Practice Essentials
Deep venous thrombosis (DVT) is the presence of coagulated blood—a thrombus—in one of the deep venous conduits that return blood to the heart. The clinical conundrum is that symptoms (pain and swelling) are often nonspecific or absent. However, if left untreated, the thrombus may become fragmented or dislodged and may migrate to obstruct the arterial supply to the lung, causing a potentially life-threatening pulmonary embolus (PE). In 1856, Virchow described the classic triad of predisposing factors for DVT—namely, venous stasis, injury of the vascular wall, and a hypercoagulable state. [1] Events or conditions that alter the equilibrium of one or more of these factors may produce DVT.
Lower extremity DVT is the most common venous thrombosis. Incidence rates for lower extremity DVT range from 88 to 112 per 100,000 person-years and increase with age. Rates of recurrent venous thromboembolism (VTE) range from 20 to 36% during the 10 years after an initial event. [2] One out of 20 people will develop a DVT sometime during their lifetime. DVTs account for approximately 600,000 hospitalizations per year in the United States, and they occur more commonly in those older than 40 years. Incidence is increased among males and in the African American population. [3] DVT is the underlying source of 90% of acute PEs. [4]
Risk factors such as older age, malignancy, inflammatory disorders, and inherited thrombophilia are associated with higher risk of VTE. Patients with signs or symptoms of lower extremity DVT such as swelling or a cramping or pulling discomfort in the thigh or the calf should undergo pretest probability, followed by D-dimer testing and venous ultrasonography. A normal D-dimer level less than 500 ng/mL excludes acute VTE when combined with a Wells DVT score of 1 or less. [2]
Postthrombotic syndrome (PTS) occurs in 25-50% of patients 3-6 months after DVT diagnosis. [2] Other than the immediate threat of PE, the risk of long-term major disability from PTS is high. [5, 6, 7, 8, 9] Risk of recurrent thrombosis and severity of PTS are related to the number and the anatomic sites of involved venous segments. The worst PTS occurs when the initial thrombus involves the iliac or iliocaval outflow segment and when multiple segments are involved. [10]
Over a few months, most acute DVTs evolve to complete or partial recanalization, and collaterals develop. [11, 12, 13, 14, 15, 16, 17]
(See the images below.)
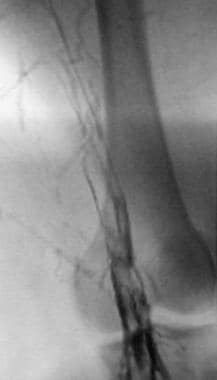 Lower extremity venogram shows outlining of an acute deep venous thrombosis in the popliteal vein with contrast enhancement.
Lower extremity venogram shows outlining of an acute deep venous thrombosis in the popliteal vein with contrast enhancement.
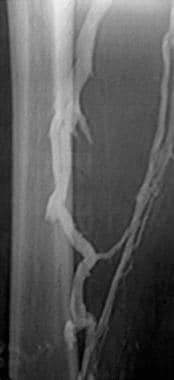 Lower extremity venogram shows a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of 2 parallel veins, when, in fact, it is 1 lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
Lower extremity venogram shows a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of 2 parallel veins, when, in fact, it is 1 lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
Anatomic variants that result in a diminished or absent inferior vena cava (IVC) or iliac veins may contribute to venous stasis. An underlying anatomic cause is identified in 60-80% of patients with iliocaval thromboses, . The best-known anomaly is compression of the left common iliac vein at the anatomic crossing of the right common iliac artery.
(See the image below.)
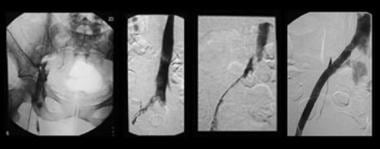 Sequential images show treatment of iliofemoral deep venous thrombosis due to May-Thurner (Cockett) syndrome. Far left, View of the entire pelvis shows iliac occlusion. Middle left, After 12 hours of catheter-directed thrombolysis, an obstruction at the left common iliac vein is evident. Middle right, After 24 hours of thrombolysis, a bandlike obstruction is seen; this is the impression made by the overlying right common iliac artery. Far left, After stent placement, the image shows wide patency and rapid flow through the previously obstructed region. Note that the patient is in the prone position in all views. (Right and left are reversed.)
