Practice Essentials
Esophageal atresia (EA) is a condition in which the proximal and distal portions of the esophagus do not communicate. EA with or without tracheoesophageal fistula (TEF) remains one of the most common gastrointestinal neonatal malformations (2.43 cases per 10,000 births). [1] The upper segment of the esophagus is a dilated, blind-ending pouch with a hypertrophied muscular wall that typically extends to the level of the second to fourth thoracic vertebra. The distal esophageal portion is an atretic pouch with a small diameter and a thin muscular wall; it extends a variable distance above the diaphragm. [2, 3, 4, 5, 6, 7, 8, 9]
Five types of EA and TEF have been described. The most common abnormality is EA with a distal TEF (84%). Isolated atresia with no fistula is the next most common finding (8%), followed by H-type TEF (no atresia) (4%). EA with proximal and distal fistulas (3%) and EA with a proximal fistula (1%) are less common. The frequencies given for each type are calculated from a summary of 6 long-term studies. [10, 7, 11, 12, 13, 14, 15, 16]
The condition may occur alone, but additional congenital malformations such as VACTERL (vertebral defects, anal atresia, cardiac defects, TEF, renal anomalies, and limb abnormalities) and CHARGE (coloboma, heart defects, atresia choanae, growth retardation, genital abnormalities, and ear abnormalities) are not uncommon. [4] Cardiac defects are observed particularly frequently. [17, 18] Prenatally, patients with EA may present with polyhydramnios, mostly in the third trimester, which may be a diagnostic clue to EA. Additionally, approximately 50% of patients with EA/TEF will have congenital anomalies such as VACTERL or CHARGE. [4, 17, 18]
H-type fistula is an uncommon anomaly consisting of congenital tracheoesophageal fistula not associated with esophageal atresia. It presents with coughing, choking, and cyanosis during feeding and/or recurrent pneumonia. [19]
(See the images below.)
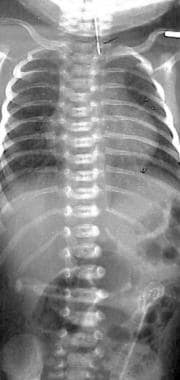 Esophageal atresia (EA) with distal tracheoesophageal fistula (TEF). Frontal view of the chest and abdomen of a neonate demonstrates a tube in the proximal pouch in this patient with EA. The presence of bowel gas implies the presence of a distal TEF, making this the most common type of EA/TEF.
Esophageal atresia (EA) with distal tracheoesophageal fistula (TEF). Frontal view of the chest and abdomen of a neonate demonstrates a tube in the proximal pouch in this patient with EA. The presence of bowel gas implies the presence of a distal TEF, making this the most common type of EA/TEF.
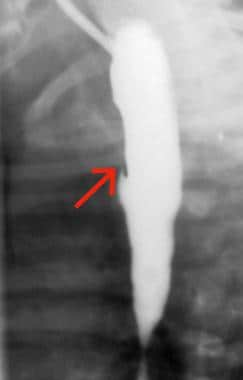 H-type tracheoesophageal fistula (TEF). Oblique barium esophagogram demonstrates a fistula (arrow) arising from the anterior esophagus and extending anterosuperiorly to the trachea.
H-type tracheoesophageal fistula (TEF). Oblique barium esophagogram demonstrates a fistula (arrow) arising from the anterior esophagus and extending anterosuperiorly to the trachea.
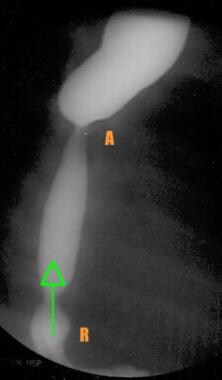 Barium esophagogram obtained in a patient 3 weeks after esophageal repair shows the relative narrowing at the anastomotic site (A). Reflux (arrow) is seen with an associated sliding hiatal hernia (R).
Barium esophagogram obtained in a patient 3 weeks after esophageal repair shows the relative narrowing at the anastomotic site (A). Reflux (arrow) is seen with an associated sliding hiatal hernia (R).
