Practice Essentials
A transjugular intrahepatic portosystemic shunt (TIPS) is a percutaneously created connection within the liver between portal and systemic circulations. A TIPS is placed to reduce portal pressure in patients with complications related to portal hypertension. [1, 2] This procedure has emerged as a less invasive alternative to surgery in patients with end-stage liver disease. [3] TIPS is now regarded as a well-established percutaneous means of decreasing portal hypertension. Major clinical indications for TIPS include refractory variceal hemorrhage and refractory ascites. The shunt itself is created by placing a stent between the portal vein and the hepatic vein. [4]
In patients with liver cirrhosis, esophageal varices is present in 85% and the rupture rate is 10-15%. Variceal hemorrhage is associated with a 40% mortality and a 70% recurrence rate. In patients with acute variceal bleeding, endoscopy is recommended within 12 hours of admission by the American Association for the Study of Liver Disease and the European Association for the Study of the Liver. TIPS creation may be considered in patients with bleeding that cannot be controlled endoscopically and in those at high risk for rebleeding. [5, 6, 7]
(See the images below.)
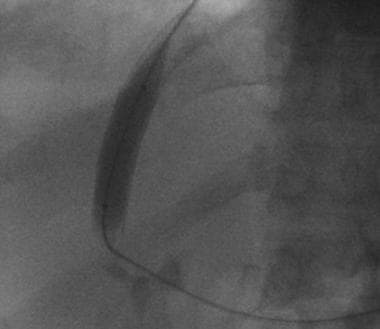
 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A curved catheter is placed into the right hepatic vein.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A curved catheter is placed into the right hepatic vein.
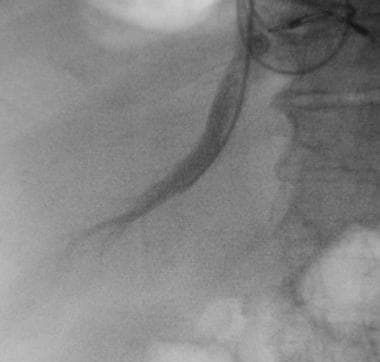
 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Image demonstrates advancement of a Colapinto needle into the right portal vein.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Image demonstrates advancement of a Colapinto needle into the right portal vein.
The goal of TIPS placement is to divert portal blood flow into the hepatic vein, so as to reduce the pressure gradient between portal and systemic circulations. Shunt patency is maintained by placing an expandable metal stent across the intrahepatic tract.
Transjugular intrahepatic portosystemic shunt implantation is an effective and safe treatment for portal hypertension in patients with hepatocellular carcinoma (HCC), according to one study. Portal hypertension and HCC are major complications of advanced liver cirrhosis. Forty HCC patients with portal hypertension who were treated with TIPS were included in the analysis. No severe procedure-related complications and no deterioration of liver function were observed. [3]
Although TIPS is relatively safe, procedural or shunt-related morbidity can reach 20%, and procedural complications have a fatality rate of 2%. Delayed recognition and treatment of TIPS complications can lead to life-threatening clinical scenarios. Complications vary from stent migration or malpositioning to nontarget organ injury, TIPS dysfunction, encephalopathy, or liver failure. [8]
Several pediatric diseases such as extrahepatic portal vein obstruction, biliary atresia, alpha1-antitrypsin deficit, and autoimmune hepatitis can lead to cirrhosis and portal hypertension in children. Interventional radiology procedures can facilitate diagnosis and treatment of diseases associated with liver cirrhosis and portal hypertension in the pediatric population. These procedures include image-guided liver biopsy, mesenteric-intrahepatic left portal vein shunts, balloon-occluded retrograde transvenous obliteration, transjugular intrahepatic portosystemic shunts, and splenic embolization. [9]
In a study of patients from the National Inpatient Sample (Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project) who underwent TIPS, Helzberg et al found that Black patients had significantly higher in-hospital mortality and longer lengths of stay. Non-Hispanic Black patients had a 20% mortality rate, about twice the rate of all other racial/ethnic groups, which ranged from 9 to 10.8%. Black patients were significantly more likely to undergo delayed endoscopy, and non-white patients generally had longer wait times from admission to TIPS. [10]
ALTA recommendations
The Advancing Liver Therapeutic Approaches (ALTA) Consortium, which focuses on management of portal hypertension, have published recommendations on TIPS, including the following [11] :
-
Prior to TIPS creation, recommend that a gastroenterologist or hepatologist should be involved in the initial decision to place an emergent or nonemergent TIPS, with subsequent consultation by an interventional radiologist or other proceduralist with competency in TIPS. If center expertise is not available, we recommend referral to an expert center.
