Practice Essentials
Embolization is defined as the "therapeutic introduction of various substances into the circulation to occlude vessels, either to arrest or prevent hemorrhaging; to devitalize a structure, tumor, or organ by occluding its blood supply; or to reduce blood flow to an arteriovenous malformation." [1] Venous malformations are the most common vascular malformation and are generally found in the head and neck area or in the extremities. Accurate diagnosis is necessary to establish cause and determine treatment. The International Society for the Study of Vascular Anomalies has divided vascular malformations into simple and combined types. The simple types include capillary, venous, and lymphatic malformations. Combined types include arteriovenous malformation and arteriovenous fistula. Doppler ultrasound can identify low flow of venous malformations, and MRI can identify lesion margins and invasion into other structures. [2, 3]
Embolization, or embolotherapy, is performed by radiologists who have completed advanced postresidency training (fellowship) in interventional radiology (see the images below). [4, 5, 6, 2, 7, 8]
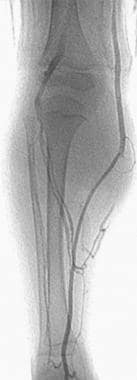 A lower-extremity venogram (digital subtraction angiogram) in a patient with a venous malformation in the upper calf. The deep veins are patent but appear to be displaced by the vascular malformation.
A lower-extremity venogram (digital subtraction angiogram) in a patient with a venous malformation in the upper calf. The deep veins are patent but appear to be displaced by the vascular malformation.
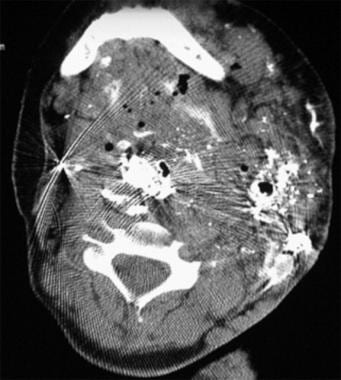 Axial CT image through the upper part of the neck after percutaneous cyanoacrylate embolization. The patient was a young girl with an extensive head and neck venous malformation resulting in significant airway obstruction, which required a tracheostomy.
Axial CT image through the upper part of the neck after percutaneous cyanoacrylate embolization. The patient was a young girl with an extensive head and neck venous malformation resulting in significant airway obstruction, which required a tracheostomy.
Embolization may have 3 therapeutic goals:
An adjunctive goal (eg, preoperative, adjunct to chemotherapy or radiation therapy)
A curative goal (eg, definitive treatment such as that performed in cases of aneurysms, arteriovenous fistulae [AVFs], arteriovenous malformations [AVMs], and traumatic bleeding)
A palliative goal (eg, relieving symptoms, such as those of a large AVM, which cannot be cured by using embolotherapy alone)
Medical conditions treated by using embolotherapy can be grouped as follows:
Vascular anomalies (eg, AVM, AVF, venous malformation [VM], lymphatic malformation [LM], and hemangioma)
Hemorrhage (eg, pseudoaneurysms and GI tract, pelvic, posttraumatic, epistaxis, and hemoptysis bleeding)
Other conditions (eg, tumors, varicoceles, and organ ablation)
Embolization Materials And Substances
Materials used in embolization include coils, ethanol, sodium tetradecyl sulfate, cyanoacrylate, polyvinyl alcohol (PVA), microspheres, and gelatin sponge (Gelfoam), among others. [9, 10, 11, 12, 13]
Coils
Coils can be grouped into microcoils and macrocoils. Macrocoils, also called Gianturco coils, were first introduced in 1975. Coils have the advantage of being precisely positioned under fluoroscopic control. Occlusion occurs as a result of coil-induced thrombosis rather than mechanical occlusion of the lumen by the coil. To increase the thrombogenic effect, Dacron wool tails are attached to coils. The coils are available in many sizes and may be delivered through commonly used angiographic catheters (4-5F). [14]
Microcoils (platinum coils) can be delivered through microcatheters (2.2-3.5F). They can be particularly useful when superselective coil embolization is required. Microcoils are highly thrombogenic, radiopaque, and biocompatible. Again, the thrombogenic effect primarily results from the addition of silk or synthetic fibers, not from the coil. [15, 16]
Collateralization is a potential disadvantage of coil embolization, and it can result in the persistence of flow into the vascular territory of the vessel that was embolized with the coil. Additionally, when proximal occlusion occurs with coil embolization, repeat intervention via the same artery becomes difficult, if not impossible. Mechanically and electronically detachable coils are currently available.
Ethanol
Ethanol (absolute alcohol) is the most commonly used liquid agent. Embolization with absolute alcohol has a direct toxic effect on the endothelium that activates the coagulation system and causes the microaggregation of red blood cells.
In the treatment of vascular malformations, ethanol has demonstrated its curative potential compared with the palliative effect seen with other embolic agents. Occlusion of the lumen occurs within minutes or days. Ethanol can be damaging if it reaches the capillary bed of any given tissue (eg, skin), and it usually causes significant soft-tissue swelling, which may subsequently cause compartment syndrome (nerve compression).
When absolute alcohol is mixed with a contrast medium and when small catheters are used, superselective vascular embolization can be safely performed under fluoroscopic guidance. Ethiodized oil (Ethiodol), an oily contrast medium, is used most commonly.
If large amounts of absolute alcohol enter the systemic circulation, toxic effects can occur. These include central nervous system depression, hemolysis, and cardiac arrest. Slow, careful injections by using balloon occlusion arterial catheters for delivery and by applying manual compression on the draining veins (or tourniquet control) or balloon occlusion of the draining system may decrease alcohol washout from the lesion and reduce acute systemic toxicity. Ethanol 1 mg/kg is the maximum amount that can be injected during a single session.
Sodium tetradecyl sulfate
Sodium tetradecyl sulfate (Sotradecol) is another sclerosant. This contains 2% benzyl alcohol and is commonly used for VMs and varices. Use of this agent is less painful for the patient, and it is considered to be less toxic then absolute alcohol. Therefore, some lesions can be treated without general anesthesia.
Sodium tetradecyl sulfate can be used as a sclerosant in various concentrations (1-3%); however, manufacture of this agent has been discontinued in the United States. This author has begun using ethanolamine oleate (Ethamolin) instead of sodium tetradecyl, with the same indications.
Cyanoacrylate
Cyanoacrylate, or N-butyl-2-cyanoacrylate (NBCA), is a rapidly hardening liquid adhesive often referred to as glue. The substance hardens (polymerizes) immediately on contact with blood or other ionic fluid. Polymerization results in an exothermic reaction that destroys the vessel wall. [4, 17, 18] Cyanoacrylate glues form flexible polymers with strong adhesive bonds to soft tissues. They are combined with either tantalum powder or ethiodized oil, which prolongs polymerization time, opacifies the liquid agent, and allows visualization under fluoroscopy. [18]
Penetration of the capillary bed causes severe tissue injury. Because of the rapid polymerization, coaxial catheterization, precise positioning of the delivery catheter, and considerable skill are required for NBCA embolization. When a suitable location is reached by using a microcatheter, the catheter is flushed with 5% dextrose to clear it of any blood or contrast medium.