Imaging modalities
Plain radiographs provide a great deal of information, including findings for EA confirmation and depiction of the position of the aortic arch, as well as the presence of any vertebral or other associated anomalies. Barium studies performed after the surgical placement of a gastrostomy tube may be used to evaluate the gap length and associated GI abnormalities, such as duodenal atresia or malrotation; however, radiographs may not always demonstrate the presence of a fistula.
In an analysis by Morini et al of over 370 neonates with EA/TEF, chest radiograph and echocardiography were almost uniformly performed in all patients. Esophagoscopy was performed in only 8% of patients, and bronchoscopy was performed in 37%. Only 14% of the patients without a distal TEF had a bronchoscopy. [20]
In neonates in whom EA and/or TEF is suspected, posteroanterior and lateral plain chest radiographs should be obtained first. The patient's inability to pass a rigid nasogastric tube from the mouth to the stomach is diagnostic of EA and/or TEF, but this finding should be confirmed with radiographic visualization of the tube coiled in the proximal pouch.
In a study by Rassiwala et al, prediction of gap length by vertebral level of arrest of the nasogastric tube in the upper pouch in a preoperative chest radiograph was found to correlate well with gap length measured intraoperatively. Patients were divided into A, B, and C groups based on gap length (gap length >2.1 cm; >1-≤2 cm; and ≤1 cm, respectively). In group A, nasogastric tube arrest at T1-T2 and T2-T3 vertebral level corresponded in 39 of 41 (95.12%) patients. In group B, tube arrest at T2-T3 and T3-T4 corresponded in 44 of 44 (100%) patients. In group C, tube arrest at T3-T4 and T4 corresponded in 31 of 33 (93.93%) patients. [21]
Contrast-enhanced studies are rarely indicated because of the risk of aspiration, but they may be necessary to identify or locate a fistula. Only an experienced radiologist should perform contrast-enhanced studies with fluoroscopic control. [22] Endoscopy and/or bronchoscopy may be performed to locate or rule out TEFs.
Upper-pouch TEF occurs with EA in less than 1% of all cases of EA/TEF but could be easily missed after birth. In a study by Houben et al, upper TEFs were initially missed but ultimately detected early with upper pouch esophagogram (UPEG) and tracheobronchoscopy (TBS). [23]
Sonographic evaluation will reveal the first findings suggestive of a congenital anomaly, but it is not conclusive. Many conditions involve polyhydramnios and a small or absent stomach bubble on ultrasonography. [24] Visualization of a dilated proximal pouch is suggestive of esophageal atresia, but further tests are necessary to confirm the diagnosis. In addition, sonograms may not give any indication of EA when it is present, and often, a fistula is not seen.
Prenatally, ultrasonographic findings may suggest a diagnosis of isolated esophageal atresia or tracheoesophageal fistula; however, prenatal EA detection rates are low, and if suspected, the diagnosis must be confirmed postnatally. A systematic review and meta-analysis of 23 studies found prenatal ultrasound had a sensitivity of 41.9%, a specificity of 99.9%, a positive likelihood ratio of 88.1, a negative likelihood ratio of 0.58. and a diagnostic odds ratio of 153.7. By itself, ultrasonography is a poor diagnostic tool for identifying esophageal atresia prenatally, as well as having a high rate of false positive diagnoses. Magnetic resonance imaging and amniotic fluid analysis have high diagnostic accuracy for esophageal atresia. [25]
Esophageal atresia with transposition of the great arteries has been diagnosed with prenatal ultrasound combined with MRI. [26, 27, 28]
Etiologies and factors
The etiologies of EA and TEF are still largely unknown, but many theories concerning their origins have been proposed. [29] The trachea and esophagus are foregut derivatives. During the fourth gestational week, lateral mesodermal ridges form in the proximal esophagus; the fusion of these grooves in the midline separates the esophagus from the trachea around the 26th day of gestation.