-
Recommend contrast-enhanced multiphasic cross-sectional imaging (CT/MRI) to assist with TIPS planning.
-
Recommend domprehensive echocardiography to assess for abnormalities in cardiac structure, function, and right ventricular systolic pressure.
-
In patients with cirrhosis undergoing emergent TIPS, suggest at least a liver ultrasound with Doppler to evaluate the patency of the portal venous system and consideration of a limited (bedside) echocardiogram, evaluating left ventricular ejection fraction and right ventricular systolic pressure.
-
The absolute contraindications to elective TIPS include severe congestive heart failure, severe untreated valvular heart disease, moderate-to-severe pulmonary hypertension (based on invasive measurements) despite medical optimization, uncontrolled systemic infection, refractory overt HE, unrelieved biliary obstruction, and lesions (eg, cysts) or tumors in the liver parenchyma that preclude TIPS creation.
-
For patients undergoing TIPS placement, recommend the use of an ePTFE lined stent graft with controlled expansion, which allows the operator to tailor the amount of portosystemic shunting based on the indication, target gradient, and patient comorbidities.
-
The use of general anesthesia, deep sedation, or conscious sedation may all be appropriate for TIPS placement, and their use will vary depending on the patient risk factors and local practices.
-
Recommend the use of the free hepatic vein or IVC pressure as the systemic pressure when measuring the portosystemic gradient before and after TIPS placement.
-
In patients undergoing TIPS placement who are potentially eligible for liver transplant, recommend positioning the stent as to not interfere with the portal and hepatic vein anastomoses, presuming that this does not detrimentally affect TIPS function or patency. This positioning includes leaving a segment of unstented main portal vein and not extending the TIPS stent into the right atrium.
-
In all patients undergoing TIPS creation, routine labs (complete blood count, comprehensive metabolic panel, and PT/INR) should be obtained on the day following TIPS creation. Hemoglobin/hematocrit labs may be obtained on the same day of TIPS creation, depending on institution and/or operator discretion.
-
In patients who have undergone TIPS creation for management of varices, either Doppler ultrasound findings suggesting TIPS dysfunction, or persistence or recurrence of portal hypertensive complications, should prompt TIPS venography and manometry +/− intervention. Ultrasound findings suggesting TIPS dysfunction include alterations in intrahepatic portal vein direction of flow, abnormal flow velocities within the TIPS, and persistent (eg, >6 wk post-TIPS) or recurrent ascites.
-
In patients who have undergone TIPS creation for management of ascites and/or hepatic hydrothorax, persistence or recurrence of portal hypertensive complications should prompt TIPS venography and manometry +/− intervention. Medical decision-making should be individualized in patients with well-controlled ascites and/or hepatic hydrothorax and ultrasound findings suggesting TIPS dysfunction.
-
Recommend counseling patients that TIPS is associated with a risk of overt HE in approximately 25–50% of recipients. Patient-specific risk factors for development of post-TIPS overt HE include prior history of overt HE, advanced age, advanced liver dysfunction, hyponatremia, renal dysfunction, and sarcopenia.
History of TIPS
In 1969, Rosch first described the establishment of a percutaneous tract between the portal vein (PV) and the hepatic vein via a jugular approach. [12] He used serial dilators to create a shunt within the liver and advocated the use of silicone tubing across the hepatic parenchyma to create a functional shunt. Further progress was made in 1977, with the work of Reich and coworkers, who used a 9-mm cryoprobe to create a larger parenchymal tract in a swine model. [13] These early pioneers realized that intraparenchymal tracts, either unsupported or supported with a silastic stent, had poor patency and were usually complicated by complete occlusion within a short time.