Under real-time fluoroscopic control, a mixture of NBCA and oily contrast medium is delivered. As soon as a cast of the vascular tree is seen fluoroscopically, the delivery microcatheter is quickly removed so that the catheter tip does not adhere to the vessel. Again, the catheter is flushed quickly with 50% dextrose so that it can be reused during the same procedure.
A foreign-body inflammatory reaction is the primary disadvantage of the use of this embolic material.
Cyanoacrylate glue deposition can be unpredictable, with complications including nontarget embolization, venous migration, microcatheter blockage, and catheter retention. [18]
Polyvinyl alcohol
PVA is obtained by the reticulation of PVA (Ivalon) with formaldehyde. PVA is available as particles with a large range of sizes. For sizes as large as 710 μm, a microcatheter can be used as a delivery catheter.
Successful PVA-particle embolization depends on the formation of a thrombus in which a large proportion of the embolized vessel is filled with thrombus rather than PVA particles. Histologically, this agent causes intraluminal thrombosis associated with an inflammatory reaction, with subsequent organization of the thrombus. PVA is considered a permanent embolic agent because of the low frequency of recanalization of the embolized vessels. PVA is not absorbable, and it likely produces permanent occlusion.
PVA is usually administered in a mixture of contrast medium and isotonic sodium chloride solution under fluoroscopic guidance. Aggregation of PVA particles can be minimized by using dilute contrast medium in a matched-density suspension; for example, Omnipaque and sodium chloride solution can be used in a ratio of 1:0.4 for contour particle suspension. PVA particles have a tendency to aggregate within the vessel once administered, potentially leading to an occlusion that is more proximal than intended. Diluted mixtures advance more distally, whereas concentrated mixtures cause more proximal occlusions.
Tris-acryl gelatin microspheres
Microspheres (Embosphere) are biocompatible, hydrophilic, nonresorbable, and precisely calibrated particles produced from an acrylic polymer and impregnated with porcine gelatin. Microspheres are available in sizes of 40-1200 µm, and they are supplied in apyrogenic sterile sodium chloride solution.
To provide the desired clinical outcome, appropriately sized microspheres and delivery catheters must be chosen to best match the size of the target vessel. For example, when AVMs are being embolized, choose a particle size that occludes the nidus without passing into the systemic circulation. These particles typically do not aggregate, and this is a distinct advantage of microspheres compared with PVA particles.
Microspheres can tolerate temporary compression of 20-30% to facilitate their passage through the delivery catheter. When a coaxial technique is used, a 2.5-3.0F microcatheter allows the passage of microspheres as large as 700 µm for embolization.
Because microspheres are not radiopaque, contrast enhancement must be used to monitor embolization under fluoroscopic guidance. Microspheres are considered permanent embolic particles.
Gelfoam
Gelfoam is a sterile gelatin sponge intended for application to bleeding surfaces for hemostasis or for use as a temporary intravascular embolic material. It is a water-insoluble, off-white, nonelastic, porous, and pliable material. Gelfoam may be cut without fraying, and it can absorb and hold many times its weight in blood and other fluids.
Gelfoam is usually absorbed completely (depending on the amount used, degree of saturation with blood, and site at which it is used), with little tissue reaction. When used as an embolic material, the vessel recanalizes within a few weeks. Gelfoam is supplied in a sterile envelope enclosed in an outer peelable envelope. It is available in sizes from 12 mm to 6 cm.
Other materials
Other less commonly or previously used materials include balloons, microfibrillar collagen (Avitene), autologous materials, ethylene vinyl alcohol, alginates, phosphoryl choline, sodium morrhuate, hot contrast material, and 50% dextrose.
Vascular Anomalies
Vascular anomalies are grouped into 2 categories: hemangiomas and vascular malformations. Vascular malformations are categorized further as high-flow lesions (AVM, AVF), low-flow lesions (capillary malformation, VM, LM), or combined vascular malformations. Embolotherapy with a variety of embolic materials is commonly used in the treatment of vascular anomalies.
Hemangioma
Hemangiomas are benign tumors that require no treatment in most patients. In rare patients, embolization may be necessary (particularly in patients in whom therapy is needed urgently) because of spontaneous hemorrhage or functional abnormality caused by the extreme size of the lesion or the particular anatomic location or because of significant congestive heart failure. In addition, embolotherapy is considered useful prior to surgical resection in select patients and in patients in whom a hemangioendothelioma causes Kasabach-Merritt phenomenon (platelet trapping). Embolotherapy of a hemangioma or hemangioendothelioma can be performed with the use of particles. [19]
The goal of embolotherapy is to block a large percentage of the tumor vessels, thereby preventing further trapping and destruction of the platelets and hastening involution of the lesion. Some tumors may require several sessions of embolotherapy because of revascularization of the tumor. Infantile hepatic hemangioendotheliomas, a variant of infantile hemangioma, usually are multiple and frequently are complicated by congestive heart failure. In the clinical setting, the goal of embolotherapy is to reduce hepatic arterial flow, which sufficiently relieves high-output cardiac failure. A variety of embolic materials have been used. Coils are an infrequently used embolic agent for hemangioma. In some patients, embolization of other nearby arteries (eg, intercostals) may also be necessary.
Arterial portography should be performed to exclude the possibility of feeders from the portal system. A rare entity called noninvoluting hemangioma, which is found in the adult population, may also respond to transluminal embolization (usually with particles such as PVA or microspheres). This procedure is performed mostly to improve the cosmetic appearance.
CT-guided radiofrequency ablation combined with transcatheter arterial embolization has been found to be successful in treating large hepatic hemangiomas (≥10 cm). Mean diameter of the hemangiomas decreased from 13.0 ± 2.2 to 7.1 ± 2.0 cm after embolization, and out of 15 hepatic hemangiomas, 14 (93.3%) showed no enhancement on CT or MRI, indicating complete ablation. [20]
Arteriovenous malformation
AVMs are typically characterized by a nidus of abnormal vessels in which shunting of arterial blood to veins occurs. These vascular anomalies are usually present during childhood but often demonstrate a sudden increase in size in response to trauma, hormones, or other stimuli. Although a clinical grading system has been used among surgeons, no grading system has been developed for imaging. Most AVMs can be managed by transcatheter embolization and/or sclerotherapy. [21] Some focal AVMs can be treated by surgical excision. Proximal embolization or ligation of the feeding arteries usually worsens matters because of subsequent recruitment of collaterals, and transluminal embolization of the nidus cannot be performed afterward. The collaterals that develop are more problematic in terms of transcatheter treatment; however, embolization of the feeding arteries may be indicated prior to surgical excision. [22, 23, 24, 25]
Whenever possible during primary embolotherapy, the nidus should be embolized. In the author's practice, absolute alcohol is most commonly used, and it is the most effective agent in the treatment of AVMs. If the nidus cannot be reached through the feeding arteries, direct percutaneous cannulation of the nidus can be attempted. In selected patients with AVMs, another therapeutic approach is embolic occlusion of the venous outflow.
