy and 649 who had UFE. Average follow-up was 8.6 years for the hysterectomy cohort and 4.6 years for the UFE cohort. In this study, 85% of UFE patients reported relief from fibroid symptoms, versus 95% of hysterectomy patients, and 23% of UFE patients required further treatment for fibroids. [23, 24]
The Embolisation versus Hysterectomy (EMMY) trial, a prospective randomized comparison in 177 women, found that UFE is a good alternative to hysterectomy; both procedures led to improved health-related quality of life at 24-month follow-up. [25]
Gabriel-Cox et al studied 5-year outcomes of UFE in 562 women; overall, the rate of subsequent hysterectomy was 19.7%. However, the rate in women who had had unilateral UFE was 39.2%; this was the only identified factor that predicted subsequent hysterectomy. [26]
A systematic review and meta-analysis of 4 randomized, controlled trials with 515 patients reported that the short-term advantages of UFE over surgery include less blood loss, shorter hospital stay, and quicker resumption of work. [9] The mid- and long-term advantages are similar, although there is a higher reintervention rate after UFE.
Treatment failure can be defined as the absence of demonstrable clinical benefit after successful embolization of both right and left uterine arteries. Possible explanations for treatment failure include the following:
-
Incomplete embolization
-
Luminal recanalization
-
Extremely large uterine fibroids
-
Presence of uterine leiomyosarcoma
-
Presence of a coexisting disorder (eg, adenomyosis)
-
Persistence of collateral sources of arterial blood supply to fibroids (eg, ovarian artery, round ligament artery)
Potential Complications
Immediate complications
Immediate complications include complications related to iodinated contrast material administration; femoral arterial catheterization; and postembolization syndrome.
There are also complications related to nontarget or inadvertent embolization of vessels other than the uterine artery. Branches of the anterior division of the internal iliac artery are at risk for nontarget embolization during UFE. In particular, inadvertent embolization of the vesical artery, which sometimes arises from a common trunk with the uterine artery, may increase the risk of bladder necrosis.
It may be possible to prevent nontarget embolization by performing the following:
-
Ensure a thorough knowledge of pelvic arterial anatomy and potential variants
-
Proceed carefully, using an angiographic technique that includes the following: position the catheter into the descending segment of the uterine artery and confirm this position with an arteriogram prior to embolization; inject embolic materials under fluoroscopic guidance to prevent reflux into other arteries; and flush the embolization catheter before performing additional arteriograms
A fatal pulmonary embolus, which occurred 20 hours after UFE, was reported in a 65-year-old patient. [27] The source of the thrombus was believed to be the deep pelvic veins, and it may have occurred either secondary to the mass effect of the fibroid or from diminished arterial flow after embolization that resulted in stasis and thrombosis within pelvic veins.
To reduce the risk of PE after UFE, patients should ambulate as soon as possible to prevent the development of lower extremity deep venous thrombosis (DVT) and possible pulmonary embolism (PE). In addition, compression stockings have been recommended for patients with a history of smoking or oral contraceptive use, since these patients are at increased risk for thrombotic events. While these measures are important to reduce the risk of DVT and PE in many patients, they will not help if the source of a PE is from the pelvic veins.
Inadvertent end-organ damage (ie, uterus) can result in ischemic injury or infection, both of which may require hysterectomy for treatment.
Infection or, more specifically, pyometrium with acute endometritis was one of the earliest reported complications of UFE. High fever and persistent pain led to hysterectomy in an early case report. Since then, infection has been reported in less than 1% of patients. Some infected patients have been successfully treated with antibiotics, while others required hysterectomy. In one patient, infection resulted in multiorgan failure and death. All patients with prolonged fever (>7-10 days) should be evaluated for possible uterine infection. Diagnosis may be aided by the performance of a pelvic examination, lab tests (including CBC, blood and urine cultures), and pelvic CT scan.
Ischemic injury of the uterus is reported in less than 1% of patients. These patients present with pelvic pain persisting several weeks beyond the expected postembolization syndrome. Classic teaching states that the uterus receives approximately two thirds of its blood supply from the uterine arteries and approximately one third from the ovarian arteries, which originate from the abdominal aorta distal to the renal arteries. One possible explanation for this complication is that embolization of the uterine arteries places the uterus at risk for ischemic injury and infarction in cases in which there is potentially inadequate collateral blood flow from the ovarian arteries. Patients experiencing this complication may require further treatment (eg, hysterectomy) to relieve associated pain and discomfort.
Submucosal fibroids, particularly if pedunculated, are at increased risk for expulsion from the uterus after embolization-induced infarction. This particular complication has been reported in approximately 1-2% of patients. While this may occur spontaneously without clinically significant sequelae, a hysteroscopic resection or other treatment may be required to remove tissue retained within the uterus to prevent subsequent infection.
