Background
Breast cancer is the most common type of cancer in women in the United States, accounting for 1 of every 3 cancers diagnosed. A woman's chance of developing invasive breast cancer at some time in her life is approximately 1 in 8 (12.4%). It is one of the leading causes of cancer mortality among women in the United States. [1]
Because of early detection, intervention, and postoperative treatment, breast cancer mortality has been decreasing. Mammography is the preferred screening examination for breast cancer. It is widely available, well-tolerated and inexpensive. Randomized controlled trials have demonstrated a mortality benefit for women from 40 to 74 years old. Some studies have shown that mammography may be particularly beneficial for women who are 80 years of age and older. [2, 3]
The earliest sign of breast cancer can be an abnormality depicted on a mammogram, before it can be felt by the woman or her physician. When breast cancer has grown to the point where physical signs and symptoms appear, the patient feels a breast lump (usually painless).
Screening mammography accounts for the greatest contribution to early detection and decrease in breast cancer mortality, although its use has resulted in a minor increase in the number of in situ cancers detected. [1] (See the image below.)
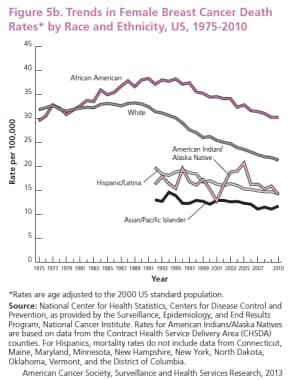 Trends in female breast cancer death rates by race and ethnicity, US, 1975-2010. Since the widespread adoption of screening mammography in the later 80s, the death rate from breast cancer has decreased 34%. Courtesy of the American Cancer Society (American Cancer Society. Breast Cancer Facts and Figures 2013-2014. Atlanta: American Cancer Society, Inc.).
Trends in female breast cancer death rates by race and ethnicity, US, 1975-2010. Since the widespread adoption of screening mammography in the later 80s, the death rate from breast cancer has decreased 34%. Courtesy of the American Cancer Society (American Cancer Society. Breast Cancer Facts and Figures 2013-2014. Atlanta: American Cancer Society, Inc.).
In the UK Age trial, breast cancer mortality in the first 10 years after diagnosis was significantly lower (rate ratio [RR] 0.75) in women who received annual screening mammography from age 40-49 years than in those invited for screening at age 50 years and every 3 years thereafter. During the remainder of the 17-year follow-up period, however, reduction in breast cancer mortality was not evident (RR 1.02). [4]
A large-scale, population-based, observational study by García-Albéniz et al concluded that continuing annual breast cancer screening past age 75 years did not result in substantial reductions in 8-year breast cancer mortality compared with stopping screening. The study used data from 1,058,013 women enrolled in Medicare across the United States. [5]
In women aged 70 to 74 years, continued screening resulted in a slightly reduced 8-year rate of breast cancer death: 2.7 deaths per 1,000 women, compared with 3.7 for those who stopped screening. In those aged 75 to 84 years, comparable figures were 3.8 versus 3.7 deaths per 1,000 women (hazard ratio, 1.00 [CI, 0.83 to 1.19]). [5] Although breast cancer was diagnosed more often in women who continued screening, that did not translate to a significant reduction in deaths because breast cancer is less successful treatment in older women. [6]
Ultrasonography, nuclear medicine study, and MRI have adjuvant roles in breast cancer imaging and staging. In patients of any age with increased breast density, which may mask small cancers, additional tests such as digital breast tomosynthesis (DBT), ultrasonography, nuclear medicine study, and MRI may be useful.
Breast Cancer Staging
To stage cancer, the American Joint Committee on Cancer first places the cancer in a letter category using the tumor, nodes, metastasis (TNM) classification system. The stage of a breast cancer describes its size and the extent to which it has spread. The staging system ranges from stage 0 to stage IV according to tumor size, lymph nodes involved, and distant metastasis.
T indicates tumor size. The letter T is followed by a number from 0 to 4, which describes the size of the tumor and whether it has spread to the skin or chest wall under the breast. Higher T numbers indicate a larger tumor and/or more extensive spread to tissues surrounding the breast.
-
TX: The tumor cannot be assessed.
-
T0: No evidence of a tumor is present.
-
Tis: The cancer may be LCIS, DCIS, or Paget disease.
-
T1: The tumor is 2 cm or smaller in diameter.
-
T2: The tumor is 2-5 cm in diameter.
-
T3: The tumor is more than 5 cm in diameter.
-
T4: The tumor is any size, and it has attached itself to the chest wall and spread to the pectoral (chest) lymph nodes.
N indicates palpable nodes. The letter N is followed by a number from 0 to 3, which indicates whether the cancer has spread to lymph nodes near the breast and, if so, whether the affected nodes are fixed to other structures under the arm.
-
NX: Lymph nodes cannot be assessed (eg, lymph nodes were previously removed).
-
N0: Cancer has not spread to lymph nodes.
-
N1: Cancer has spread to the movable ipsilateral axillary lymph nodes (underarm lymph nodes on the same side as the breast cancer).
-
N2: Cancer has spread to ipsilateral lymph nodes (on the same side of the body as the breast cancer), fixed to one another or to other structures under the arm.
-
N3: Cancer has spread to the ipsilateral mammary lymph nodes or the ipsilateral supraclavicular lymph nodes (on the same side of the body as the breast cancer).
M indicates metastasis. The letter M is followed by a 0 or 1, which indicates whether the cancer has metastasized (spread) to distant organs (eg, lungs or bones) or to lymph nodes that are not next to the breast, such as those above the collarbone.