AVM flow has been shown to decrease substantially after embolization, primarily relating to the total number of pedicles embolized. One study showed that after a single embolization, AVM flow decreased by an average of 29%, but that after all embolizations were completed, flow decreased by 75%. The total number of pedicles embolized following all sessions predicted final flow, rather than the number of pedicles embolized per session, probably because of flow redistribution after partial embolization.` [26]
Cervicofacial AVMs
For dental arcade AVMs, spontaneous or catastrophic hemorrhage during tooth extraction is a common presentation. Embolization of the AVM in this region requires superselective catheterization of the involved branches of the external carotid artery and other regional arterial branches (eg, thyrocervical trunk) using microcatheters and the coaxial technique. [5, 27]
Embolotherapy can be performed with NBCA, alcohol, particles, and/or microcoils, depending on the nature and extent of the malformation. Gelfoam can also be used for preoperative embolization. Patients with dental AVMs associated with acute bleeding and loose teeth should undergo embolization immediately prior to tooth extraction. Possible complications of embolotherapy of cervicofacial AVMs include stroke, nerve paralysis, skin necrosis, infection, blindness, and pulmonary embolism.
Extremity AVMs
In the extremities, AVMs can be diffuse and may involve the entire extremity (Parkes-Weber syndrome). Extremity AVMs typically present with extremity-length discrepancy, high cardiac output, pain, and ulceration. Extremity lesions can be treated with multiple embolizations and/or surgical resections or amputation of the extremity. Incidentally, some small extremity AVMs can be identified in the elderly population and usually are of uncertain etiology and clinical significance (see the images below).
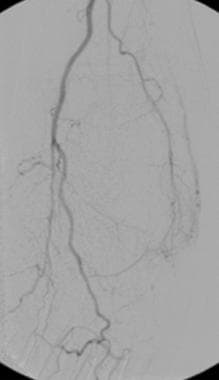 Digital subtraction angiogram shows a small arteriovenous malformation in the lateral aspect of the foot. A small feeder vessel is seen arising from the widely patent posterior tibial artery. An early draining vein is demonstrated clearly. Delayed opacification of the dorsalis pedis artery (not seen) is secondary to proximal anterior tibial arterial disease. These small arteriovenous malformations are occasionally encountered during arteriography in the extremities of elderly patients. The importance of the lesions is unclear in this patient population. If a similar lesion were encountered in a young child, it would be a more significant finding, one possibly requiring treatment.
Digital subtraction angiogram shows a small arteriovenous malformation in the lateral aspect of the foot. A small feeder vessel is seen arising from the widely patent posterior tibial artery. An early draining vein is demonstrated clearly. Delayed opacification of the dorsalis pedis artery (not seen) is secondary to proximal anterior tibial arterial disease. These small arteriovenous malformations are occasionally encountered during arteriography in the extremities of elderly patients. The importance of the lesions is unclear in this patient population. If a similar lesion were encountered in a young child, it would be a more significant finding, one possibly requiring treatment.
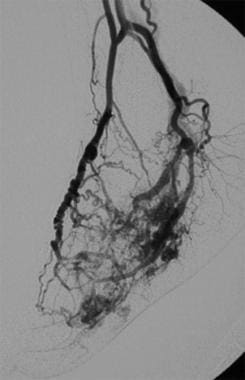 Arteriovenous malformation in the foot. This is an example of an extensive form of arteriovenous malformation. In most patients, the lesions require aggressive treatment by means of surgical excision, transcatheter embolization, or both. The feeders arise from large-caliber foot arteries, and obvious arteriovenous connections (nidus) are seen. Most of these are centered in the midfoot.
Arteriovenous malformation in the foot. This is an example of an extensive form of arteriovenous malformation. In most patients, the lesions require aggressive treatment by means of surgical excision, transcatheter embolization, or both. The feeders arise from large-caliber foot arteries, and obvious arteriovenous connections (nidus) are seen. Most of these are centered in the midfoot.
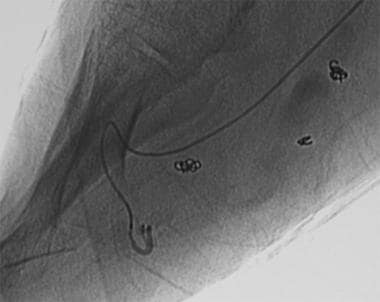 Transluminal embolization using a microcatheter. These lesions usually require embolotherapy with precise embolization techniques. Small feeders are catheterized by using a microcatheter and embolized with embolic material (eg, alcohol) under continuous fluoroscopic control. The tip of the microcatheter is in a tiny plantar feeding artery on this image.
Transluminal embolization using a microcatheter. These lesions usually require embolotherapy with precise embolization techniques. Small feeders are catheterized by using a microcatheter and embolized with embolic material (eg, alcohol) under continuous fluoroscopic control. The tip of the microcatheter is in a tiny plantar feeding artery on this image.
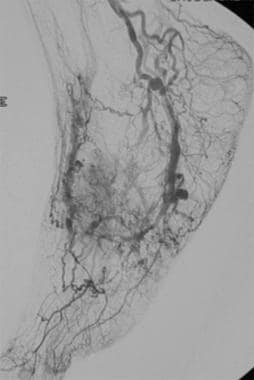 Postembolization arteriogram. After successful embolization, significant improvement is seen because most of the arteriovenous connections have disappeared.
Postembolization arteriogram. After successful embolization, significant improvement is seen because most of the arteriovenous connections have disappeared.
A detailed preembolization angiographic examination is important for mapping the feeders and draining veins. During the injection of contrast material, the injection rate and duration should be adjusted so that arteriovenous connections can be identified accurately. A high injection rate in a short period is appropriate for AVMs.
Selective catheterization with microcatheters is required to reach the nidus of the AVM (see the images below).
 Arteriovenous malformation in the foot. This is an example of an extensive form of arteriovenous malformation. In most patients, the lesions require aggressive treatment by means of surgical excision, transcatheter embolization, or both. The feeders arise from large-caliber foot arteries, and obvious arteriovenous connections (nidus) are seen. Most of these are centered in the midfoot.