Notochord abnormalities, desynchronous esophageal mesenchymal and epithelial growth rates, neural crest cell involvement, and incomplete tracheoesophageal separation resulting from a lack of apoptosis are some of the conditions theorized for EA embryogenesis. Similarly, incomplete tracheoesophageal septation, lateral ridge fusion failure, and tracheal and esophageal proximity have been suggested as possible explanations for the origin of TEF. In addition, other contributors to the development of EA and/or TEF include vascular insufficiencies; genetic factors; vitamin deficiencies; drug and alcohol exposures; and viral, chemical, and external physical events. First-trimester use of benzodiazepines had been reported to increase the risk for esophageal atresia. [30]
According to these theories, several factors appear to alter the rate and timing of cell growth and proliferation in the embryonic foregut. These events most likely occur before the 34th day of gestation. Other organs, such as the remainder of the intestinal tract, the heart, the kidneys, the ureters, and the skeletal system, are also developing around this time, and they are vulnerable to developmental irregularities as well. In fact, although over 90% of children born with EA survive, the associated comorbidities and complications adversely affect long-term quality of life. [2]
Complications
Fistula recurrence between the esophagus and trachea is observed in 3-14% of patients treated for EA with TEF, EA without TEF, or H-type TEF. Fistulas usually recur within a few months, but they may recur as late as 2 years after surgery.
An anastomotic leak with local inflammation and erosion at the previous repair site, ischemia, and surgical dissection too near the trachea may cause a recurrent fistula. This condition should be suspected when choking episodes occur during feeding and/or recurrent pneumonia is observed. The best methods of diagnosis are bronchoscopy and esophagography under videofluoroscopic guidance with the patient in the prone position and with bolus injections of contrast agent into a nasoesophageal tube. Fistulas do not close spontaneously and require surgical division and ligation. About 10-20% of cases recur after the first TEF recurrence.
Esophageal strictures occur in 40% of children after surgical EA repair. Strictures may be diagnosed with barium swallow examination or esophagoscopy. Although barium swallow studies aid in stricture reduction by dilating the anastomotic site, decreasing the size discrepancy between the 2 segments, and loosening the fibrosis of healing, they are not completely effective, and dilations are required for resolution. Dilation is 90% effective, but strictures that do not respond to dilation must be resected surgically.
Gastroesophageal reflux (GER) is a common complication, occurring in 40-70% of patients after EA repair. [3] Symptoms of GER include coughing, apnea, recurrent pneumonia, failure to thrive, and stricture formation. [31] A barium swallow examination may demonstrate GER, which is caused by tension, dysmotility of the lower esophagus, and an altered angle of Hiss resulting from distal esophageal mobilization.
Special concerns
The use of contrast agents for examining children with esophageal atresia and/or tracheoesophageal fistula is of special concern because of the risk of aspiration. If an agent is used, the type of contrast material should be considered carefully. [32]
Barium possibly enables the best visualization; however, extraluminal barium has a risk of causing a granulomatous and fibrotic reaction that can result in fibrous mediastinitis.
Aqueous low-osmolality agents are preferred. These agents have no deleterious effects on the GI system, but they are more expensive than standard agents. Aqueous contrast agents are preferred in neonates, premature infants, and children with a suspected esophageal perforation. They remain in the GI tract for prolonged periods, and they are not absorbed, because they are hypertonic and hyperosmolar.
Unfortunately, aqueous contrast agents are diluted quickly and are less fluoroscopically visible because of their decreased coating ability; therefore, as many as 15-25% of thoracic perforations and 50% of cervical perforations may not be detected.
Hyperosmolar agents should not be used because they cause marked irritation and pulmonary edema if they are aspirated.
In addition, the use of hypertonic and hyperosmolar agents may result in hypovolemia due to the displacement of intravascular fluids into the GI tract, severe dehydration, and pneumonitis caused by significant irritation of the trachea and bronchi.
Only experienced radiologists should perform contrast-enhanced examinations in children with EA and/or TEF.
Radiography
The findings on posteroanterior and lateral chest images will confirm a diagnosis of esophageal atresia (EA) by displaying a coiled nasogastric tube (placed for determination of EA) in the proximal esophageal pouch of a child with EA. The location of the aortic arch can also be discerned. [33, 34, 35, 36, 37, 38, 39, 40]
Radiographic signs of a right-sided aortic arch include the following:
-
A right ascending aorta, indicated by an opaque shadow on the right side of the mediastinum
-
A right-sided tracheal indentation and/or deviation
-
A dilated inlet of the aberrant left subclavian artery, which resembles a diverticulum and appears as a round shadow to the left of the trachea at the site of the normal aortic knot
Any vertebral anomalies may be visualized, and some cardiac anomalies may be suggested. Aspiration pneumonia (especially in the right upper lobe) and patchy atelectasis are frequently present. Barium studies performed by means of a surgically placed gastrostomy tube are useful for evaluating the gap length and visualizing additional GI anomalies. Esophageal motility disturbances are present in children with EA and can be visualized with videofluoroscopy. Peristaltic discontinuity often exists in a 6-15 cm segment extending above and below the anastomosis. Aside from these general findings, the radiographic observations in children with EA and/or TEF vary depending on the type of anomaly present. These observations include isolated atresia, EA with a distal fistula, EA with a proximal fistula, and isolated tracheoesophageal fistula (TEF) (H-type fistula).