Technical failure
Technical failure in UFE can be defined as an inability to successfully catheterize and embolize both the right and the left uterine arteries. While not considered a true complication by some, an inability to successfully complete this procedure is certain to be perceived as a complication by both the patient and the referring gynecologist, since it will likely necessitate surgical intervention for definitive treatment. Based on published and presented reports, the technical failure rate for UFE is 1-2%.
Possible explanations for technical failure include the following:
-
Anatomic variation
-
Anatomic deviation secondary to the mass effect produced by large uterine fibroids
-
Operator inexperience
-
Persistent effects of GnRH agonist (eg, leuprolide acetate) at the time of embolization (as described below)
Hormonal changes
Postprocedure amenorrhea has been reported in approximately 2-14% of patients. Transient amenorrhea may occur in the presence of normal hormone levels secondary to endometrial atrophy. Often, a spontaneous return to normal menstruation occurs within 3-6 months.
The existence of collateral pathways between the uterine and ovarian arteries also makes it possible for the embolic materials injected into a uterine artery to enter ovarian arterial circulation.
Early menopause after UFE has been described. In a randomized trial in premenopausal women who underwent either UFE or hysterectomy for menorrhagia from uterine fibroids, Hehenkamp et al found increased levels of follicle-stimulating hormone and decreased levels of anti-mullerian hormone (a reliable marker of ovarian reserve in some patients). For older women with less ovarian reserve, these hormonal changes might hasten the onset of menopause; in younger women, the changes might affect fertility. [28]
These events provide one possible explanation for permanent amenorrhea after UFE. However, most women do not demonstrate a change in their basal follicle-stimulating hormone levels, and, in the absence of this change, the true cause-and-effect relationship between UFE and permanent amenorrhea remains unproved.
Fertility
There remains considerable uncertainty as to the optimal treatment for women with fibroids who wish to maintain their fertility. Concerns have been raised regarding abnormal placentation post UAE possibly leading to higher risks of early miscarriage and postpartum hemorrhage. A prospective multicenter study by Holub et al found that the risk of spontaneous abortion was significantly increased after UFE, as compared with laparoscopic uterine artery occlusion. [29]
A review by Zupi and colleagues emphasized that a desire for future pregnancy is a relative contraindication for UAE, as the lack of data in the literature cannot ensure a good pregnancy outcome. [30] In a randomized controlled trial comparing UAE and myomectomy, surgical removal had a more favorable outcome than UAE in terms of pregnancy rate (78% vs 50%), delivery rate (48% vs 19%), and abortion rate (23% vs 64%). [31]
Alternative Radiologic Treatment
Myolysis is a minimally invasive procedure targeting the destruction of fibroids via a focused energy delivery system. High frequency magnetic resonance-guided focused ultrasound surgery (MRgFUS) is thermal ablation using MRI to visualize and define the target. Ultrasonic energy is directed to a point inside the fibroid, and coagulation tissue necrosis is induced. In theory, damage to surrounding tissue is minimal but, in fact, the impact on critical neighboring structures cannot be excluded. [32]
A study of 359 women treated with MRgFUS showed improved scores on the Uterine Fibroid Symptoms Quality of Life questionnaire at 3 months that persisted for up to 24 months (P< .001). Overall, this procedure is well tolerated, although risks include localized pain and heavy bleeding, and the risk of additional procedures was high. [33] In a pilot randomized, placebo-controlled trial, 30% of women underwent further fibroid surgery or procedures 2 years after MRgFUS. [34] However, screening and MRI-based prediction models for assessing therapeutic responses may reduce the risk of treatment failure. [35]
Hyperintensive MRI images are associated with reduced treatment success compared with hypointensive images of fibroids. [30] The principal limitations to the use of MRgFUS are that only a fraction of patients with fibroids meet the inclusion criteria, future fertility may be compromised, and the procedure is costly.
-
Nonselective pelvic angiogram demonstrating the tortuous and dilated uterine arteries supplying a hypervascular uterine fibroid.
-
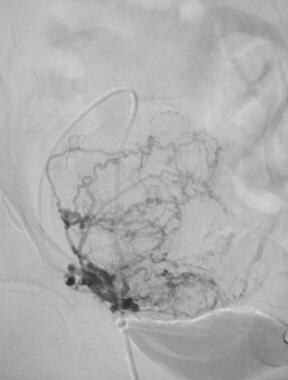
Selective angiogram of the left uterine artery prior to embolization.
-
Selective angiogram of the right uterine artery prior to embolization.
-
Nonselective angiogram of the pelvis after embolization demonstrating occlusion of both the right and left uterine arteries.