Colapinto reported the first clinical use of TIPS in a patient with cirrhosis that involved a 12-mm angioplasty balloon to expand the intrahepatic tract. However, this technique also resulted in poor patency. [14] Long-term TIPS patency was not accomplished until metallic stents were introduced in the 1980s. This led to the establishment of TIPS as a percutaneous alternative to use of a surgical portosystemic shunt.
The TIPS procedure has gained worldwide acceptance. Technical refinements have resulted in reduced morbidity and improved clinical success. Advances in stent technology and the introduction of polytetrafluoroethylene-covered stents led to further increases in clinical patency and improved long-term results with TIPS. [4]
The Gore VIATORR TIPS Endoprosthesis has FDA approval for the treatment of portal hypertension and can be used in both de novo and revision procedures. The expanded polytetrafluoroethylene graft lining reduces bile and mucin permeation, thereby improving patency. It also reduces ingrowth of tissue into the graft, which can be advantageous for subsequent liver transplantation. [15, 16]
Indications
The United States National Digestive Diseases Advisory Board has established a set of clinical indications for TIPS placement. [17] These accepted indications are as follows:
-
Acute variceal bleeding that cannot be successfully controlled with medical treatment, including sclerotherapy
-
Recurrent and refractory variceal bleeding or recurrent variceal bleeding in patients who cannot tolerate conventional medical treatment, including sclerotherapy and pharmacologic therapy
Unproven but promising indications include the following:
-
Therapy for refractory ascites [18]
-
Portal decompression in patients with hepatic venous outflow obstruction (Budd-Chiari syndrome), [19] hepatic hydrothorax, or hepatorenal syndrome
Unproven uses include the following:
-
Initial therapy of acute variceal hemorrhage
-
Initial therapy to prevent initial or recurrent variceal hemorrhage
-
Reduction of intraoperative morbidity during liver transplantation
Absolute contraindications include the following:
-
Right-sided heart failure with increased central venous pressure
-
Polycystic liver disease
-
Severe hepatic failure
Relative contraindications include the following:
-
Active intrahepatic or systemic infection (bacteria can colonize the stent, causing persistent infection)
-
Severe hepatic encephalopathy poorly controlled with medical therapy
-
Hypervascular hepatic tumor
-
Portal vein (PV) thrombosis (although PV thrombus may make the procedure more technically demanding, it is not an absolute contraindication to TIPS placement)
Patient Preparation for TIPS Placement
All patients undergoing TIPS placement should receive prophylactic broad-spectrum antibiotics. Appropriate resuscitation with fluid and blood products is indicated prior to the procedure. Portal vein (PV) patency should be confirmed prior to attempted TIPS placement. [20]
At the author's institution, all potential patients for TIPS placement undergo preoperative evaluation with duplex sonography to determine if the PV is patent. If Doppler sonographic findings are inconclusive, arterial portography via the splenic or superior mesenteric arteries can be performed to evaluate PV anatomy and patency. In the presence of reversed intrahepatic portal flow, the PV may not fill during arterial portography, however. Magnetic resonance venography may have a role in assessing PV patency prior to TIPS. An alternative approach in a patient with inconclusive sonograms can be wedged hepatic venography with CO2 from the jugular vein.
Patients with cirrhosis often have coagulopathy, and severe coagulopathy should be addressed before any invasive procedure is undertaken. Platelets are routinely administered when platelet counts are less than 50,000 mm3, and fresh frozen plasma (FFP) is used with an international normalized ratio (INR) greater than 2.0.
TIPS Placement Technique
Most patients can safely undergo TIPS placement with intravenous conscious sedation involving short-acting benzodiazepines and opiates. Some institutions prefer use of general anesthesia during TIPS procedures because of the prolonged nature of the procedure and the degree of discomfort that many patients experience during transvenous punctures. This technique is fairly standard and has been well described in the scientific literature, with some minor variations related to the interventionalist or the center at which the procedure is performed.
Commercially available sets specifically designed for TIPS placement are available in the United States, such as the following:
-
Colapinto transjugular cholangiography liver biopsy set. This includes a 16-gauge Colapinto puncture needle.
-
Rosch-Uchida transjugular liver access set. This includes a 14-gauge needle with a trocar.
-
AngioDynamics transjugular access set.
-
Ring transjugular intrahepatic access set.