-
MX: Metastasis cannot be assessed
-
M0: No distant metastasis to other organs is present
-
M1: Distant metastasis to other organs has occurred
The reduction in breast cancer mortality is attributed to early detection and shift from late stage to early stage disease at the time of diagnosis.
X-ray Mammography
Mammography is a special type of x-ray imaging used to create detailed images of the breast. It is estimated that 48 million mammograms are performed each year in the US. Mammography uses low dose x-rays, achieved by using targets made of low atomic weight alloys (eg, molybdenum and rhodium). Filters made of aluminum, molybdenum, beryllium, rhodium, or palladium are used to eliminate photons that do not contribute to the image, thereby minimizing the radiation dose to the patient.
Breast compression is necessary to flatten the breast so that the maximum amount of tissue can be imaged and examined. It also allows for a lower x-ray dose and immobilization of the breast to reduce motion blur. Compression also reduces x-ray scatter, which may degrade the image. Breast compression may cause some discomfort, but it should not cause any significant pain.
Randomized controlled trials in the 1970s and 80s used high-contrast, high-resolution (with single-sided emulsion) film to demonstrate findings smaller than 100 µm, such as microcalcifications. Nearly all film-based units in the United States have since been replaced with digital mammography systems.
In 2005, the American College of Radiology Imaging Network (ACRIN) published results of a multicenter trial comparing the diagnostic performance of film-screen to digital mammography. [7] The results indicated equivalent overall performance for the 2 modalities. However, in subgroup analysis, digital mammography performed better for women with dense breasts, premenopausal women, and women younger than 50 years. Since then, there has been a gradual national and international shift away from film-screen to digital mammography.
Mammography plays a major role in the early detection of breast cancers, detecting about 75% of cancers at least a year before they can be felt. Mammography uses low-dose ionizing radiation. Patients receive less radiation from a mammogram than from background environmental sources each year. The significant reduction in breast cancer mortality far outweighs the risks and inconvenience of the test.
Screening x-ray mammography
There are 2 types of mammography examinations: screening and diagnostic. Screening mammography is done in asymptomatic women. Early detection of small breast cancers by screening mammography greatly improves a woman's chances for successful treatment.
A screening examination consists of 2 images of each breast in the cranial-caudal (CC) and medio-lateral-oblique (MLO) projections that are viewed together. For screening mammography, each breast is imaged separately.
See the images below.
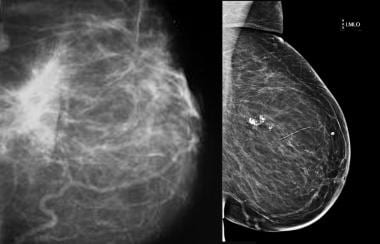 Traumatic fat necrosis. Mammogram shows traumatic fat necrosis following removal of a lesion. The stellate lesion has a halo center.
Traumatic fat necrosis. Mammogram shows traumatic fat necrosis following removal of a lesion. The stellate lesion has a halo center.
Research has shown that regular mammograms may decrease the risk of late-stage breast cancer in women 80 years of age and older. [2, 3]
Some studies suggest that mammography screening should not just be based on age and family history of breast cancer but also on breast density, history of breast biopsy, and beliefs about the benefits and risks of the screening. [8] Even though breast density represents a lower risk than other risk factors such as family history, it is more common in the general population. [9] However, only 10-25% of all breast cancers occur in the high-risk population. Delayed or less frequent screening of the 75-90% of the United States population at average risk of developing breast cancer would result in late detection and many unnecessary deaths.
Diagnostic X-ray mammography
Diagnostic mammography is performed in symptomatic women, such as when a breast lump or nipple discharge is found during self-examination or when an abnormality is found during screening mammography. Diagnostic mammography uses specialized views to determine exact size and location of breast abnormalities and to image the surrounding tissue and lymph nodes. Typically, several additional views of the breast are acquired and interpreted during diagnostic mammography. Thus, diagnostic mammography is slightly more expensive than screening mammography. In most cases, however, diagnostic mammography confirms that potential abnormalities found at screening mammography or physical exam are benign.
A diagnostic mammogram consists of supplemental views tailored to the specific problem. These supplemental views can include latero-medial (LM) and medio-lateral (ML), exaggerated CC, magnification, spot compression, and others. Special skin markers are sometimes used to identify certain lesions, skin abnormalities, the nipple, and other areas.
American College of Radiology Breast Imaging Reporting and Data System
The American College of Radiology (ACR) has established the Breast Imaging Reporting and Data System (BI-RADS) to guide the breast cancer screening and diagnostic routine. BI-RADS is the product of a collaborative effort between members of various committees of the ACR with cooperation from the National Cancer Institute, the Centers for Disease Control and Prevention, the FDA, the American Medical Association, the American College of Surgeons, and the American College of Pathologists. [10]
The BI-RADS atlas provides a standardized system for performing breast imaging examinations, interpreting the findings, reporting the results, communicating recommendations to patients and providers, and auditing statistical performance. There are separate guidance chapters for mammography, ultrasound, and magnetic resonance imaging (MRI). According to the ACR, the BI-RADS system is intended to guide radiologists and referring physicians in the breast cancer decision-making process that facilitates patient care.
BI-RADS categories or levels are used to standardize interpretation of mammograms among radiologists. They are useful for statistical analysis of mammography practice, and BI-RADS results are compiled in the National Mammography Database in the United States to help refine mammographic procedures everywhere.
Interpretation of mammograms
The quality of the mammograms should be assessed, and if not optimal, repeat examinations may be ordered. Mammograms of the right and left breasts are displayed on a high resolution monitor with previous comparable projections. Lighting should be homogeneous, and adequate viewing conditions should be maintained. The mammograms are inspected carefully. The search is done systematically through similar areas in both breasts. The goal of the radiologist is to determine whether the findings are normal, benign, or suspicious enough to warrant tissue sampling.