Sequential images show treatment of iliofemoral deep venous thrombosis due to May-Thurner (Cockett) syndrome. Far left, View of the entire pelvis shows iliac occlusion. Middle left, After 12 hours of catheter-directed thrombolysis, an obstruction at the left common iliac vein is evident. Middle right, After 24 hours of thrombolysis, a bandlike obstruction is seen; this is the impression made by the overlying right common iliac artery. Far left, After stent placement, the image shows wide patency and rapid flow through the previously obstructed region. Note that the patient is in the prone position in all views. (Right and left are reversed.)
Conclusive diagnosis historically required invasive and expensive venography, which is still considered the criterion standard. Diagnosis can now be obtained noninvasively by means of ultrasound examination. Validation of the simpler and cheaper D-dimer test as an initial screening test has revealed a rapid, widely applicable screening that may reduce the rate of missed diagnosis. Algorithms are based on pretest probabilities and D-dimer results. [18] As many as 40% of patients with low clinical suspicion and a negative D-dimer result require no further evaluation. [19, 20]
The point-of-care ultrasound DVT (POCUS DVT) examination can provide rapid bedside diagnosis and treatment of lower extremity DVT. Waiting Doppler ultrasonography results result in delays in treatment with anticoagulation agents. [21]
DVT is a common complication of acute stroke, and hemorrhagic stroke is associated with a higher incidence of DVT. Poststroke paralysis is a common contributor to DVT of the lower extremities. [22]
Image-Guided Technique
Access to the popliteal vein is usually obtained with ultrasonographic guidance, although the common femoral, tibial, or internal jugular vein may be used. When thrombolysis is planned, ultrasonography and use of a micropuncture 21-gauge needle are recommended to minimize bleeding risk. Diagnostic venography is performed to identify the extent of DVT; fluoroscopic guidance provides the most accurate and straightforward means of placing infusion catheters or devices. A sheath is placed, and a multiple–side-hole catheter or wire is used to deliver the drug and to maximize exposure of the lytic to the surface area of the thrombus.
Computed Tomography
Spiral multidetector-row CT venography (CTV) from the popliteal fossa to the pelvis offers good diagnostic accuracy and correlation with sonographic findings. Radiation dose, cost, and scanning time, as well as an explosion in the number of requests for CT scanning at most hospitals, have made it prohibitive to use CT to evaluate extremity DVT alone. [23, 24, 25, 26]
Studies of multidetector-row CTV showed that venous phase scanning after arterial phase scanning is feasible and may be cost-effective. In practice, adding indirect CTV to the now relatively standard CT pulmonary arteriography for suspected PE leads to additional diagnosis of thrombotic disease in only a few patients. However, it results in an incremental increase of 15-38% for diagnosis of VTE. For patients in the emergency department, the yield is relatively low, and management is unlikely to be changed, because deep venous thrombosis is rarely identified in the absence of PE. In the converse, in an oncology population, CTV added to CT pulmonary angiography (CTPA) resulted in a 20% increase in detection of thrombotic disease. CTV showed DVT isolated to iliac or pelvic veins in 4.5%. [25, 26, 27, 28]
CTV is useful for evaluating for DVT versus other causes of leg swelling in patients with equivocal or negative Doppler sonographic results and for obtaining additional information on patients with known DVT before endovascular treatment. CTV reliably depicts the extent of thrombi and underlying anatomic abnormalities, and it may help in defining the chronicity of the lesion. Increased attenuation of the thrombus (>60 HU) and an increased diameter of the vessel (>150% the diameter of the contralateral normal vein) are correlated with acuity and serve as predictors of successful CTV.
In a retrospective review of the predictive factor of DVT in gynecologic cancer patients with lower extremity edema, Kim and associates concluded that CTV is a useful diagnostic tool, particularly in high-risk patients, and should be included as part of the initial evaluation. [29]
Limitations
CT requires the use of iodinated contrast agent, to which some patients are allergic. In addition, renal insufficiency is a contraindication because of the large dose of contrast agent needed. The radiation dose for bilateral lower extremity CTV is 3-8 mSv (less than that for abdominal CT).