At the author's institution, the right internal jugular vein is accessed, usually with ultrasonographic guidance. The left jugular vein may be used if the right vein is unsuitable for any reason. Techniques involving a femoral venous approach have been described, but these are used much less commonly and are technically more demanding. [21, 22, 23]
With standard catheter exchange, a 5F catheter with a multipurpose curve is placed into the right hepatic vein. The catheter is wedged in a peripheral branch of the right hepatic vein. Wedged hepatic venography is then performed with carbon dioxide gas to opacify the portal venous system.
(See the images below.)
 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A curved catheter is placed into the right hepatic vein.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A curved catheter is placed into the right hepatic vein.
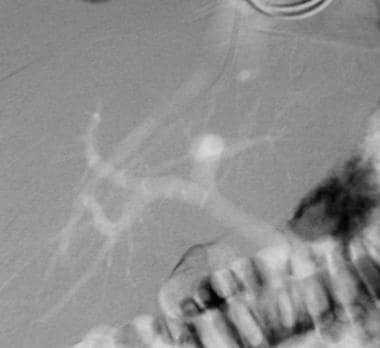 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A wedged hepatic venogram obtained by using the digital subtraction technique obtained with CO2 gas demonstrates the location of the portal vein. The catheter is wedged in a branch of the right hepatic vein.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A wedged hepatic venogram obtained by using the digital subtraction technique obtained with CO2 gas demonstrates the location of the portal vein. The catheter is wedged in a branch of the right hepatic vein.
Typically, a 50-mL manual injection of CO2 is used. This procedure usually reveals the location of the main PV, as well as the locations of left and right branches. Frequently, more than one CO2 injection is required to obtain a good portogram. If wedged injection fails to fill the PV, an occlusion balloon catheter may be used. Biplane CO2 wedged hepatic venograms and biplane fluoroscopy can be used.
With wedged hepatic venogram images used as a guide, the Colapinto needle is advanced through the wall of the right hepatic vein and is directed anteroinferiorly to access the right PV.
(See the image below.)
 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Image demonstrates advancement of a Colapinto needle into the right portal vein.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Image demonstrates advancement of a Colapinto needle into the right portal vein.
Some have advocated injecting CO2 as the 21-gauge fine needle is advanced through the liver to more definitively guide the puncture. Once the portal vein has been cannulated, CO2 is injected into the parenchymal tract to exclude transgression of the bile duct or hepatic artery. If the bile duct has been transgressed, the access may be abandoned; if a small branch has been transgressed, a covered stent such as the Gore VIATORR TIPS Endoprosthesis is placed.
The Gore Viatorr polytetrafluoroethylene-covered stent (in 316 patients) was compared with standard uncovered stents (in 157 patients) for measurement of shunt function and clinical efficacy by Tripathi et al. Investigators found that the Viatorr covered stent had lower rates of shunt insufficiency (8% vs 54%), variceal bleeding (6% vs 11%), and hepatic encephalopathy (22% vs 32%). Mortality rates were similar for the 2 groups. [24] Clark et al concluded that covered stents do not mitigate postshunt hepatic dysfunction and do not improve survival. [25]
If a good wedged venogram cannot be obtained, an appropriate site for puncture from the hepatic vein to the PV is selected based on a thorough understanding of hepatic anatomy. The right hepatic vein lies superior and posterior to the PV bifurcation, and the right PV usually courses lateral to the T11 vertebral body. The portal bifurcation may be extrahepatic in a large percentage of patients. An extrahepatic puncture can lead to life-threatening hemorrhage, whereas puncture of a peripheral portal venous branch can create an angle that is too acute for successful TIPS placement.
From the right hepatic vein, the needle is usually aimed anteromedially and caudally, then is advanced 3-4 cm within the liver. Several punctures may be necessary for success. Large-volume paracentesis before TIPS in a patient with massive ascites can facilitate cannulation of the hepatic vein and puncture of the PV.
The needle is gently aspirated as it is withdrawn across the parenchymal tract. Once portal venous blood is freely aspirated, contrast material is injected through the needle to verify the point of entry into the vessel. An intrahepatic access site with entry into the right PV at least 1 cm from the main PV bifurcation is desired. A guidewire and a catheter are advanced into the PV, and portal venography is performed.