First, breast symmetry, size, general density, and glandular distribution are observed. Next, a search for masses, densities, calcifications, architectural distortions, and associated findings is performed. For masses, the shape, margins, and density are analyzed. The features of benign and malignant masses can be similar. Benign masses are often round or oval with circumscribed margins. Malignant lesions tend to have irregular, indistinct, or spiculated margins. Malignancies tend to have density greater than that of the normal breast tissue. The presence of very low density fat in a lesion often indicates benign findings such as oil cysts, lipomas, galactoceles, and hamartomas.
Calcifications can also be the first sign of cancer or a harmless process in the breast. Benign calcifications are usually larger than calcifications associated with malignancy. They are usually coarser, often round with smooth margins, and more easily seen. Benign calcifications tend to have specific shapes: eggshell calcifications in cyst walls, tramlike in arterial walls, popcorn type in fibroadenomas, large and rodlike with possible branching in ectatic ducts, and small calcifications with a lucent center in the skin.
Calcifications associated with malignancy are usually small (< 0.5 mm) and often require high-resolution magnification imaging with digital zooming for accurate assessment. They tend to have a pleomorphic or heterogeneous shape or a fine granular, fine linear, or branching (casting) shape.
The distribution of the calcification can provide clues to the underlying process and should be specified as grouped, clustered, linear, segmental, regional, or diffuse.
Special findings may be encountered, such as a linear density that might represent a duct filled with secretions or a reniform-shaped mass with a radiolucent center that is typical of an intramammary lymph node.
Associated findings are then taken into account. These include skin or nipple retraction, skin thickening (which may be focal or diffuse), trabecular thickening, skin lesions, axillary adenopathy, and architectural distortion.
Diagnostic views are used to determine where each lesion is in the breast. These may be described as central, retroareolar, in a quadrant, or, more precisely, at a clock position. The breast is viewed as the face of a clock with the patient facing the observer. The depth of the lesion is assigned to the anterior, middle, or posterior third of the breast.
If previous examination results are available, their comparison is useful in assessing disease progress.
All of these findings are considered together, a final impression is formed, and a BI-RADS category is assigned.
Breast density
Breast density is strictly a mammographic finding. Density has no relationship to the physical exam. It represents the ratio of glandular tissue (white on a mammogram) to fat (dark on a mammogram). The radiologist evaluates the density and categorizes it into one of 4 categories according to the BI-RADS atlas: A, B, C or D. Category A represents a breast that is composed almost entirely of fat, and category D represents a breast that is composed almost entirely of glandular tissue.
Breast density also impacts interpretation of mammograms and the risk of developing breast cancer. Data show that the sensitivity for breast cancer detection is inversely related to density. It is as high as 98% in fatty breasts (category A) and 50-65% in dense breasts (category D). In addition, the risk of developing breast cancer increases with breast density. The relative risk of developing breast cancer in women with very dense category D breasts is 4 times greater than in women with fatty category A breasts. In the United States, 50% of women have breast density category C or D.
For women whose mammogram reveals dense breast tissue, 21 US states have laws requiring that the women be notified and be advised to discuss supplemental imaging with their provider. However, a prospective cohort study found that only a minority of women with dense breasts have high interval cancer rates. The authors concluded that supplemental imaging should not be justified on the basis of breast density alone. [11]
Kerlikowske et al reported that women at high risk can be identified by combining 5-year breast cancer risk, as determined with the Breast Cancer Surveillance Consortium (BCSC) risk calculator, with breast density as categorized with the Breast Imaging Reporting and Data System (BI-RADS). High interval cancer rates were observed for women with a 5-year BCSC risk of 1.67% or greater and extremely dense breasts or a 5-year risk of 2.50% or greater and heterogeneously dense breasts. However, study participants who met those criteria accounted for only 24% of all women with dense breasts. The highest rate of advanced-stage breast cancer, >0.4 case per 1000 examinations, was seen in women with 5-year BCSC risk of 2.50% or greater and heterogeneously or extremely dense breasts. Such patients comprised 21% of all women with dense breasts. [11]
Breast Imaging Reporting and Data System
The American College of Radiology (ACR) established the Breast Imaging Reporting and Data System (BI-RADS) to guide the breast cancer diagnostic routine. BI-RADS is the product of a collaborative effort between members of various committees of the ACR in cooperation with the National Cancer Institute (NCI), the Centers for Disease Control and Prevention (CDC), the FDA, the American Medical Association (AMA), the American College of Surgeons (ACS), and the College of American Pathologists (CAP). [12] The BI-RADS system includes categories or levels that are used to standardize interpretation of mammograms among radiologists. For referring physicians, the BI-RADS categories indicate the patient’s risk of malignancy and recommend a specific course of action.
Summary of BI-RADS assessment categories
BI-RADS assessment categories can be summarized as follows [12] :
-
Category 0 - Need additional imaging evaluation
-
Category 1 - Negative
-
Category 2 - Benign finding, noncancerous
-
Category 3 - Probably benign finding, short-interval follow-up suggested
-
Category 4 - Suspicious abnormality, biopsy considered
-
Category 5 - Highly suggestive of malignancy, appropriate action needed
-
Category 6 - Known cancer, appropriate action should be taken
Category 0 is a temporary category that means additional imaging is needed before assigning a permanent BI-RADS assessment category. Most category 0 findings are shown to be benign after additional imaging is completed. This category is most often used when the radio9logist discovers something on a screening mammogram and wants to apply diagnostic views to make a decision.