Claustrophobia, extreme patient girth, certain metallic implants, or inability to remain immobile can produce nondiagnostic studies, although these factors generally are less important with CT than with magnetic resonance imaging (MRI).
In preliminary studies, CTV findings that were used to exclude iliofemoral thrombi had sensitivity equivalent to that of ultrasonography. Studies in which indirect CTV was compared with venography showed 100% sensitivity and 96 to 97% specificity. Contrast enhancement of vessels to greater than 60 HU is desired. In these studies, CTV required 80% less contrast agent when compared with venography.
In an intensive care unit (ICU) setting, combined CTPA and CTV yielded a 25% incidence of nondiagnostic DVT studies because of inadequate contrast opacification or because of artifacts due to metallic hardware.
The main disadvantage of indirect CTV is poor attenuation of deep veins, which is usually associated with differences in circulation time of contrast medium reaching the limbs. Image quality may be compromised because of lower density contrast between background and veins. Three-dimensional (3D) images produced by direct CTV are better than those obtained by indirect CTV, but the presence of artifacts may result in false-positive diagnoses, especially in cases of small, localized thrombus. [30]
False-positive findings include tumor thrombus and/or invasion (pelvis and cava), compression by an extrinsic mass (usually detected), inflow defects from unopacified blood (usually seen at the iliac confluence to the IVC, or as brachiocephalic confluence or inflow at the renal vein), and poorly timed CT scanning with indeterminate findings. False-negative findings include small thrombi (< 1 cm) when CT is performed at large gaps or intervals (ie, 5 mm of every 20 mm scanned) to reduce the radiation dose. This technique reduces sensitivity as well.
Magnetic Resonance Imaging
Findings on magnetic resonance venography (MRV) depend on the sequence used. If nonenhanced (flow, bright blood) or contrast-enhanced (gadolinium-enhanced) images are obtained, they reveal a bright rim around a dark intraluminal filling defect. Although MRI is highly sensitive and relatively specific, the cost of the examination, its technical complexity, and lack of general availability limit the use of MRV as a screening tool. The specific primary indication for MRV is as an alternative to CT (particularly for patients with allergy to contrast material, those with renal failure, and those for whom evaluation of the iliocaval veins is required for questionable sonographic findings); MRV is also indicated for preinterventional evaluation of the extent of a thrombus. [31, 32, 33, 34, 35]
Limitations
MRI cannot be used for patients with ferromagnetic implants or for those who depend on metallic devices that cannot be placed in the imaging unit.
Claustrophobia, extreme patient girth, certain metallic implants, or inability to remain immobile can produce nondiagnostic studies.
MRV is effective and accurate, with sensitivity and specificity for iliac and femoral DVT approaching 100%, when compared to venography, and sensitivity of 92% for detecting isolated calf vein thrombus. In addition, pelvic veins that are nearly impossible to visualize on sonography and are difficult to view by other means are consistently imaged well with MRV.
In general, MRI findings are subject to many artifacts that simulate vascular disease. Adjacent metallic objects, inadequate contrast enhancement, turbulent or sluggish venous blood flow, inflow from another vein into a vessel filled with contrast agent, and reflux (reversal) of venous blood flow may affect the signal received, depending on the machine and the protocol chosen. False-positive findings may result from slow or turbulent flow, an adjacent pulsatile structure, or hypointense inflow defects.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). This disease has occurred in patients with moderate to end-stage renal disease after they were given a gadolinium-based contrast agent to enhance MRI or magnetic resonance angiography (MRA) scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Ultrasonography
Ultrasonography is currently the first-line imaging examination for DVT because of its relative ease of use, absence of irradiation or contrast material, and high sensitivity and specificity in institutions with experienced sonographers. [36, 37, 38] Compression ultrasonography entails imaging the calf to the groin in the axial plane with a 5- to 10-MHz transducer. Compression is intermittently applied to induce complete coaptation of the walls of the patent vein. If the vein does not compress, it is occluded. Attempts are made to visualize iliac and pelvic veins.