Pressure measurements are obtained in the PV as well as in the right atrium. The difference between measured pressures yields the portosystemic gradient. If the pressure gradient is significantly elevated (>12 mm Hg), the TIPS is placed. If the gradient is not elevated, the presence of a competitive shunt such as a spontaneous splenorenal shunt must be evaluated. Spontaneous shunts can be used to lower the portosystemic gradient, but they are not true vessels and lack normal vascular integrity, which poses a risk for rupture.
The intrahepatic parenchymal tract is dilated with an 8- or 10-mm high-pressure balloon such as the Conquest high-pressure balloon. Images of the initial balloon waist are saved because they show the locations of the PV entry site and the hepatic vein exit site. A self-expanding metallic stent such as the Wallstent is then deployed across the tract and is dilated to the desired diameter with use of an angioplastic balloon.
(See the image below.)
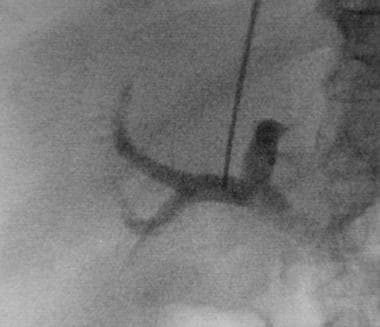
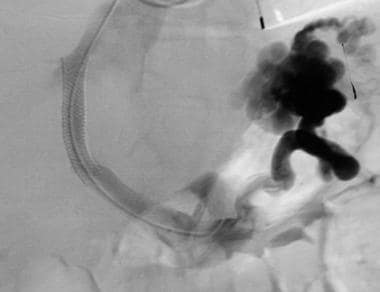 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A TIPS (10 X 68 mm Wallstent dilated with 10 mm X 4 cm balloon) has been placed. Note flow through the Wallstent and filling of the splenorenal shunt. The intrahepatic portal flow became reversed after TIPS placement.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A TIPS (10 X 68 mm Wallstent dilated with 10 mm X 4 cm balloon) has been placed. Note flow through the Wallstent and filling of the splenorenal shunt. The intrahepatic portal flow became reversed after TIPS placement.
Proper stent placement is crucial, especially if the patient is undergoing subsequent orthotopic liver transplantation. The stent should form a smooth and gentle curve and must extend adequately into the portal and hepatic veins. Overextension of a stent into the PV, the hepatic vein, or the inferior vena cava can be problematic for the transplant surgeon. The Wallstent significantly shortens as it is deployed, and ability of the surgeon to predict and achieve appropriate final stent placement improves with experience. Self-expanding nitinol stents that minimally shorten with deployment are available from multiple manufacturers, and one of these may be used to attain more predictable stent placement.
The stent may have to be dilated 8-12 mm, depending on the indication for TIPS and the stent used. Postplacement venography is performed, and pressures are measured to confirm adequate stent positioning, good flow through the TIPS, and reduction in the portosystemic gradient. If needed, additional stent placement or further balloon dilation can be performed to obtain a final portosystemic gradient of 8-12 mm Hg. Postplacement venography revealing persistent variceal filling indicates that insufficient decompression has been achieved with the stent. These varices can be selectively catheterized and embolized with coils.
Complications and Advanced Techniques
Complications
Major complications associated with TIPS can result in additional therapy, prolonged hospitalization, permanent adverse sequelae, or even death. Intracardiac echocardiography (ICE) has been shown to decrease needle passes during TIPS, thereby decreasing complication rates in high-risk patients undergoing TIPS. [26]
A large number of complications can occur during and after placement of a TIPS. [27, 28] Complications related to the puncture site include pneumothorax, vessel or tissue injury, and arteriovenous fistula formation. Ultrasonographic guidance for the jugular puncture can minimize these complications. Placement of the catheter in the right atrium can cause serious cardiac dysrhythmias. Cardiac monitoring should be continued throughout the TIPS procedure and during the initial postprocedural period.
Complications can occur during creation of the intrahepatic tract. The needle can injure the hepatic artery or bile ducts. Capsular tears can result in life-threatening hemorrhage when they occur in association with a hepatic artery puncture. Portal venous puncture in an extrahepatic location can result in significant bleeding complications. Puncture of the right PV at least 1-2 cm distal to the PV bifurcation more often than not ensures that an intrahepatic TIPS has been created.