Treatment by BIRADS category
Each BI-RADS level has an appropriate management or follow-up plan associated with it. For example, if a referring doctor sees a mammogram report with a category 3 assigned to it, he or she knows the recommendation is for the woman to undergo follow-up mammography in 6 months. [12]
If used correctly and consistently, each BI-RADS category has the risks of malignancy and the associated plan of management or follow-up shown in Table 1 below.
Table 1. Risk of malignancy and care plan by BI-RADS category (Open Table in a new window)
Category |
Description |
Risk of Malignancy |
Care Plan and Comments |
1 |
Negative |
5 in 10,000 |
Continue annual screening mammography for women 40 years of age or older. |
2 |
Benign finding, noncancerous |
5 in 10,000 |
Continue annual screening mammography for women 40 years of age or older. This category is for cases with a characteristically benign finding (eg, cyst, fibroadenoma). |
3 |
Probably benign finding |
< 2% |
Usually, 6-month follow-up mammography is performed. Most category 3 abnormalities are not evaluated with biopsy. |
4 |
Suspicious abnormality |
25-50% |
Most category 4 abnormalities are benign but may require biopsy. |
5 |
Highly suggestive of malignancy |
75-99%, depending on how individual radiologists define categories 4 and 5 |
Classic signs of cancer are seen on the mammogram. All category 5 abnormalities are typically evaluated with biopsy; if the results are benign, repeat biopsy is done to ensure correct sampling. |
Postoperative mammograms
Women who had previous surgery for breast cancer may still require breast cancer screening with mammography. If a woman had a total mastectomy, then continued annual screening of the other breast is recommended because the patient is at higher risk of developing cancer in the remaining breast. If she had subcutaneous or nipple-sparing mastectomy or partial mastectomy or lumpectomy, then annual screening is recommended for the treated breast. The first mammogram of the treated breast is often performed 6 months postoperatively to provide a baseline for the new postoperative and radiation changes. Thereafter, the mammogram may be performed every 6-12 months for screening and follow-up.
Women with breasts augmented by implants may be a special challenge. Four special screening views are added to the 4 standard views. The implant must be pulled aside so the underlying breast tissue can be imaged. MRI may be useful for assessing silicone implant integrity in this group of patients. MRI is not recommended for screening of average-risk patients with implants. Rupture of saline implants can be determined with standard mammograms.
False-positive and false-negative results
False-positive results may arise when benign microcalcifications are regarded as malignant. Tissue summation shadows may appear as local parenchymal distortion; this may be erroneously called suspicious tissue. A benign circumscribed lesion may show signs suggestive of malignancy, along with other findings, such as an irregular border and no halo sign.
According to data from the Breast Cancer Detection Demonstration Project, the false-negative rate of mammography is approximately 8-10%. Approximately 1-3% of women with a clinically suspicious abnormality, a negative mammogram, and a negative sonogram may still have breast cancer.
Possible causes for missed breast cancers include dense parenchyma obscuring a lesion, poor positioning or technique, perception error, incorrect interpretation of a suspect finding, subtle features of malignancy, and slow growth of a lesion.
Birdwell et al performed a multicenter study and found that, on prior mammograms with missed cancers, 30% of the lesions were calcifications, with 17 of 49 clustered or pleomorphic. Approximately 70% were mass lesions, with 40% spiculated or irregular. For calcifications and masses, the most frequently suggested reasons for possible miss were dense breasts (34%) and distracting lesions (44%). [13]
Some cancers (eg, mucinous carcinomas) may have well-defined borders and mammographic features suggestive of benignancy.
The false-positive rate of recommendation for biopsy is 4.3-6.7/1000, and 64-84/1000 need additional imaging after screening. Using an annual screening regimen, 61% of patients will receive a recall from screening after 10 years, and 7% will have a recommendation for biopsy. [14]
DCIS has increased from 2.4/100,000 to 27.7/100,000 since screening. Fifty percent of all recurrences after DCIS are invasive. Long-term follow-up of low-grade DCIS treated only by biopsy without definitive excision or radiation therapy is associated, at 30 years, with a 30-60% incidence of invasive cancer. [15]
Digital breast tomosynthesis
Digital breast tomosynthesis (DBT), also known as 3D mammography, uses the same compression and views as 2D mammography and adds a 3D volume acquisition. The examination requires only a few additional seconds for each view, and most women will not be able to tell the difference between having a 2D or 3D exam. A traditional 2D exam displays the entire volume of the breast on a single 2D image. When viewed in this manner, the normal dense parenchyma can cause some normal tissue to look like possible cancer and can also hide cancers. DBT addresses some of these challenges of 2D mammography.
The 3D volume of the entire DBT acquisition can be displayed on a monitor and viewed as slices as thin as 1 mm. This ability theoretically allows the radiologist to see cancers that might be obscured, improving sensitivity. It should also allow the radiologist to separate normal tissue and avoid an unnecessary recall, improving specificity. The research has confirmed the improved performance of this test. In a multicenter reader study published in 2013, the sensitivity and specificity for breast cancer was found to be significantly better with DBT than with 2D digital mammography. [16]
In a reader study with an enriched population, 2-view tomosynthesis outperformed standard 2D mammography in terms of accuracy, as measured by the area under the ROC curve, for both masses and microcalcifications. [17]
Skaane and colleagues concluded that the combination of tomosynthesis and digital mammography resulted in a significantly higher cancer detection rate (27%, P =.001) and reduction in false-positive findings (15%, P< .001), as compared with digital mammography alone (N = 12,621). [18]
In one study, the addition of 2-view tomosynthesis to conventional digital mammography during screening examinations resulted in a 29.7% decrease in recall rates (P< .01; N = 13,158). The greatest reduction in recall rates occurred in patients with dense breasts and in those younger than 50 years. [19]
Feng and colleagues demonstrated that DBT, on average, delivers twice the radiation dose to the breast than 2D digital mammography. [20] This isn’t surprising, since the DBT examination begins with the 4 standard views of 2D screening mammography, followed by 4 tomosynthesis views. However, the dose remains below the limit set by the FDA for screening mammography. In addition, Hologic has developed a software-based reconstruction algorithm that transforms the DBT images into a 2D image. If approved and instituted, the dose for a DBT exam would be the same as that for a 2D exam, with higher sensitivity and specificity.