Arteriovenous malformation in the foot. This is an example of an extensive form of arteriovenous malformation. In most patients, the lesions require aggressive treatment by means of surgical excision, transcatheter embolization, or both. The feeders arise from large-caliber foot arteries, and obvious arteriovenous connections (nidus) are seen. Most of these are centered in the midfoot.
 Transluminal embolization using a microcatheter. These lesions usually require embolotherapy with precise embolization techniques. Small feeders are catheterized by using a microcatheter and embolized with embolic material (eg, alcohol) under continuous fluoroscopic control. The tip of the microcatheter is in a tiny plantar feeding artery on this image.
Transluminal embolization using a microcatheter. These lesions usually require embolotherapy with precise embolization techniques. Small feeders are catheterized by using a microcatheter and embolized with embolic material (eg, alcohol) under continuous fluoroscopic control. The tip of the microcatheter is in a tiny plantar feeding artery on this image.
 Postembolization arteriogram. After successful embolization, significant improvement is seen because most of the arteriovenous connections have disappeared.
Postembolization arteriogram. After successful embolization, significant improvement is seen because most of the arteriovenous connections have disappeared.
Significant complications of embolotherapy possibly include skin necrosis (blisters), nontarget embolization (which also includes pulmonary emboli), and systemic sclerosant toxicity if a liquid agent (eg, alcohol) is used.
Pulmonary AVMs
Pulmonary AVMs also are called pulmonary AVFs. They have a high association with Osler-Weber-Rendu syndrome (also called hereditary hemorrhagic telangiectasia syndrome). Symptoms may include dyspnea, cyanosis, and clubbing. Paradoxic embolization may cause stroke or brain abscess. This anomaly can be classified as simple or complex on the basis of the number of feeding and draining veins. In simple lesions, a single artery and vein are involved; in complex lesions, 2 or more supplying arteries and 1 or more draining veins are involved.
Most pulmonary AVMs (80%) are simple. Although a surgical approach (thoracotomy and resection) is the traditional mode of therapy, transcatheter embolization is currently a preferred alternative. Transcatheter embolization offers significantly reduced morbidity and mortality rates, particularly in hereditary hemorrhagic telangiectasia syndrome.
Possible complications of embolotherapy include nontarget embolization in the systemic circulation (through the AVM shunt) or in other noninvolved pulmonary arteries. Therefore, properly sizing the coil to the feeding (afferent) artery is essential. A preliminary detailed angiography is essential for mapping the feeders and draining veins, paying particular attention to the size of the feeders. Basically, a successful embolization is accomplished by nesting 1 or more coils in the feeding artery, which occludes flow through the AVM shunt.
Generally, feeding arteries larger than 3 mm should be embolized. Feeders smaller than 3 mm have low risk of paradoxic embolization and should be left out unless they are simple and straightforward technically. A coil that is 2- to 3-mm larger than the feeding artery is usually selected. If the feeding artery is large in caliber (>12 mm), a balloon occlusion of the proximal artery via a second groin puncture can be used for a more controlled coil deployment. Postembolization syndrome can occur and is characterized by pleuritic chest pain, pleural fluid, atelectasis, fever, and leukocytosis.
Arteriovenous fistula
AVFs are relatively large arteriovenous connections and may be congenital or secondary to trauma, surgery, or underlying vascular abnormality (eg, neurofibromatosis). [28] AVFs may be seen in any part of the body. A patient may present with cardiac failure, localized growth disturbances, neurologic deficits, and ischemic changes. Unlike AVMs, AVFs can be cured with embolotherapy. The embolotherapy technique used depends on the size, location, and hemodynamics of individual lesions. The goal of embolotherapy is to occlude the fistula and the immediate draining vein.
Embolization can be achieved by using coils, balloons, or tissue adhesives. Gelfoam or particles are not appropriate for use in AVF embolization. Detachable coils or balloons are ideal because these embolic materials can be positioned optimally before they are detached. Balloons also have the advantage of conforming to the size and shape of the abnormal vessels. Microcoils have several advantages over macrocoils. They are nonferromagnetic, they are delivered via microcatheters, and they cause no significant artifacts on subsequent MRI studies.
Microcoils can be dislodged; however, this complication can be minimized by performing flow-control techniques (eg, balloon occlusion, tourniquet, or blood pressure cuff control). The other disadvantage of coil embolization is that the clot that forms around the coil may dissolve, resulting in recanalization. Appropriate nesting of several coils (packing) can minimize recanalization. Also, thrombosis can be augmented by soaking the coils in thrombin before deployment or by injecting sclerosants around the coils. If adequate coil packing cannot be accomplished, tissue adhesive can be effectively used in combination with coils. Covered stents have great potential for replacing coil embolization of the involved vessel in the treatment of AVFs.
Venous malformation
VMs are the most common type of vascular malformations and can vary significantly in size and clinical presentation. [2] These lesions (usually distinguished by a bluish discoloration with swelling and pain) may also be associated with systemic syndromes such as blue rubber-bleb nevus syndrome or Maffucci syndrome. Sinus pericranii (communication between intracranial and extracranial venous drainage) is also commonly associated with craniofacial VMs. These lesions can be treated with embolotherapy (sclerotherapy) and/or surgical excision. The type of treatment used depends on the morphology, size, and location of the malformation.
A preliminary venogram is usually obtained to evaluate the deep venous system and to determine if any communication exists between the VM and the extremity veins. In particular, a venogram is performed on extremity VMs (see the image below).
 A lower-extremity venogram (digital subtraction angiogram) in a patient with a venous malformation in the upper calf. The deep veins are patent but appear to be displaced by the vascular malformation.
A lower-extremity venogram (digital subtraction angiogram) in a patient with a venous malformation in the upper calf. The deep veins are patent but appear to be displaced by the vascular malformation.
The lesion is localized by using ultrasonography, and the largest-appearing cystic portion of the lesion is selected. Then, the lesion is accessed by using real-time ultrasonographic guidance and a small Angiocath needle (typically, 20-22 gauge). The lesion is studied with contrast agent injections under fluoroscopy as well as digital subtraction angiography (see the image below).
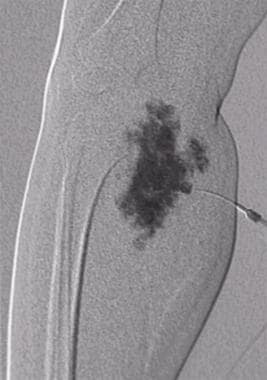 Direct intralesional contrast agent injection into a vascular lesion in the upper calf. This type of contrast agent filling, with or without opacification of the draining veins, is characteristic of the venous malformation. Contrast material outlines the malformation without opacification of the draining veins. Because no draining veins are present, a sclerosant agent can be safely injected into the lesion (sclerotherapy).