A major concern for a newly placed TIPS is new-onset or worsened encephalopathy, which occurs in about 25% of treated patients. [29, 30] Patients with preprocedural hepatic encephalopathy (HE) or Child Class C cirrhosis are more likely to have this complication. Shunt diameter and degree of portosystemic gradient reduction are related to the development of encephalopathy. Most often, HE can be medically treated with lactulose and dietary protein restriction, although shunt revision to a smaller diameter or intentional shunt thrombosis may be necessary. Patients often have HE immediately after shunt placement, and symptoms diminish as the shunt undergoes fibrous changes and resultant narrowing over time.
In a retrospective case analysis of 136 patients post TIPS by Masson et al, HE developed in 34.5% of patients and frequencies were similar with covered and uncovered stents. The most significant predictive factor was the presence of pre-TIPS HE. Minimal encephalopathy occurred in 49% of patients at 26-month follow-up; 10.3% of patients developed post-TIPS encephalopathy that required liver transplantation or contributed to death. Study authors concluded that although post-TIPS HE is rather common, it is usually short-lived and well managed if patients are carefully selected for the procedure. [31]
The second most common complication associated with TIPS placement is shunt stenosis and occlusion. Early shunt thrombosis (often within 24 hr) is usually believed to be secondary to extension of the intrahepatic tract across a bile duct. Such early shunt occlusions can be treated with balloon dilation of the stent. Use of covered (polytetrafluoroethylene [PTFE], polyester) stents to improve primary and secondary shunt patency may prove helpful.
Deterioration of the patient's hemodynamic status is another concern after TIPS placement because acute increases in cardiac output and in central venous and pulmonary wedge pressures can result in acute pulmonary edema and congestive heart failure. [32] Patients should be closely monitored after the procedure until their hemodynamic status is stabilized. [33]
In a study, by Bettinger et al, of morbidity and mortality associated with TIPS placement in 389 patients, procedure-related complications occurred in 42 patients (10.8%), with intraperitoneal bleeding in 8 patients (2.1%) and infection in 14 patients (3.6%). Shunt- and disease-related complications consisted of HE (1-year incidence, 29%), nonprocedural infection (8.7%), and acute hepatic decompensation (4.1%). Nine patients (2.3%) died during the index hospital stay from procedure-related causes (2 patients, 0.5%), shunt-related causes (4 patients, 1%), or disease-related causes (3 patients, 0.8%). A total of 23 patients (5.9%) died within 4 weeks after TIPS implantation. The 1-year probability of survival was 67.7% and was negatively associated with severe HE and acute hepatic decompensation. [34]
In a discharge-weighted national estimate of 83,884 TIPS procedures performed in the United States, 12.3% of patients died during hospitalization. Number of diagnoses and number of procedures showed positive correlations with in-hospital death. Patients with diagnosed acute respiratory failure, acute kidney failure, hepatic encephalopathy, or esophageal variceal bleeding were found to be at considerably higher risk for in-hospital death. Comorbidity measures with highest risk for in-hospital death were fluid and electrolyte disorders, coagulopathy, and lymphoma. [35]
Advanced TIPS techniques
Various techniques to facilitate portal venous access, including ultrasound guidance, have been described. If standard portal venous access fails, the author prefers to percutaneously place a transhepatic Chiba needle, which can be used as a target under fluoroscopy to advance the Colapinto needle. This approach adds an extra step to the procedure, and it is usually not appropriate in patients with massive perihepatic ascites. Alternatively, puncture of a patent umbilical vein can provide access into the portal system. An approach for creating a TIPS by using a combination of transfemoral access to the hepatic vein with transmesenteric access to the portal system via minilaparotomy has been described. [36]
If direct PV and right atrial pressures (measured to determine the portosystemic gradient) show a less-than-expected gradient, a competitive shunt (spontaneous splenorenal or large varices) should be excluded by advancing the catheter into the splenic or mesenteric vein and injecting a bolus of contrast agent with digital imaging.