After surgical repair of EA and/or TEF, plain radiographs show the condition of the repair and any developing complications. Mild narrowing at the anastomotic site is a common finding, but more severe obstructive strictures may also be observed. Esophagography can be used to demonstrate an asymptomatic leak. In addition, a dilated proximal esophageal segment (particularly if the repair included myotomy) and distal esophageal stenosis are noted postoperatively.
The finding of a coiled nasogastric tube at radiographic examination confirms the presence of esophageal atresia (EA). Occasionally, the tube may coil because of extrinsic compression on the esophagus, and repeat placement should be attempted. If the tube passes to the stomach, the possibility of the tube passing through a tracheoesophageal fistula (TEF) must be investigated. The infant's cry should be profoundly affected if the tube passes through the vocal cords on the way through a distal TEF and into the stomach.
In a study by Rassiwala et al, prediction of gap length by vertebral level of arrest of the nasogastric tube in the upper pouch in a preoperative chest radiograph was found to correlate well with gap length measured intraoperatively. Patients were divided into A, B, and C groups based on gap length (gap length >2.1 cm; >1-≤2 cm; and ≤1 cm, respectively). In group A, nasogastric tube arrest at T1-T2 and T2-T3 vertebral level corresponded in 39 of 41 (95.12%) patients. In group B, tube arrest at T2-T3 and T3-T4 corresponded in 44 of 44 (100%) patients. In group C, tube arrest at T3-T4 and T4 corresponded in 31 of 33 (93.93%) patients. [21]
On occasion, determination of the side of the aortic arch may be difficult on plain images. Echocardiography, magnification, high-kilovoltage techniques, computed tomography (CT), or ultrasonography may be used to detect its location. Fistula identification may be difficult in some cases; further testing with endoscopy, bronchoscopy, or tracheoscopy may be necessary. If tracheal filling is observed with the use of a contrast material, these methods can aid in determining whether this observation is the result of a fistula or of aspiration.
Appropriate follow-up examinations of any concomitant congenital anomalies may be required, depending on the type of abnormality present. Although videofluoroscopy demonstrates dysmotility, the dysfunction is quantified with manometry and scintigraphy. Fluoroscopy is the best method for the identification of anastomotic leaks.
(See the images below.)
 Esophageal atresia (EA) with distal tracheoesophageal fistula (TEF). Frontal view of the chest and abdomen of a neonate demonstrates a tube in the proximal pouch in this patient with EA. The presence of bowel gas implies the presence of a distal TEF, making this the most common type of EA/TEF.
Esophageal atresia (EA) with distal tracheoesophageal fistula (TEF). Frontal view of the chest and abdomen of a neonate demonstrates a tube in the proximal pouch in this patient with EA. The presence of bowel gas implies the presence of a distal TEF, making this the most common type of EA/TEF.
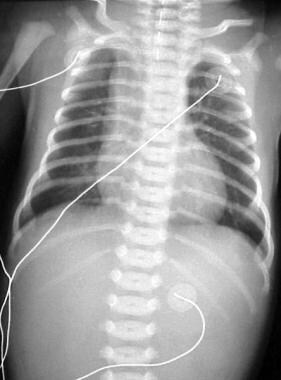 Isolated esophageal atresia (EA). Frontal view of the chest and abdomen demonstrates a catheter in the proximal pouch in this patient with EA. Note the absence of bowel gas in this patient with EA, but it is not associated with a tracheoesophageal fistula (TEF).
Isolated esophageal atresia (EA). Frontal view of the chest and abdomen demonstrates a catheter in the proximal pouch in this patient with EA. Note the absence of bowel gas in this patient with EA, but it is not associated with a tracheoesophageal fistula (TEF).