Some protocols use gray-scale ultrasonography alone, whereas others include Doppler interrogation. The Society of Radiologists in Ultrasound recommends a comprehensive duplex ultrasonography protocol from thigh to ankle with Doppler at selected sites rather than a limited or complete compression-only examination. [39]
(See the images below.)
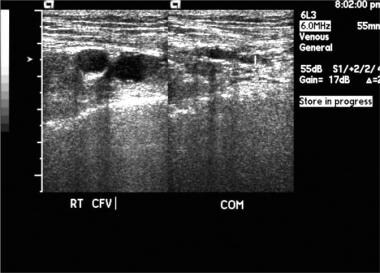 Popliteal vein thrombosis with normal compression of the common femoral vein. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
Popliteal vein thrombosis with normal compression of the common femoral vein. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
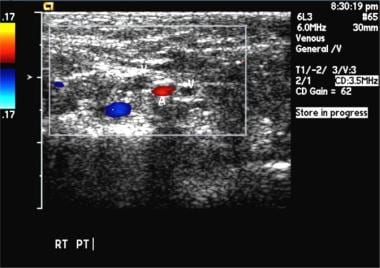 Bilateral popliteal vein thrombosis with normal compression of the superficial femoral vein. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
Bilateral popliteal vein thrombosis with normal compression of the superficial femoral vein. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
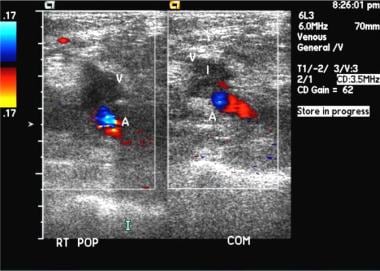 Popliteal vein thrombosis. Duplex sonogram shows absent flow. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
Popliteal vein thrombosis. Duplex sonogram shows absent flow. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
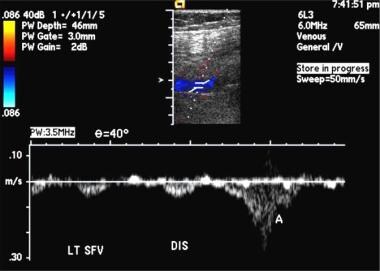 Popliteal vein thrombosis. Gray-scale images show compression failure. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
Popliteal vein thrombosis. Gray-scale images show compression failure. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
Regarding clinical outcomes, the negative predictive value at 3 months after compression ultrasonography yields normal results is 97-98% and is greater than 99% with serial ultrasonography. A more comprehensive study includes color Doppler imaging. In addition to compressibility, evaluation includes assessment for incomplete color filling, flow augmentation (vein patency peripheral to the transducer), and respiratory variation (patency central to the transducer). An acutely thrombosed vein (within the first 2 weeks after thrombus forms) is commonly dilated, with a diameter greater than that of the adjacent artery. This is the most accurate parameter for assessing the age of the DVT. In addition to assessing for normal flow, distal augmentation maneuvers such as squeezing the calf are performed during Doppler evaluation to further assess vein patency. While the distal augmentation maneuver is performed, there should be a sharp “spike” of augmented anterograde venous flow. Blunted or absent flow augmentation suggests venous obstruction distal to the level of interrogation. Evidence of retrograde flow in the venous system after a distal augmentation maneuver indicates valvular incompetence of the vein, which occurs secondary to prior DVT or PTS. [40]
A negative single, complete duplex color sonogram of the entire lower extremity obtained to assess suspected DVT has a negative predictive value of 99.5%.
Specific findings include the following:
-
Incompressibility: A thrombosed vein does not compress.
-
Loss of augmentation: Loss of appropriately increased flow when the lower extremity is compressed implies an obstruction (clot) between the transducer and the compressed area.
-
Visualization: DVT may be directly visualized as moderately echoic to hyperechoic masses separate from anechoic fluid.
-
Doppler flow: Doppler color flow imaging can depict absent or abnormal flow in an area where an isoechoic clot might not be visible.
-
Below-the-knee thrombus: A clot below the popliteal vein level remains elusive in duplex scanning. It is challenging to detect, and detection is operator dependent.