Direct intralesional contrast agent injection into a vascular lesion in the upper calf. This type of contrast agent filling, with or without opacification of the draining veins, is characteristic of the venous malformation. Contrast material outlines the malformation without opacification of the draining veins. Because no draining veins are present, a sclerosant agent can be safely injected into the lesion (sclerotherapy).
Subsequently, sclerotherapy is performed by using ethanol (absolute alcohol) or sodium tetradecyl mixed with a contrast medium (Ethiodol or iodinated contrast) under real-time fluoroscopic control. [29]
Hemorrhage
Several types of hemorrhage can be treated with embolization. Examples include hemoptysis; epistaxis; and GI tract, postpartum pelvic, posttraumatic, and iatrogenic hemorrhage (eg, postbiopsy or nephrostomy tube insertion).
GI hemorrhage
Major causes of upper GI tract hemorrhage are ulcer disease, gastritis, Mallory-Weiss tears, and varices. The most common causes of a lower GI tract hemorrhage are angiodysplasia, diverticulosis, and bleeding after endoscopic biopsy. If the bleeding source is identified on arterial angiogram (see the images below), the patient is treated by using either intra-arterial vasopressin infusion (Pitressin) or embolization of the bleeding mesenteric artery. [30, 31]
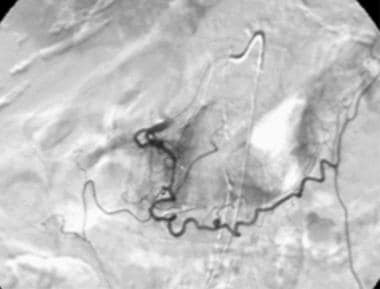 Contrast material injection in the gastroduodenal artery via a microcatheter demonstrates a tiny saccular aneurysm in a patient who presented with upper gastrointestinal bleeding.
Contrast material injection in the gastroduodenal artery via a microcatheter demonstrates a tiny saccular aneurysm in a patient who presented with upper gastrointestinal bleeding.
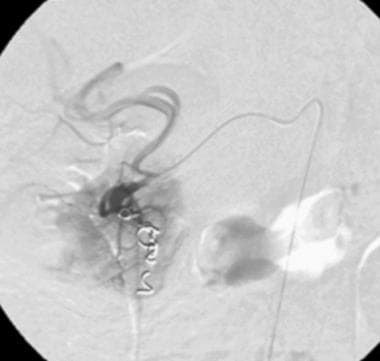 Postembolization with coils. The involved proximal segment of the gastroduodenal artery was embolized with several microcoils. The patient's bleeding episode ceased immediately.
Postembolization with coils. The involved proximal segment of the gastroduodenal artery was embolized with several microcoils. The patient's bleeding episode ceased immediately.
Embolization is usually the first line of treatment in patients with upper GI tract bleeding and is used as the second line of treatment in patients with lower GI tract bleeding (usually used if vasopressin treatment fails).
Embolization in the arcades proximal to the bleeding vasa recta is recommended to minimize the risk of bowel necrosis. The most commonly used embolic agents are coils (macrocoils or microcoils) and Gelfoam pieces (torpedoes). Coil embolization is particularly helpful if the bleeding is caused by focal vascular abnormalities such as a false aneurysm. Some interventional radiologists have also used PVA, although the use of PVA or other particles should be avoided because of the risk of bowel infarction. After embolization, control angiography is performed to determine if bleeding (contrast material extravasation) continues via any collaterals. The primary advantage of embolotherapy is the immediate cessation of bleeding without prolonged catheterization (unlike vasopressin infusion therapy).
Pelvic hemorrhage
Intractable pelvic hemorrhage, either posttraumatic or postpartum, should be approached in a similar interventional fashion. A surgical approach to control active bleeding in acute trauma setting is usually not favored. Detailed angiographic examination with superselective injections in the branches of the internal iliac artery is mandatory. Embolization can be performed by using an autologous blood clot, Gelfoam torpedoes, PVA particles, cyanoacrylate (glue), coils, or detachable balloons. At the capillary level, embolization with small particles or Gelfoam powder is contraindicated (because of the elimination of collateral flow, which results in massive tissue necrosis). [32, 33]
A specific anatomic relationship between the fracture site and the affected vessel often allows embolization of the branch (frequently the obturator artery) even when no obvious bleeding site is detected. In patients with intractable puerperal hemorrhage in whom no bleeding site is noted on angiograms, limited embolization of the branches of the anterior division of the internal iliac artery (particularly of the uterine artery) usually is performed with temporary agents (either autologous blood clot or Gelfoam). In particular, liquid or particle embolization of the inferior gluteal branch of the anterior division should be avoided to minimize the possibility of sciatic nerve injury. (This branch supplies muscles of the thigh and buttocks and the sciatic nerve.) In addition, embolization of the posterior division of the internal iliac artery should be avoided because of the risk of gluteal necrosis.
Hemoptysis
Hemoptysis is considered massive when at least 300 mL of blood is lost in less than 24 hours, and it may be life threatening. Common causes of massive hemoptysis are cystic fibrosis, bronchiectasis, tuberculosis, and aspergillosis. Malignancy is rarely a cause. Surgical intervention is usually not feasible because of severe pulmonary disease; therefore, embolization of the bleeding bronchial arteries can be life saving. [34]
Bronchial arteries are variable. They usually arise from the descending thoracic aorta between thoracic vertebrae T4 and T7. The right bronchial artery arises from the intercostobronchial trunk in most patients (>90%). The left bronchial artery usually arises directly from the aorta and is multiple in most patients. Occasionally, the right and left bronchial arteries arise from a common trunk.
Although some bronchial branches may supply the spinal cord, the most important of these branches is the artery of Adamkiewicz. This artery usually arises from an intercostal or lumbar artery on the left. Other spinal branches from the right intercostobronchial artery, thyrocervical, or costocervical arteries may be identified. A preliminary thoracic aortogram may be performed, which usually shows abnormal bronchial arteries. An aortogram helps in outlining the bronchial anatomy.
Because of potential source of collaterals, a subclavian arteriogram also is obtained, particularly if the upper lung field is involved. Then, the bronchial arteries are catheterized and studied with selective injections. The most common appearance of the abnormal bronchial artery (the bleeding source) is increased caliber of the bronchial artery with some hypervascularity over the lung field (see the image below).
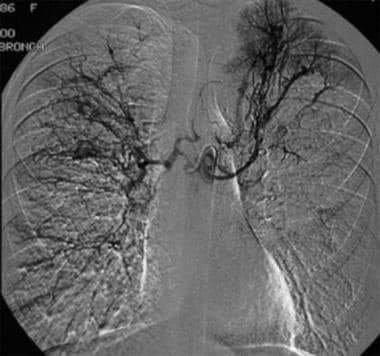 Contrast agent injection in the common bronchial artery demonstrates prominent bronchial arteries bilaterally, with patchy hypervascularities over the lung fields, particularly in the left upper lung. This is the most commonly seen angiographic appearance in patients with hemoptysis. Contrast agent extravasation is encountered only rarely.