 H-type tracheoesophageal fistula (TEF). Oblique barium esophagogram demonstrates a fistula (arrow) arising from the anterior esophagus and extending anterosuperiorly to the trachea.
H-type tracheoesophageal fistula (TEF). Oblique barium esophagogram demonstrates a fistula (arrow) arising from the anterior esophagus and extending anterosuperiorly to the trachea.
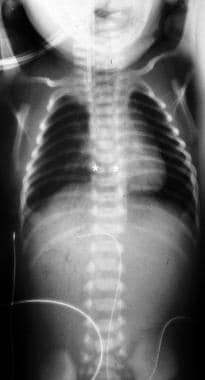 Vertebral, anorectal, cardiac, tracheal, esophageal, renal, and limb (VACTERL) association. Frontal radiograph in a patient with esophageal atresia (EA) without a tracheoesophageal fistula (TEF). Note the catheter in the proximal pouch and the butterfly vertebra (asterisks) at the level of T8 in this patient with associated VACTERL.
Vertebral, anorectal, cardiac, tracheal, esophageal, renal, and limb (VACTERL) association. Frontal radiograph in a patient with esophageal atresia (EA) without a tracheoesophageal fistula (TEF). Note the catheter in the proximal pouch and the butterfly vertebra (asterisks) at the level of T8 in this patient with associated VACTERL.
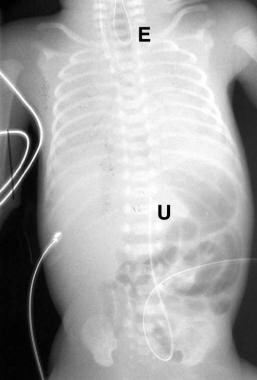 False-positive finding of esophageal atresia (EA). Image demonstrates a feeding tube coiled in the proximal esophagus (E). An umbilical arterial catheter (U) is noted at the level of T11. The catheter was repositioned and extended to the stomach, with no alteration of the infant's cry (see next image).
False-positive finding of esophageal atresia (EA). Image demonstrates a feeding tube coiled in the proximal esophagus (E). An umbilical arterial catheter (U) is noted at the level of T11. The catheter was repositioned and extended to the stomach, with no alteration of the infant's cry (see next image).
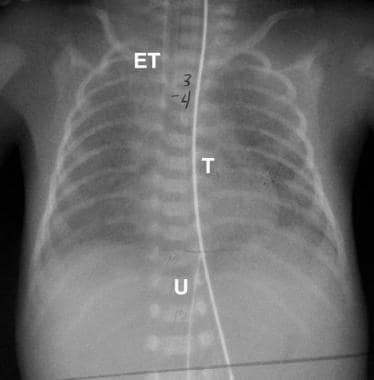 False-positive finding of esophageal atresia. Follow-up image in the patient in the previous image demonstrates the tube (T) extending to the stomach. The endotracheal tube (ET) and umbilical arterial catheter (U) are also identified.
False-positive finding of esophageal atresia. Follow-up image in the patient in the previous image demonstrates the tube (T) extending to the stomach. The endotracheal tube (ET) and umbilical arterial catheter (U) are also identified.
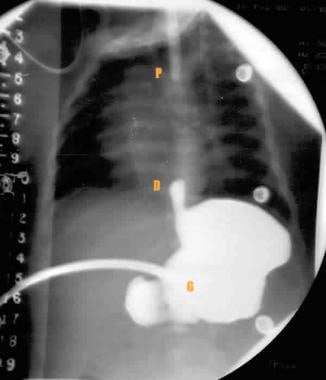 Gapogram shows the location of the proximal pouch (P), which is suggested by the position of the catheter. The distal pouch location (D) is visualized with the reflux of contrast material through a previously placed gastrostomy tube (G). The distance between the proximal and distal pouches is measured on the adjacent radiopaque ruler.
Gapogram shows the location of the proximal pouch (P), which is suggested by the position of the catheter. The distal pouch location (D) is visualized with the reflux of contrast material through a previously placed gastrostomy tube (G). The distance between the proximal and distal pouches is measured on the adjacent radiopaque ruler.
 Barium esophagogram obtained in a patient 3 weeks after esophageal repair shows the relative narrowing at the anastomotic site (A). Reflux (arrow) is seen with an associated sliding hiatal hernia (R).