Limitations
Patient size limits the use of sonography because large patients are difficult to scan with accuracy. Good-quality sonograms depend on the experience of the technologist performing the examination. Iliac and pelvic veins are not imaged consistently with sonography.
For patients with clinically suspected disease, compression ultrasonography is 95-99% sensitive for proximal venous thrombus compared to contrast venography. For isolated calf vein thrombus, sensitivity decreases to below 50%. The high accuracy of ultrasonography versus venography for diagnosis of proximal DVT has been shown in asymptomatic patients. For clinical evaluations in which anticoagulation was withheld on the basis of negative serial compression sonograms, the incidence of thromboembolic complications was 0.07 to 1.5% at 6-month follow-up. [19, 20]
Because of limitations of diagnostic studies of the proximal veins, serial scans are required to ensure that calf vein DVT is not progressing. A few cases have become positive over 7-day follow-up. However, because of cost and because of patient compliance issues with follow-up testing, investigators evaluated the usefulness of single, complete lower extremity compression sonography. The technical-failure rate was 1.5%; these cases required additional study. The outcome evaluated was thromboembolic complications at 3 months, which occurred in 0.2 to 0.8% of studies.
Visualization of the iliac or proximal thrombus is often difficult. In the presence of thigh swelling or an abnormal common femoral vein, evaluation of the central iliocaval veins is warranted. Interposed bowel gas may compromise duplex ultrasonography, and CTV or MRV is a useful adjunct. Visualization at the adductor canal is similarly difficult, and a focal thrombus may not be identified; however, this has not compromised the clinical relevance of a negative study. If findings are indeterminate, extremity venography remains the diagnostic criterion standard.
DVT of the lower extremities can be associated with significant morbidity and may progress to PE and PTS. A systematic review examined the accuracy of diagnostic tests for first-episode and recurrent DVT of the lower extremities, including proximal compression ultrasonography, whole-leg US, serial US, and high-sensitivity quantitative D-dimer assays. For any suspected DVT, pooled estimates for sensitivity and specificity of proximal compression were 90.1% and 98.5%, respectively. For whole-leg US, pooled estimates were 94.0% and 97.3%; for serial US, pooled estimates were 97.9% and 99.8%; and for D-dimer, pooled estimates were 96.1% and 35.7%. [41]
False-positive findings may result from a technical error in scanning or from interpretation of chronic DVT as acute DVT. However, use of compression ultrasonography with consideration of venous diameter is highly sensitive in identifying recurrence. [42, 43] False-negative findings may result from inadequate scanning due to the size of the patient's leg; edema; or inexperience of the technologist, who must carefully scan each segment. In addition, iliac or pelvic DVT may be missed because of overlying bowel gas, which is the major limitation of duplex scanning in patients with DVT. For most patients, iliac or pelvic DVT cannot be completely excluded.
Femoral vein duplication is a congenital variant that poses a pitfall in diagnosis. If a patent femoral vein is identified, an occluded duplicated vein may be missed if the anomaly is not recognized.
Nuclear Imaging
Radiolabeled peptides that bind to various components of a thrombus have been investigated. Apcitide, a technetium-labeled platelet glycoprotein IIb/IIIa receptor antagonist, is approved for diagnostic studies of DVT. Other peptides in development include fragments of fibronectin with a distinct fibrin-binding domain and analogs of laminin and thrombospondin, which bind to platelet receptors. [44, 45]
Costs of the tests and inability to visualize the anatomy of the area of involvement (which many clinicians prefer) have led to underuse of scintigraphy. The radiation dose is 6.8 mSv—equivalent to lower extremity CTV.
Foci of increased activity indicate an acute thrombus in that location. This scanning technique is used in institutions where practitioners have experience and confidence in using the technique.