DBT was approved by the FDA in 2011. Many centers across the United States and Europe are replacing their 2D mammography machines with DBT because of the increased sensitivity and specificity, although there are no data regarding a mortality benefit. Regarding DBT, the ACR has stated that “breast tomosynthesis has shown to be an advance over digital mammography, with higher cancer detection rates and fewer patient recalls for additional testing.” [21]
For women of any age, the ACP does not recommend the following low-value screening strategies [22] :
-
Annual mammography
-
MRI
-
Tomosynthesis
-
Regular systematic breast examination
In contrast to the ACP recommendation against the use of tomosynthesis (3D mammography) in breast cancer screening, the American College of Radiology (ACR) states that, “breast tomosynthesis has shown to be an advance over digital mammography, with higher cancer detection rates and fewer patient recalls for additional testing.” The ACR notes that further studies will be needed to determine which subgroups of women are likely to benefit most from tomosynthesis screening. [23]
In the Screening with Tomosynthesis Or standard Mammography-2 (STORM-2) study—a prospective population-based screening study in 9672 women that compared integrated 3D mammography with 2D mammography—3D mammography detected more cases of breast cancer than 2D mammography but increased the percentage of false-positive recalls in sequential screen-reading. [24] Thus, the benefit of significantly increased breast cancer detection with tomosynthesis screening must be weighed against the possible risk of overdiagnosis.
Digital subtraction and contrast-enhanced digital mammography
Contrast enhanced digital mammography (CEDM) was invented to address the decreased sensitivity of mammography in dense breasts and the high cost of MRI. It was approved by the FDA in 2011. It is not being used for screening but is being used sparsely for cancer staging or neoadjuvant follow-up in places where MRI may not be available. Contrast resolution of CEDM is lower than that of CT or MRI. Subtraction can be performed by temporal or dual-energy techniques. [25]
Temporal subtraction requires immobilization and compression for both pre- and post-contrast images taken minutes apart. Compression can prevent blood flow and contrast enhancement. For these reasons, temporal subtraction has largely been abandoned. Dual-energy acquires 2 identical images after contrast injection in full compression a few seconds apart. Dual-energy takes advantage of the difference in the atomic density of tissue, as compared to the contrast. [26]
Two images are acquired, and the low-density breast tissue is subtracted; however, the high energy of contrast persists, allowing any enhancing abnormality to be more visible. In a recent study comparing CEDM to MRI, the results indicated very high sensitivity for the index lesion with both modalities, but MRI detected more satellite tumors and CEDM had fewer false positives. [27]
In a study that looked at the role of contrast-enhanced spectral mammography (CESM) and digital breast tomosynthesis (DBT), in the “T” staging of histologically proven breast cancer before planning for treatment management, DBT showed the highest accuracy in size assessment (n = 69, 70.4%) compared to CESM (n = 49, 50%) and regular mammography (n = 59, 60.2%). CESM presented the least performance in assessing calcifications, yet it was most sensitive in the detection of multiplicity (92.3%), followed by DBT (77%) and regular mammography (53.8%). The combined analysis of the three modalities provided an accuracy of 74% in the “T” staging of breast cancer.
Other uses of radiography in breast cancer
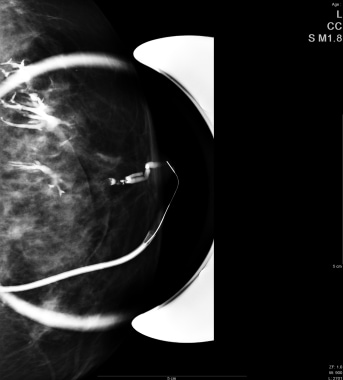
A ductogram, or galactogram, is used to acquire images of the lumen of an individual duct and can help determine the cause of nipple discharge. In this procedure, a fine plastic tube (30-gauge) is placed into the opening of the suspected duct in the nipple. A small amount (0.1 to 0.3 cc) of iodinated contrast medium is injected, which outlines the shape of the duct on a mammogram and shows whether a mass is present inside the duct.
See the image below.
Ultrasound
The role of ultrasonography in breast imaging is a subject of ongoing discussion. Ultrasound is widely accepted as the method of choice for the diagnostic differentiation of cysts from solid masses and for guidance in interventional procedures. Indeed, ultrasound can confidently diagnose a harmless cyst as the cause of a palpable lump or mammographic mass, thereby obviating the need for biopsy. The combination of a normal mammogram and a normal sonogram has a negative predictive value greater than 98%. However, if there is a concerning physical exam finding, palpation-guided biopsy may still be indicated. The use of sonography as an adjuvant to mammography may increase accuracy by up to 7.4%. [28, 29, 30]
On sonograms, solid lesions with smooth or gently lobulated margins that are sharply defined, with homogeneous hypoechoic contents and an orientation parallel to the chest wall, are usually benign. Solid hypoechoic lesions with irregular margins, an orientation perpendicular to the chest wall, acoustic shadowing are suspicious, and biopsy is indicated to rule in or rule out malignancy. Solid hypoechoic lesions with irregular and poorly defined margins and with shadowing and vertical orientation are considered to be probably malignant. The lesions may show infiltration into the surrounding fatty tissue or other features associated with malignancy. [28, 31, 32, 33] Some cancers can mimic benign tumors and appear well defined.