Barium esophagogram obtained in a patient 3 weeks after esophageal repair shows the relative narrowing at the anastomotic site (A). Reflux (arrow) is seen with an associated sliding hiatal hernia (R).
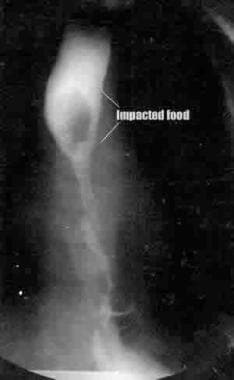 Stricture with food bolus. Frontal view from a barium swallow examination in a patient with a repaired EA shows a stricture at the anastomotic site, with a bolus of food proximal to the stricture.
Stricture with food bolus. Frontal view from a barium swallow examination in a patient with a repaired EA shows a stricture at the anastomotic site, with a bolus of food proximal to the stricture.
Isolated atresia
Isolated atresia findings include the following:
-
A dilated, air-filled, blind-ending proximal pouch, which often displaces the trachea anteriorly.
-
A gasless abdomen may be depicted. Air is normally present in the stomach 15 minutes after birth. [41]
-
The lower pouch is visualized with barium or air refluxed through a gastrostomy.
EA with a distal fistula
Findings of EA with a distal fistula include the following:
-
Gaseous distention of the stomach and small bowel (caused by air passing through the fistula).
-
An airless abdomen if the fistula is occluded or obliterated.
-
Excessive air in the esophagus; however, some air in the esophagus is normal in neonates and children.
EA with a proximal fistula
Findings of EA with a proximal fistula include the following:
-
On plain radiographs, this may have an appearance similar to that of isolated EA.
-
A gasless abdomen may be depicted.
-
Note: A barium swallow examination may fail to demonstrate this anomaly.
-
Fistula visualization requires rapid-sequence or videofluoroscopic studies during cautious filling of the proximal pouch.
Isolated tracheoesophageal fistula (TEF) (H-type fistula)
Isolated TEF (H-type) includes the following:
-
Recurrent pneumonia may be present, with a widespread pneumonia pattern.
-
Fistula delineation can be difficult.
-
Excessive air may be present in the esophagus.
-
Contrast-swallow is the study of choice for diagnosis. An isotonic, nonionic iodinated contrast agent is the contrast of choice; dilute barium can be used as an alternative contrast agent. If the patient is intubated or the contrast swallow demonstrates tracheal contrast without visualization of a fistula, an esophagogram with a feeding tube should be performed. [42] Using cross-table fluoroscopy with the patient lying prone on a waist-high footstep with the fluoroscopic table erect, serial injections of contrast are administered through a nasoesophageal catheter as the catheter is withdrawn proximally. [43] Tracheal filling is noted at the site of the fistula.
-
When the patient is intubated, care should be taken that the endotracheal tube tip is above the thoracic inlet, as the endotracheal tube may occlude the tracheal fistula opening.
Computed Tomography
CT is not typically used in the evaluation of esophageal atresia (EA) and tracheoesophageal fistula (TEF); however, CT does allow 3-dimensional (3D) examination of the esophagus in relation to its adjacent structures. Its use in patients with EA and/or TEF has increased. [44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]
Axial images can be difficult to interpret; a fistula may be demonstrated only partially or missed. Direct sagittal CT has been used in newborns to accurately diagnose EA and TEF. This method enables visualization of the entire length of the esophagus, complete with atresias, fistulas, and gap length.
Three-dimensional CT with virtual endoscopy provides similar benefits, in addition to facilitating the understanding of complex anatomic relationships. Virtual endoscopy can be used to traverse stenoses, unlike traditional endoscopy. Cardiac and respiratory motion artifacts, however, are problems. Although this technique provides an image of the anatomic relationships that is easy to understand, it does not provide any additional information beyond that on axial images.
CT imaging may also be used to identify the location of the aortic arch, but other methods typically are used. After surgical correction of EA and/or TEF, helical ultrafast CT can be used to quantify tracheomalacia, a ubiquitous finding. In addition, abnormal tracheal shape and size, an abnormally broad posterior tracheal wall, and abundant air and fluid within the esophagus have been recorded with postoperative CT.