Contrast agent injection in the common bronchial artery demonstrates prominent bronchial arteries bilaterally, with patchy hypervascularities over the lung fields, particularly in the left upper lung. This is the most commonly seen angiographic appearance in patients with hemoptysis. Contrast agent extravasation is encountered only rarely.
Contrast agent extravasation, shunting from bronchial to pulmonary arteries, or aneurysmal changes in the involved bronchial artery rarely are identified.
Embolotherapy is usually performed with particles (PVA or embospheres) and Gelfoam pledgets. When the decision is made to use particles, appropriately sized particles should be used. Sizes are usually 500-710 µm for PVA and 500-800 µm for embospheres. Use of coils is inappropriate, and absolute alcohol or cyanoacrylate is no longer used for bronchial artery embolization because of the risk of tissue necrosis (bronchial and/or esophageal).
Embolization is performed as selectively as possible (when necessary, by using a microcatheter and coaxial technique) to minimize tissue necrosis and nontarget embolization (eg, spinal artery). Special care should be taken to prevent reflux by injecting the embolic material slowly under continuous fluoroscopic control. Gelfoam pledgets/torpedoes usually are used to occlude the abnormal artery more proximally after particle embolization.
Epistaxis
Intractable epistaxis is a nosebleed that does not respond to conservative treatment (nasal spraying of vasoconstrictors, nasal packing, blood transfusion). Etiologies include uncontrolled hypertension with or without superficial mucosal abnormality (eg, Osler-Weber-Rendu syndrome).
Epistaxis can be treated by either surgical means (eg, cautery, vascular ligation) or endovascular embolotherapy. Internal carotid arteriograms are obtained to exclude aneurysms. Then, the external carotid artery is catheterized, and control angiography is performed initially to map the vascular anatomy and to check for the presence of a collateral supply to the intracranial circulation. [35]
During catheterization of the external carotid artery and its branches, vasospasm is a common problem. Nitroglycerin can be used to treat this. The target branch is usually the pterygopalatine division of the internal maxillary artery, which is distal to the origin of the meningeal and temporal arteries. By using a microcatheter, the pterygopalatine division is catheterized and embolized with particles (most commonly PVA). If bleeding is not caused by a neoplastic entity, embolotherapy can be performed in the 250- to 500-µm range. If a neoplastic entity is the cause, the capillary bed needs to be embolized. This embolization can be accomplished by using smaller particles (150-250 µm).
Although the procedure is considered safe if performed by an experienced physician, possible complications can occur. These include ischemia, pain, cranial nerve damage, blindness, and stroke.
Posttraumatic hemorrhage
Posttraumatic hemorrhage can be due to either a blunt or penetrating injury to a vessel, typically arteries in the extremities with penetrating injuries or associated fractures or arteries to the organs (eg, renal arteries, after blunt trauma). Some patients may present after an orthopedic procedure, such as total hip replacement. Pelvic fracture–related uncontrollable pelvic bleeding is a common indication for embolotherapy. An angiographic study is mandatory, not only to aid selecting in the appropriate subsequent embolization procedure but also in planning for possible future surgical interventions. [36]
Coil embolization is appropriate in extremity branch arteries responsible for the bleeding because it offers a fast and permanent occlusion of the vessel. The bleeding vessel should be embolized proximal and distal to the site of arterial injury. Avoid embolization of arteries that endangers limb viability. Hemorrhage or AVF formation after organ biopsy (particularly renal biopsy), or iatrogenic traumatic hemorrhage, is a common complication (ie, iatrogenic traumatic hemorrhage) that can be treated with embolization.
Pseudoaneurysm
Pseudoaneurysms occur secondary to trauma or infection and consist of the leakage of blood into the confined perivascular space at the site of a vessel wall disruption. A good alternative to surgical repair is transluminal and/or percutaneous embolotherapy. Embolotherapy is the treatment of choice, especially when pseudoaneurysms are not accessible or when the patient is not a surgical candidate because of sepsis or other medical conditions. [37]
Embolic materials used for pseudoaneurysms include coils, detachable balloons, thrombin, Gelfoam, and NBCA. In large-neck pseudoaneurysms, a stent placement combined with coil embolization has been described. If the involved vessel cannot be catheterized or if a pseudoaneurysm is close to the skin surface (typically a pseudoaneurysm in the groin after cardiac catheterization), the pseudoaneurysm can be directly punctured with a fine needle (eg, 22 gauge), and thrombin can be injected. When the involved artery is embolized with coils, the artery also should be embolized distal to the origin of the pseudoaneurysm so that collaterals do not fill the aneurysm.
Other Conditions Treated With Embolization
Malignant tumors
Indications for embolotherapy in neoplastic conditions include preoperative embolization and palliative embolization, which alleviates symptoms, reduces further dissemination, and increases the response to other treatment modalities (eg, radiation therapy). Embolotherapy can be used for many types of malignant tumors. [6] Renal malignancy is the most common type of tumor treated with embolotherapy. In particular, tumors extending into the hilum or other adjacent structures for which surgical removal is difficult are treated by using embolotherapy. In these patients, prior embolization of the tumor shrinks the mass and minimizes blood loss during surgical removal.
Unresectable tumors can be made operable by means of embolotherapy. If the entity is in its end stage (disseminated metastatic deposits), the technique can be used for palliation to control pain and hematuria. Other reported malignancies in which embolotherapy has been used include pelvic malignancies and bone tumors. Hemorrhage resulting from malignancy or radiotherapy (eg, due to radiation cystitis) can be controlled by using embolotherapy.
Uterine fibroids
Uterine leiomyomata or fibroids commonly are found in women in their 30s and 40s. Symptomatic fibroids can be treated by means of medical (medication), surgical, or interventional measures. Medical treatment consists of the use of several medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], birth control pills, gonadotropin-releasing hormone analogues [Lupron], and antiprogesterones [mifepristone]). Surgical treatment of fibroids includes hysterectomy or myomectomy, both of which require general anesthesia and hospitalization. A myomectomy is a local treatment and has a high recurrence rate.
Transcatheter embolotherapy is a well-recognized therapeutic alternative to surgery with a high patient satisfaction rate and positive therapeutic results. Particles (either PVA or microspheres) are the embolic agents most often used in patients with uterine fibroids. Particle sizes of 355-500 µm and 500-710 µm are used most commonly. Embolization is usually performed with an initial angiographic examination of the internal iliac arteries, followed by catheterization of the uterine arteries and the delivery of particles under fluoroscopic guidance (see the images below).