Radionuclide venography (RNV) is considered an alternative test for DVT, and one study found that RNV and venous ultrasonography show moderate agreement for detection of lower limb DVT. RNV can simultaneously evaluate the 2 lower limbs. Absent or reduced radiotracer activity in a deep venous segment or the presence of radiotracer activity in multiple collateral veins is highly predictive of the presence of DVT. A high negative predictive value suggests that patients with normal RNV have a very low probability of having DVT. [46]
Angiography
Venograms typically are performed to idenfity inflammation in the deep veins of the legs or in the abdominal cavity. Such inflammation might indicate the presence of a clot. [47] The classic finding of acute thrombus is an intraluminal filling defect in the contrast opacified vein. Lack of opacification of a vein or a venous segment indicates occlusion. Occlusion is consistent with an acute or chronic thrombus. Findings of intraluminal septation, webs, or stenoses are consistent with a healed or remote DVT. In chronic DVT, recanalization can result in a linear filling defect in the vein, sometimes termed the tram-track pattern. The vein appears as if it were 2 small, paired veins.
Until the 1980s, venography was the criterion standard examination for DVT. This procedure is now uncommonly performed because of patient discomfort from needle puncture, the potential for infiltration of contrast agent at the injection site or allergy to the agent, and costs in time and infrastructure necessary to perform the examination. The development of highly sensitive, noninvasive ultrasonography and impedance plethysmography protocols for DVT has relegated the use of venography to specific indications.
Venography remains the examination of choice when absolute determination of the presence and extent of thrombus is needed. This study is often required in obese patients, in patients with severe leg edema, or in those for whom results of noninvasive tests are equivocal or negative in the setting of high clinical suspicion. If technically adequate, this study offers a high degree of confidence. Technical limitations include poor IV access in the foot, poor contrast opacification of deep veins (contrast material shunted to superficial veins, injection too slow, poor tourniquet compression), motion artifact, and excessive muscular contractions or spasms.
An IV line is placed in a dorsolateral foot vein, and several tourniquets (placed at the ankle and below and above the knee) or the reverse Trendelenburg position is used to shunt contrast material into the deep venous system. The pelvis is imaged by compressing the femoral vein while the leg is elevated or while the table is moved from the reverse Trendelenburg to the Trendelenburg (head-down) position. Compression is then released while the external iliac vein is rapidly imaged.
Images are obtained from the foot to the pelvis, and detailed images of the entire deep venous system, including paired tibial veins, iliacs, and IVC, can be obtained. The internal iliac vein in the pelvis is not imaged, and a clot in this area cannot be excluded. The mean radiation dose for a single extremity is 6 mSv.
Matsumoto et al studied hybrid CT-angiography to facilitate lower extremity sharp venous recanalization and noted that stent patency was confirmed at 3-month follow-up, with patient-reported improvement in severe PTS. [48]
-
Sequential images show treatment of iliofemoral deep venous thrombosis due to May-Thurner (Cockett) syndrome. Far left, View of the entire pelvis shows iliac occlusion. Middle left, After 12 hours of catheter-directed thrombolysis, an obstruction at the left common iliac vein is evident. Middle right, After 24 hours of thrombolysis, a bandlike obstruction is seen; this is the impression made by the overlying right common iliac artery. Far left, After stent placement, the image shows wide patency and rapid flow through the previously obstructed region. Note that the patient is in the prone position in all views. (Right and left are reversed.)
-
Lower extremity venogram shows outlining of an acute deep venous thrombosis in the popliteal vein with contrast enhancement.
-
Lower extremity venogram shows a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of 2 parallel veins, when, in fact, it is 1 lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
-
Two views of a commercially available thrombectomy device. The Helix Clot Buster (Microvena Corp, Plymouth, MN) works by creating a vortex with a spinning self-contained propeller that macerates the clot. No thrombolytic agent (ie, tissue plasminogen activator) is necessary when this device is used, but adjunctive thrombolytic medications can be useful. Competing devices are available from Boston Scientific, Cordis Endovascular, Possis Medical, and other manufacturers.
-
Popliteal vein thrombosis with normal compression of the common femoral vein. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
-
Bilateral popliteal vein thrombosis with normal compression of the superficial femoral vein. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
-
Popliteal vein thrombosis. Duplex sonogram shows absent flow. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.
-
Popliteal vein thrombosis. Gray-scale images show compression failure. Image courtesy of Very Special Images, with permission from Dr. Lennard A. Nadalo.