According to a Canadian study, aberrant right subclavian artery (ARSA) and right aortic arch (RAA) have an incidence of 12% and 6%, respectively, in patients with EA/TEF. The authors recommended a CT angioscan to rule out such malformations when stenting of the esophagus is indicated, before esophageal replacement surgery, and when prolonged (>2 wk) use of a nasogastric tube is considered. [55]
Diagnosis of esophageal atresia with 3D CT is highly reliable, with 100% sensitivity and specificity. [46, 56] Findings with this technique have been shown to be well correlated with surgical and/or bronchoscopic findings. CT diagnosis of tracheomalacia is reliable, but suspicions should be confirmed with biopsy or endoscopy.
Magnetic Resonance Imaging
Postnatally, MRI has no role in the routine imaging of esophageal atresia (EA) and tracheoesophageal fistula (TEF); however, MRI does offer the ability to image the entire length of the esophagus in both the sagittal and coronal planes, and its contrast resolution is superior to that of CT. MRI is used rarely to determine the location of the aortic arch, but it has been used prenatally to diagnose congenital malformations. [44, 52, 57, 58]
Unlike ultrasonography, prenatal MRI allows visualization of the entire lesion and the anatomic relationships. Fetal MRI has proven accurate for establishing or ruling out a prenatal diagnosis of EA in high-risk infants based on ultrasonographic findings; however, fetal MRI may be difficult in cases of polyhydramnios because of poor image quality.
Images obtained from MRI are highly accurate. Fetal MRI has 100% sensitivity in prenatally diagnosing esophageal atresia in high-risk infants.
Prenatal imaging and fetal center evaluation correctly identified the presence or absence of esophageal atresia in 78% of patients referred on suspicion of this condition. The presence of an esophageal pouch on fetal MRI had significant predictive value for EA. The authors of this study noted that these data may assist with evidence-based prenatal family counseling. [59, 26, 27]
Fetal MRI was shown by Hochart et al to be useful for the diagnosis of EA through the visualization of the esophageal pouch or through associated signs such as tracheal bowing. Visualization of the lower esophageal lumen was found to to be a good sign of TEF. [60]
No known variants mimic EA on fetal MRI examinations. The appearance of esophageal indentations, however, may mimic that of fistulas. If findings are questionable, postnatal imaging should be performed to confirm the presence or absence of a fistula.
Ultrasonography
Although ultrasonography has a limited role in the routine postnatal evaluation of esophageal atresia (EA) and/or tracheoesophageal fistula (TEF), prenatal sonography is a valuable screening tool for EA and/or TEF. [61, 57, 62, 63, 26, 28]
Postnatally, high-frequency ultrasonography has been used to evaluate the proximal and distal pouch as well the gap, but it has limited utility in demonstrating a fistula. Endoscopic ultrasonography produces a 5-layered image of the esophageal wall that has been used in cancer staging. The aortic arch may be located sonographically to plan for EA and/or TEF repair.
Prenatally, ultrasonographic finding of an absent or small fetal stomach bubble in combination with maternal polyhydramnios is suggestive of EA and/or TEF. [24] The diagnostic accuracy is increased if an anechoic area is present in the middle of the fetal neck; this sign differentiates EA from diseases with possible swallowing impairments.
Ultrasonographic findings prenatally may suggest a diagnosis of isolated esophageal atresia or tracheoesophageal fistula; however, prenatal EA detection rates are low, and if suspected, the diagnosis must be confirmed postnatally. A systematic review and meta-analysis of 23 studies found prenatal ultrasound had a sensitivity of 41.9%, a specificity of 99.9%, a positive likelihood ratio of 88.1, a negative likelihood ratio of 0.58. and a diagnostic odds ratio of 153.7. By itself, ultrasonography is a poor diagnostic tool for identifying esophageal atresia prenatally, as well as having a high rate of false positive diagnoses. Magnetic resonance imaging and amniotic fluid analysis have high diagnostic accuracy for esophageal atresia. [25]
The presence of a dilated blind-ending esophageal pouch on a sonogram is suggestive of EA. This pouch sign has been confirmed with direct visualization after 26 weeks' gestation, but its onset has been suggested as early as the 22nd week. [64, 65, 66] The possibility of an association between increased nuchal translucency observed in the first trimester and EA with fistula has been investigated. [67]
The pouch sign is the most reliable sonographic sign indicative of EA, and it is noted in the presence of EA with or without TEF. [64, 65, 66] The prenatal diagnosis rate of EA is low; it has been reported to be 9.2%. Unless the dilated blind-ending proximal pouch is visualized directly, suspicion requires postnatal confirmation. The positive predictive value of the finding of a small or absent fetal stomach bubble in association with maternal polyhydramnios is 56%, and the sensitivity of prenatal ultrasonography in the diagnosis of EA is 42%.