Although fibroid necrosis occurs within 72 hours after embolization, shrinkage of fibroid lesions may occur over a course of weeks. Endometrial perfusion is known to return to normal within 4 months after embolization treatment. [7]
After the procedure, some pain, fever, and nausea are common (postembolization syndrome), but these are usually tolerable and easily managed. Complications from this procedure are rare. However, infection is a risk after this procedure. The 2 most significant complications are amenorrhea and fibroid slough retention. Embolotherapy may hasten or initiate menopause in a perimenopausal patient whose ovaries depended on the contribution of uterine arterial flow.
Chemoembolization
Chemoembolization is commonly performed in hepatic malignancies. The technique is used in patients with unresectable liver tumors, particularly hepatocellular carcinoma and metastatic liver disease. To proceed with chemoinfusion/chemoembolization, the patent portal vein with hepatopetal flow is typically required. The bilirubin level should be less than 3 mg/dL to perform chemoinfusion/chemoembolization safely. [38]
Vigorous intravenous hydration is required before the procedure for at least 24 hours. Initially, a superior mesenteric arteriogram is usually obtained to demonstrate whether a hepatic artery (originating from the superior mesenteric artery) has been replaced and to demonstrate patency of the portal vein. Then, the celiac truncus and, subsequently, the common hepatic artery are catheterized and studied to exclude the replaced hepatic arteries and to outline the vascular anatomy (see the image below).
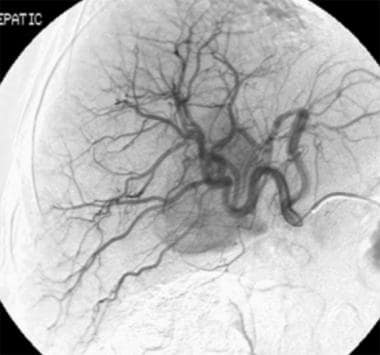 Chemoembolization. Contrast agent injection in the common hepatic artery. A tumoral stain is identified within the region of the porta hepatis.
Chemoembolization. Contrast agent injection in the common hepatic artery. A tumoral stain is identified within the region of the porta hepatis.
The involved lobar hepatic artery or, more commonly, the first- or second-order branches of this artery is subsequently catheterized by using a microcatheter (eg, Turbo Tracker), and the chemoinfusion material is injected under fluoroscopic guidance. The tip of the catheter must be placed distal to the cystic and gastroduodenal arteries. The most commonly used chemoinfusion mixture consists of 10 mL of iopamidol, 20 mL of Ethiodol, and 60 mg of doxorubicin (see the image below).
The chemoinfusion is usually followed by embolization with a slurry of gelatin sponge powder (Gelfoam).
Lidocaine is intra-arterially administered to reduce pain after the chemoinfusion/chemoembolization treatment. The delivery catheter must be positioned beyond the cystic and gastroduodenal arteries before the chemoinfusion/chemoembolization treatment is begun.
In a study by Carling et al of 115 patients with colorectal liver metastases who underwent portal vein embolization (PVE) with NBCA, a central vascular plug (CVP) in addition to NBCA embolization was associated with increased growth of the future liver remnant (FLR) compared to NBCA alone. [17]
Bridge Therapy to Liver Transplantation
As overall wait time for liver transplantation increases, so has the utilization of locoregional therapies, including chemoembolization and radioembolization, to bridge patients on the waitlist. A study of 31,609 patients found the percentage receiving at least one type of therapy increased from 42.3% in 2003 to 92.4% in 2018. The most frequently used treatment was chemoemobilization, accounting for 50% of treatments received. The use of radioembolization increased from less than 3% in 2013 to 19% in 2018. Locoregional therapy, in particular radioembolization, was associated with a reduced risk of waitlist dropout when compared to chemoembolization. [39]
Varicocele
Varicocele is a common cause of male infertility and is characterized by abnormally dilated veins in the pampiniform plexus. Primary varicoceles are due to a retrograde venous flow in the spermatic vein (due to incompetent valves), whereas secondary varicoceles are due to abdominal masses (venous outflow obstruction). Most varicoceles are located on the left side, because the left spermatic vein drains into the left renal vein, whereas the right spermatic vein drains directly into the inferior vena cava (IVC) (stress differences in anatomy). Right varicoceles (particularly unilateral) should be explored with additional imaging to exclude an abdominal mass or, less likely, situs abnormalities. [40]
Patients with varicoceles may present with a dull ache in the scrotum or groin, which is worsened by physical activity or long periods of standing, or varicoceles may be found during an infertility workup. Aside from these populations, adolescent patients without symptoms can be treated to eliminate the risk of developing testicular atrophy. After a varicocele is corrected, an improvement in fertility is expected when embolotherapy is performed for the appropriate indications.
Although both surgical correction and embolization are effective in the treatment of varicoceles, percutaneous embolization of the vein is more appropriate primarily because of its lower morbidity rate. Access can be obtained via either the internal jugular or femoral vein. With the catheter positioned well into the renal vein, a renal venogram is obtained to outline the venous structures.
Catheterization of the left spermatic vein can be problematic because of venous spasms or anatomic variants (5-19%), such as small vein size or intact valves. Catheterization of the right spermatic vein can be problematic primarily because of its acute angle to the IVC and because of variations in its origin (usually immediately anterior and inferior to the right renal vein orifice). With the catheter in the upper spermatic vein, an injection is made while the patient performs a Valsalva maneuver. The vein should be opacified all the way down to the inguinal ring to ensure that collaterals do not reconstitute a varicocele distally. If direction of flow is not antegrade and if contrast material fills the veins below the inguinal ring, the varicocele can be confirmed angiographically (see the image below).
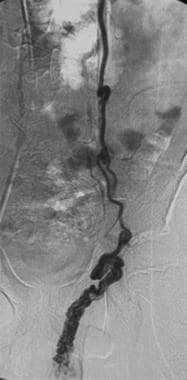 Contrast agent injection in the left spermatic vein fills the vein below the inguinal ring. This finding is consistent with a varicocele.
Contrast agent injection in the left spermatic vein fills the vein below the inguinal ring. This finding is consistent with a varicocele.
Preliminary angiographic confirmation of the condition is important before embolotherapy. False-positive results can occur if the catheter is wedged or if contrast material is injected too forcefully. During embolotherapy, the catheter is advanced further to the level of the inguinal ring, and the spermatic vein is embolized by various liquids and materials (or a combination). These materials include coils (Gianturco or microcoils), detachable balloons, and sclerosant agents such as alcohol, sodium tetradecyl, boiling contrast, or glue (see the image below).