(See the images below.)
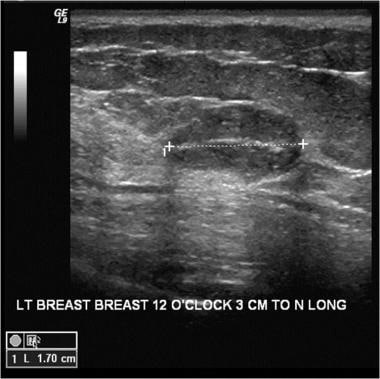 An ultrasound of a benign breast mass with circumscribed margins and oval shape; in this case, the mass is a fibroadenoma.
An ultrasound of a benign breast mass with circumscribed margins and oval shape; in this case, the mass is a fibroadenoma.
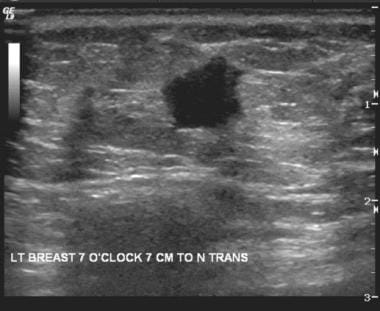 Ultrasound shows a suspicious hypoechoic mass with angular margins; in this case, the mass is an invasive ductal carcinoma.
Ultrasound shows a suspicious hypoechoic mass with angular margins; in this case, the mass is an invasive ductal carcinoma.
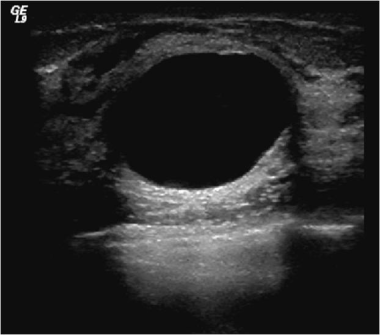 Ultrasound shows an anechoic cyst with smooth margins and enhanced through transmission. This is benign and does not need any imaging follow-up.
Ultrasound shows an anechoic cyst with smooth margins and enhanced through transmission. This is benign and does not need any imaging follow-up.
According to a multicenter trial conducted by the American College of Radiology Imaging Network, screening breast ultrasound detects 4.2 additional cancers per 1000 women with normal mammograms. [34] The advantage of ultrasound includes the low compression and lack of radiation. The major disadvantage is the high callback rate and a low positive predictive value of 10%.
A study from Korea that included over 100,000 patients reported a cancer detection rate of 3.4/1000. [35]
There is no randomized control trial showing a long-term mortality benefit associated with screening breast ultrasound. Screening breast ultrasound adds to health care costs, as a test that is performed in addition to mammography. The average callback rate from ultrasound is significantly higher than that of mammography, which also results in additional costs of diagnostic testing, time away from work, patient anxiety, and, in some cases, biopsy.
The U.S. Food and Drug Administration approved the first ultrasound system, the somo-v Automated Breast Ultrasound System (ABUS), for breast cancer screening in combination with standard mammography specifically for women with dense breast tissue. [36]
Current practice and recommendations from the American College of Radiology suggest that mammography is always the first choice for screening of all women. In addition, screening breast ultrasound should be considered in high-risk patients who cannot tolerate MRI or in intermediate-risk patients with category C or D breast density.
MRI and CT
Magnetic resonance imaging (MRI) and computed tomography (CI) may have adjuvant roles in the diagnosis of breast cancer. MRI may prove useful in screening younger women with dense breasts who are at a special high risk of developing breast cancer (eg, strong family history). CT may be used as an adjuvant for monitoring spread. Although CT imaging involves some exposure to radiation, it should be considered in patients in whom MRI is contraindicated. [30, 37]
Magnetic resonance imaging
High-resolution dynamic contrast-enhanced (DCE) MRI of the breast has recently emerged as the most sensitive (95-100%) instrument for the detection of breast cancer. The sensitivity of MRI makes it an excellent tool in specific clinical situations, such as the screening of patients at high risk for breast cancer, evaluation of the extent of disease in patients with a new diagnosis, axillary carcinoma of unknown primary, assessing treatment response during neoadjuvant chemotherapy, and detection of local recurrence in patients who have received breast-conservation therapy. [38]
MRI, however, has a significant false-positive rate, it is not readily available in all areas, and it is more expensive than mammography and sonography. Other limitations include the requirement of an intravenous gadolinium-based contrast agent, problems with claustrophobia, and longer imaging times. It also remains unclear if alterations in management plans based on MRI findings actually benefit patients.
Breast cancer almost always enhances on T1-weighted images after gadolinium enhancement. Multiple sequences, acquired before and after the administration of contrast, are compared to assess the rate enhancement and washout. The lesions are best imaged with fat-suppression techniques to eliminate the high signal intensity from fat on T1-weighted sequences. Two-dimensional (2D) or 3D techniques with gradient-echo sequences are time efficient and now largely used.
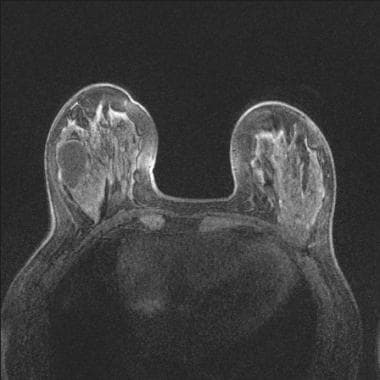 T1-weighted image with fat suppression before contrast. There is a large cyst in the right breast. The cancer in the left breast is isointense to normal tissue and very difficult to see.