Competitive shunts can be selectively embolized with coils, if necessary. Although these shunts may decrease the portosystemic gradient, they are thin walled and can spontaneously rupture, causing life-threatening hemorrhage. If no competitive shunt is found, and if the gradient is less than 12 mm Hg, TIPS is not indicated, and alternative explanations for the bleeding should be sought. Upper GI bleeding may be related to a cause other than portal hypertension in as many as 25% of cases.
(See the image below.)
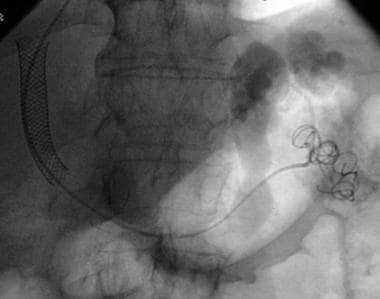 Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Coil embolization of the splenorenal shunt has been performed.
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Coil embolization of the splenorenal shunt has been performed.
An 8-mm-diameter stent is usually acceptable for patients with refractory ascites, whereas a 10-12-mm shunt may be needed for patients with life-threatening hemorrhage. Wallstents can be overdilated to about 10% larger than their nominal diameter to allow further gradient reduction. Nitinol stents cannot be overdilated in this way. In some cases, 2 parallel shunts are required to effectively lower the portosystemic gradient. Most commonly, TIPS results in complete reversal of intrahepatic PV flow and markedly reduces variceal blood flow.
Alternative techniques have been proposed that may increase success rates in cases of challenging anatomy or when resources are limited. Lukies et al found that all modified gun-sight TIPS procedures were technically successful with a single needle pass, with patency and appropriate hemodynamic flow reported at 2 weeks. Advantages of this technique included fluoroscopically guided transhepatic puncture typically using on-shelf, low-cost equipment, without the need for a dedicated TIPS set or endovascular ultrasound. Disadvantages included liver capsular puncture. [37]
Yan et al examined the safety and efficacy of microwave ablation for hepatocellular carcinoma (HCC) in the setting of TIPS. They analyzed 5 patients with TIPS who underwent percutaneous microwave ablation for HCC and noted no major adverse events. TIPS patency post ablation was 100%, as was the technical success rate and the complete response rate. [38]
Zhou et al noted that that liver toxicities and antiangiogenic effects induced by molecular targeted drugs can generate an imbalance in ammonia metabolism, elevating blood ammonia levels. TIPS may divert a partial blood supply from the liver, aggravating liver impairment and shunting ammonia-rich blood from the intestine into the systemic circulation. The authors recommend that when clinicians choose molecular targeted therapy as the second or third targeted therapy for patients who have undergone TIPS, the consequence of drug-induced HE should be considered. [39]
Clinical Results and Imaging Follow-Up
Clinical results
The technical success of TIPS placement is related to the experience and skill of the interventional radiologist. Data from 3 large centers (University of California–San Francisco, University of Pennsylvania, and Freiberg group) reveal technical success rates greater than 90%. Successful TIPS placement results in a portosystemic gradient less than 12 mm Hg and immediate control of variceal-related bleeding. [40, 41, 42] A target portosystemic gradient of 12 mm Hg is used, as varices tend not to bleed when the gradient is less than 12 mm Hg. When technical failure occurs, this is usually due to an anatomic situation that prevents acceptable portal venous puncture. Significant reduction in ascites usually occurs within 1 month of the procedure in an estimated 50-90% of cases. [18, 21, 43, 44]
Late stenosis and occlusion are usually related to pseudointimal hyperplasia (see the image below) within the stent or, more commonly, to intimal hyperplasia within the hepatic vein. In most cases, the stenotic stent can be crossed with a guidewire and recanalized with balloon dilation or repeat stent placement to improve long-term patency rates. Primary patencies after TIPS placement of 66% and 42% after 1 and 2 years have been reported. Primary-assisted patencies of 83% and 79% have been reported at 1 and 2 years, respectively, as have secondary patency rates of 96% and 90%. [21]
Mortality rates at 30 days vary among centers, and nearly all centers report few or no deaths directly related to the procedure itself. Early mortality has been shown to be related to an Acute Physiology and Chronic Health Evaluation (APACHE) score of II. Patients with severe systemic disease with an APACHE II score higher than 20 are at greater risk for early mortality. [45]
Patients with active bleeding during the procedure have increased early mortality. Thirty-day mortality rates of 3-30% have been reported; variation in this range is related to which preprocedural Child classification was assigned and whether the procedure was performed on an emergent or elective basis. [22, 46, 47, 48] LaBerge et al reported that cumulative survival rates in patients with Child grades A, B, and C, respectively, were 75%, 68%, and 49% at 1 year and were 75%, 55%, and 43% at 2 years.