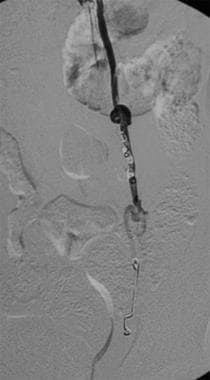 Embolotherapy was successfully performed by means of sclerotherapy with sodium tetradecyl and coil embolization, both proximally and distally.
Embolotherapy was successfully performed by means of sclerotherapy with sodium tetradecyl and coil embolization, both proximally and distally.
Particles such as PVA or embospheres are not used because they may cause testicular infarction or may cause nontarget embolization in the lungs. Some prefer a coil embolization distally (just above the inguinal ring) followed by the injection of 2 mL of sodium tetradecyl mixed with 0.5 mL of contrast agent and finally with coiling the spermatic vein more superiorly (usually within 2 cm of its origin). Communication between the spermatic vein and other veins neither constitutes a contraindication to embolotherapy nor appears to affect the results.
Organ ablation
Splenic embolization can be used as a preoperative therapy or as an alternative to the surgical removal of the spleen. Indications include posttraumatic bleeding, variceal bleeding secondary to portal hypertension or splenic vein thrombosis, hypersplenism, thalassemia major, thrombocytopenia, idiopathic thrombocytopenic purpura, Gaucher disease, and Hodgkin disease. Embolotherapy is performed with superselective catheterization/embolization of the splenic artery by using embolic particles while the tip of the catheter is beyond the caudal pancreatic artery. Careful fluoroscopic control of the splenic area is required to limit the total infarction to approximately 60% of the spleen.
Renal embolization is an alternative to surgical removal of the kidney, and indications include end-stage renal disease or renovascular hypertension requiring unilateral or bilateral nephrectomy and renal transplant with native kidneys in situ. The procedure requires selective catheterization of the renal artery with further advancement of the catheter so that the catheter is wedged or with the use of a balloon occlusion catheter to minimize the possibility of embolic material spillage into the aorta. The preferred embolic agents are particles (eg, PVA) and/or liquid agents such as ethanol or NBCA (cyanoacrylate). Postinfarction syndrome is relatively common and characterized by pain, which can be managed with narcotics. This pain usually subsides within 48-72 hours.
-
A lower-extremity venogram (digital subtraction angiogram) in a patient with a venous malformation in the upper calf. The deep veins are patent but appear to be displaced by the vascular malformation.
-
Digital subtraction angiogram shows a small arteriovenous malformation in the lateral aspect of the foot. A small feeder vessel is seen arising from the widely patent posterior tibial artery. An early draining vein is demonstrated clearly. Delayed opacification of the dorsalis pedis artery (not seen) is secondary to proximal anterior tibial arterial disease. These small arteriovenous malformations are occasionally encountered during arteriography in the extremities of elderly patients. The importance of the lesions is unclear in this patient population. If a similar lesion were encountered in a young child, it would be a more significant finding, one possibly requiring treatment.
-
Arteriovenous malformation in the foot. This is an example of an extensive form of arteriovenous malformation. In most patients, the lesions require aggressive treatment by means of surgical excision, transcatheter embolization, or both. The feeders arise from large-caliber foot arteries, and obvious arteriovenous connections (nidus) are seen. Most of these are centered in the midfoot.
-
Transluminal embolization using a microcatheter. These lesions usually require embolotherapy with precise embolization techniques. Small feeders are catheterized by using a microcatheter and embolized with embolic material (eg, alcohol) under continuous fluoroscopic control. The tip of the microcatheter is in a tiny plantar feeding artery on this image.
-
Postembolization arteriogram. After successful embolization, significant improvement is seen because most of the arteriovenous connections have disappeared.
-
Direct intralesional contrast agent injection into a vascular lesion in the upper calf. This type of contrast agent filling, with or without opacification of the draining veins, is characteristic of the venous malformation. Contrast material outlines the malformation without opacification of the draining veins. Because no draining veins are present, a sclerosant agent can be safely injected into the lesion (sclerotherapy).
-
Venous malformation following intralesional sclerosant injection. This is the expected appearance of a venous malformation after sclerotherapy. Speckled contrast agent collections are scattered throughout the malformation.
-
Axial CT image through the upper part of the neck after percutaneous cyanoacrylate embolization. The patient was a young girl with an extensive head and neck venous malformation resulting in significant airway obstruction, which required a tracheostomy.
-
Contrast material injection in the gastroduodenal artery via a microcatheter demonstrates a tiny saccular aneurysm in a patient who presented with upper gastrointestinal bleeding.
-
Postembolization with coils. The involved proximal segment of the gastroduodenal artery was embolized with several microcoils. The patient's bleeding episode ceased immediately.
-
Contrast agent injection in the common bronchial artery demonstrates prominent bronchial arteries bilaterally, with patchy hypervascularities over the lung fields, particularly in the left upper lung. This is the most commonly seen angiographic appearance in patients with hemoptysis. Contrast agent extravasation is encountered only rarely.
-
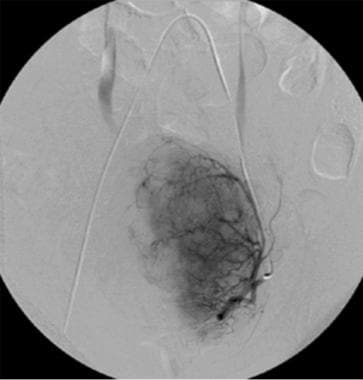
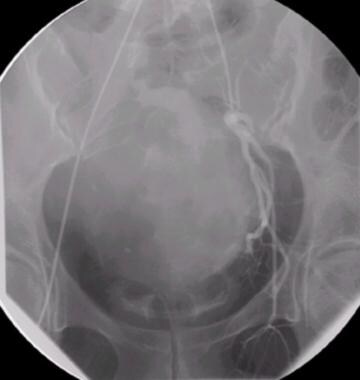
Uterine artery angiography (preembolization).
-
Uterine artery angiography (postembolization).
-
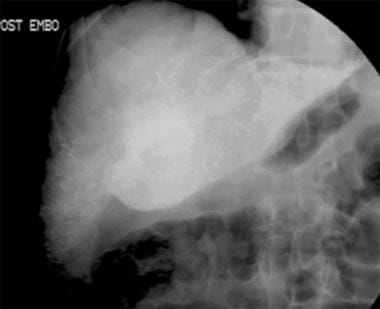
Chemoembolization. Contrast agent injection in the common hepatic artery. A tumoral stain is identified within the region of the porta hepatis.
-
Postchemoembolization.
-
Contrast agent injection in the left spermatic vein fills the vein below the inguinal ring. This finding is consistent with a varicocele.
-
Embolotherapy was successfully performed by means of sclerotherapy with sodium tetradecyl and coil embolization, both proximally and distally.