T1-weighted image with fat suppression before contrast. There is a large cyst in the right breast. The cancer in the left breast is isointense to normal tissue and very difficult to see.
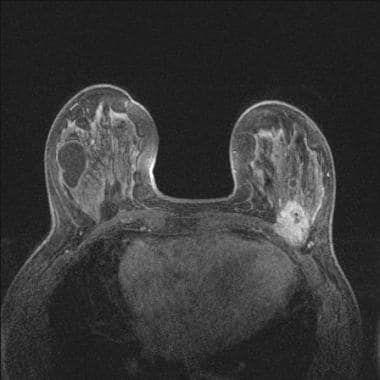 Same patient as in previous image: T1-weighted image with fat suppression after contrast clearly shows the posterior cancer invading the pectoralis major on the left.
Same patient as in previous image: T1-weighted image with fat suppression after contrast clearly shows the posterior cancer invading the pectoralis major on the left.
The moderate specificity of MRI results in the detection of some lesions that may represent cancer and can only be seen with MRI. Special techniques have been developed to biopsy such lesions, using MRI for guidance .
The generally accepted indications for breast MRI include the following:
Screening women with a lifetime risk of breast cancer >20%.
Search for primary malignancy in the breast in patients who have adenocarcinoma in an axillary lymph node but have a normal mammogram and clinical breast exam.
Evaluation of the extent of disease in the ipsilateral breast and possible synchronous cancer in the contralateral breast in women with a new diagnosis of cancer.
Follow-up of known cancer to assess response to neoadjuvant chemotherapy.
Berg et al showed that MRI was more accurate than mammography or ultrasound at depicting the full extent of disease for DCIS, invasive ductal carcinoma, and invasive lobular carcinoma. [39] Kuhl et al demonstrated that MRI is much more sensitive for the detection of DCIS than mammography (94% vs 54% respectively). [40]
Gadolinium-based contrast agents have been linked to the rare development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Breast Cancer Screening and Diagnosis include using breast MRI for screening as an adjunct to annual mammography and clinical breast examination in the following situations [41, 42] :
-
In women who have a BRCA1 or BRCA2 mutation or who have a first-degree relative who has a BRCA1 or BRCA2 mutation but who have not undergone genetic testing themselves.
-
In those who are determined to have a lifetime risk greater than 20% based on models that are highly dependent on family history.
-
In those with a history of lobular carcinoma in situ.
-
In patients who underwent radiation treatment to the chest between 10 and 30 years of age.
-
In women who carry or have a first-degree relative who carries a genetic mutation in the TP53 or PTEN genes (Li-Fraumeni, Cowden, and Bannahyan-Riley-Ruvalcaba syndromes).
According to the NCCN, MRI is specifically not recommended for screening women at average risk for breast cancer. MRI is also not generally recommended as a problem-solving tool when mammographic, sonographic, or physical examination findings are equivocal. As stated, the addition of MRI may result in additional false-positive findings and does not obviate the need for biopsy. Tissue sampling is preferred for these situations, because it will directly answer the question in a relatively rapid and inexpensive manner. [43]
Computed tomography
Dedicated breast CT scanners have been developed and tested in the research setting but are not yet in widespread clinical use. Its advantages are the speed of the method, comfort for the patient, absence of movement artifacts, easy standardization, and wide applicability. Dynamic contrast-enhanced CT of the breast has been found to be effective for the detection of intraductal extension of breast carcinoma and is thought to be useful in the preoperative assessment of the extent of disease prior to breast-conserving surgery. The lesions appear attenuating compared with fatty background, and they show early enhancement on arterial phase contrast-enhanced CT. [38, 44]
Three-dimensional (3D) helical CT can provide good information about the spread of breast cancer and could be an alternative to 3D MRI for preoperative examination of breast cancer. In vitro high-resolution helical CT can depict the internal structure of small nodes. Morphologic changes detected on helical CT help distinguish benign from malignant nodes. Tumors appear as dense lesions on CT and usually show early contrast enhancement similar to that seen with dynamic MRI. CT is less sensitive than mammography for detecting microcalcification when it is the sole manifestation of early cancer.
In one study, 3D CT depicted nearly all of the tumors and defined the correct tumor extent in most patients. Its sensitivity, specificity, and accuracy in diagnosing muscular invasion of breast cancer were 100%, 99%, and 99%, respectively. Its sensitivity, specificity, and accuracy in diagnosing skin invasion of breast cancer were 84%, 93%, and 91%, respectively. The sensitivity, specificity, and accuracy in detecting intraductal spread or DCIS were 71.9%, 83.3%, and 76.0%, respectively, for 3D CT and 87.5%, 61.1%, and 78.0%, respectively, for 3D MRI. The sensitivity rate for microcalcifications was about 59%. [38, 44]
Nuclear Medicine and Other Screening Modalities
Currently, there are 2 types of nuclear-based imaging for the breast that are FDA approved and used in clinical practice: breast-specific gamma imaging (BSGI) and positron emission mammography (PEM). Technetium-99m (99mTc)-sestamibi was the first radiopharmaceutical to be approved by the FDA for use in scintimammography and is used in BSGI. [45] A BSGI exam consists of 4 views that are identical in positioning to a screening mammogram. Each view requires 10 minutes, and the patients must stay still. The total imaging time is 40 minutes. Sestamibi must be injected intravenously, and it delivers a radiation dose to the entire body as it circulates. There is ongoing research into a reduced-dose protocol. [30, 46, 47, 48, 49]
In a retrospective study, the overall sensitivity of 99mTc-sestamibi BSGI in the detection of breast cancer was 95%. BSGI single and dual head devices are available. [50]
18F-Fluorodeoxyglucose (18F-FDG) FDG is used for PEM. PEM produces data that can be displayed as a 12-slice reconstruction. A clinical study reported a sensitivity of 93% and a specificity of 89% in patients with known cancer. [51] There are no data on screening with PEM.