Clinical imaging follow-up
The high frequency of shunt stenosis warrants close surveillance with Doppler ultrasonography or portography. Doppler ultrasonography is the primary imaging modality used for TIPS patency screening. [20] At the author's institution, patients undergo a baseline Doppler study within 24 hours of the procedure to document functional parameters, including the direction of PV flow and flow velocities throughout the shunt and within the hepatic vein. Although TIPS venography with direct portal and right atrial pressure measurements is the criterion standard for stent assessment, high sensitivity and specificity for shunt function have been reported with certain Doppler criteria. [49, 50]
-
Absent flow
-
Low peak shunt velocity (< 50 to 90 cm/s)
-
High peak shunt velocity (190 cm/s)
-
Low mean PV velocity (< 30 cm/s)
-
Return of antegrade flow in intrahepatic PVs
-
Significant change in shunt velocity (>50 cm/s) compared with the immediate postprocedural result
Surveillance ultrasonography is performed at the author's institution at 3 and 6 months after the procedure, and twice yearly thereafter. If clinical evidence of TIPS malfunction is evident, ultrasonography may be performed more frequently.
Pinter et al found that volume flow measurement using 3D/4D Doppler ultrasonography provides a potential alternative to standard pulsed wave Doppler metrics for evaluating shunt patency and for identifying cases requiring revision. [20]
According to Guo et al, linear correlation between spleen viscoelasticity and hepatic venous pressure gradient (HVPG) raises the prospect of assessment of portal pressure by magnetic resonance elastography after TIPS placement. [44]
Four-dimensional–flow magnetic resonance (MR) imaging has been deemed feasible for noninvasive longitudinal hemodynamic monitoring of hepatic blood flow before and after TIPS placement. [51]
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A curved catheter is placed into the right hepatic vein.
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A wedged hepatic venogram obtained by using the digital subtraction technique obtained with CO2 gas demonstrates the location of the portal vein. The catheter is wedged in a branch of the right hepatic vein.
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Image demonstrates advancement of a Colapinto needle into the right portal vein.
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Portal venogram obtained with a pigtail catheter shows filling of the coronary vein.
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Delayed venogram demonstrates filling of large varices.
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. A TIPS (10 X 68 mm Wallstent dilated with 10 mm X 4 cm balloon) has been placed. Note flow through the Wallstent and filling of the splenorenal shunt. The intrahepatic portal flow became reversed after TIPS placement.
-
Basic transjugular intrahepatic portosystemic shunt (TIPS) procedure. Coil embolization of the splenorenal shunt has been performed.
-
Early transjugular intrahepatic portosystemic shunt (TIPS) thrombosis. Image obtained after placement of the initial TIPS shows good flow through the shunt.
-
Early transjugular intrahepatic portosystemic shunt (TIPS) thrombosis. Sonogram obtained the day after shunt placement demonstrates thrombosis. The catheter was placed through the thrombosed TIPS without any difficulty. Note the absence of flow through the shunt and the hepatopetal portal flow.
-
Early transjugular intrahepatic portosystemic shunt (TIPS) thrombosis. After recanalization of the shunt, a Wallgraft is placed within it.
-
Early transjugular intrahepatic portosystemic shunt (TIPS) thrombosis. Good flow is restored through the TIPS after placement of the Wallgraft.
-
A parallel transjugular intrahepatic portosystemic shunt (TIPS) required in this patient to effectively decrease the portosystemic gradient.
-
Hyperplasia within a transjugular intrahepatic portosystemic shunt (TIPS) several months after placement.
-
Balloon angioplasty used to treat hyperplasia.
-
Final appearance of the transjugular intrahepatic portosystemic shunt (TIPS) after balloon dilation (see also Image 14).