Although not indicated as a screening procedure for the detection of breast cancer for average-risk patients, BSGI and PEM may play a useful role in various specific clinical indications, as in the screening of high-risk patients who cannot undergo an MRI or in evaluating tumor response to chemotherapy.
The disadvantages of BSGI and PEM include the radiation dose that extends to the whole body, the false-positive findings, and the lack of technique and equipment for BSGI-guided tissue sampling. The recommended injection for BSGI is 740–1100 MBq (20–30 mCi) of99m Tc-sestamibi, resulting in an estimated effective whole-body dose of 8.9 to 9.4 mSv. [52] For PEM, 370 MBq of 18F-FDG results in an estimated effective whole-body dose of 6.2 to 7.1 mSV. These are both much higher than the average effective dose to the breast from a mammogram, which is 0.44 mSV to 0.56 mSV. The dose from natural background radiation is 3 mSV per year.
Electrical impedance imaging (T-scan)
Electrical impedance imaging scans the breast for electrical conductivity, based on the idea that breast cancer cells conduct electricity better. It involves passing a very small electrical current through the body and detecting it on the skin of the breast with a small probe (similar to an ultrasound probe). The test does not use radiation and does not require breast compression.
Thermography
Thermography (thermal imaging) and computerized thermal imaging depend on mapping heat radiating from the breast, with the assumption that cancerous tissue produces more heat than normal breast tissue. It is not approved as a screening tool for breast cancer. It has not been shown to provide any decrease in breast cancer mortality. It is not recommended as a screening tool.
CT laser mammography
Computed tomography laser mammography is an experimental test that uses a laser to produce a 3-dimensional view of the breast. It has not yet been approved for clinical use.
Ductal lavage
During ductal lavage, breast cells are removed from a milk duct through a small flexible tube inserted into one of the ducts in the nipple. The sample is examined under a microscope to determine whether abnormal cells are present in the duct. It may be useful for screening in conjunction with mammography for women at high risk of developing breast cancer.
-
Trends in female breast cancer death rates by race and ethnicity, US, 1975-2010. Since the widespread adoption of screening mammography in the later 80s, the death rate from breast cancer has decreased 34%. Courtesy of the American Cancer Society (American Cancer Society. Breast Cancer Facts and Figures 2013-2014. Atlanta: American Cancer Society, Inc.).
-
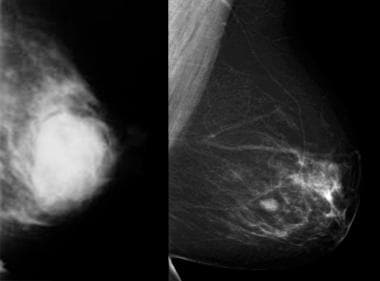
Normal four-view mammogram showing breasts that are extremely dense (BI-RADS category D).
-
Normal four-view mammogram showing breasts that are almost entirely fatty (BI-RADS category A) with very little parenchyma.
-
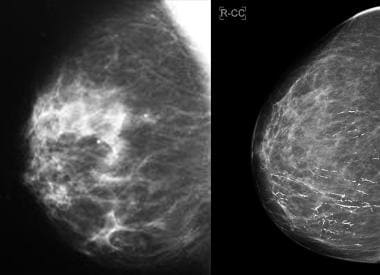
Screening mammogram depicts malignant pleomorphic microcalcifications in a linear branching distribution.
-
A four-view mammogram shows a highly suspicious mass with spiculated margins and increased density in the right lower outer quadrant: an invasive ductal carcinoma.
-
Image from a mammogram shows a benign mass: a fibroadenoma with well-defined edges and a halo sign.
-
Benign microcalcifications: secretory change.
-
Bilateral mammogram shows diffuse inflammatory carcinoma of the left breast, which appears smaller and more dense than the right breast.
-
Traumatic fat necrosis. Mammogram shows traumatic fat necrosis following removal of a lesion. The stellate lesion has a halo center.
-
Standard 2D mammogram (left) is normal. The corresponding slice from the tomosynthesis (right) taken at the same time shows a mass with architectural distortion.
-
Mammographic spot image during a ductogram shows contrast (white) within the catheter and branching ductal system.
-
An ultrasound of a benign breast mass with circumscribed margins and oval shape; in this case, the mass is a fibroadenoma.
-
Ultrasound shows a suspicious hypoechoic mass with angular margins; in this case, the mass is an invasive ductal carcinoma.
-
Ultrasound shows an anechoic cyst with smooth margins and enhanced through transmission. This is benign and does not need any imaging follow-up.
-
T1-weighted image with fat suppression before contrast. There is a large cyst in the right breast. The cancer in the left breast is isointense to normal tissue and very difficult to see.
-
Same patient as in previous image: T1-weighted image with fat suppression after contrast clearly shows the posterior cancer invading the pectoralis major on the left.
-
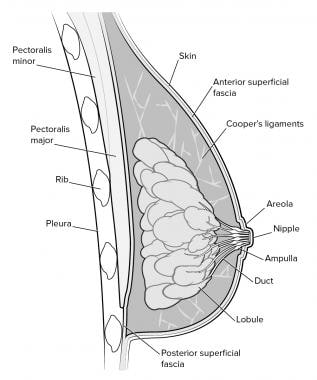
Anatomy of the breast.