Polyhydramnios alone is a poor predictor of EA. [24] Only 1 in 12 patients with polyhydramnios has EA. Similarly, a small or absent fetal stomach bubble has multiple associations in addition to EA. [24] These findings, then, are not conclusive and require further testing with the passage of a postnatal nasogastric tube from the mouth to the stomach and with plain chest radiography to confirm diagnosis. [24]
Prenatal ultrasonography can also cause many cases of fistula to be missed because fluid may pass through the fistula, contributing to the appearance of a normal, fluid-filled stomach. Sonography can depict TEF in about one third of affected fetuses.
No normal variants mimic the prenatal sonographic findings of EA and/or TEF. Transient visualization of esophageal fluid and the absence of visualization of the stomach may be seen in healthy patients. Polyhydramnios, while not normal, is a nonspecific finding.
Polyhydramnios can be seen in the following conditions:
-
Agnathia, microstomia, synotia
-
DiGeorge velocardiofacial syndrome
-
Placental insufficiency
-
Maternal diabetes mellitus
-
Chromosomal disorders, isoimmunologic disease, congenital abnormalities, multiple gestations, idiopathic conditions
-
Epignathus
-
Hydrolethalus
Nuclear Imaging
Typically, nuclear medicine is not used in the evaluation of esophageal atresia (EA) and tracheoesophageal fistula (TEF); however, it may be useful in the assessment of motility after repair. [68] Scintigraphy and radionuclide studies enable detection and quantification of esophageal transit, esophageal clearance, and gastroesophageal reflux (GER). [44]
Radionuclide esophageal transit studies are associated with a high degree of confidence, with 97.3% sensitivity and a 94.7% positive predictive value for the determination of motility dysfunctions, as well as 92.3% sensitivity and 90.9% specificity for the evaluation of GER. Gastroesophageal scintiscans are also accurate for the diagnosis of GER, with sensitivity reported to be as high as 90%.
-
Esophageal atresia (EA) with distal tracheoesophageal fistula (TEF). Frontal view of the chest and abdomen of a neonate demonstrates a tube in the proximal pouch in this patient with EA. The presence of bowel gas implies the presence of a distal TEF, making this the most common type of EA/TEF.
-
Isolated esophageal atresia (EA). Frontal view of the chest and abdomen demonstrates a catheter in the proximal pouch in this patient with EA. Note the absence of bowel gas in this patient with EA, but it is not associated with a tracheoesophageal fistula (TEF).
-
H-type tracheoesophageal fistula (TEF). Oblique barium esophagogram demonstrates a fistula (arrow) arising from the anterior esophagus and extending anterosuperiorly to the trachea.
-
Vertebral, anorectal, cardiac, tracheal, esophageal, renal, and limb (VACTERL) association. Frontal radiograph in a patient with esophageal atresia (EA) without a tracheoesophageal fistula (TEF). Note the catheter in the proximal pouch and the butterfly vertebra (asterisks) at the level of T8 in this patient with associated VACTERL.
-
False-positive finding of esophageal atresia (EA). Image demonstrates a feeding tube coiled in the proximal esophagus (E). An umbilical arterial catheter (U) is noted at the level of T11. The catheter was repositioned and extended to the stomach, with no alteration of the infant's cry (see next image).
-
False-positive finding of esophageal atresia. Follow-up image in the patient in the previous image demonstrates the tube (T) extending to the stomach. The endotracheal tube (ET) and umbilical arterial catheter (U) are also identified.
-
Gapogram shows the location of the proximal pouch (P), which is suggested by the position of the catheter. The distal pouch location (D) is visualized with the reflux of contrast material through a previously placed gastrostomy tube (G). The distance between the proximal and distal pouches is measured on the adjacent radiopaque ruler.
-
Barium esophagogram obtained in a patient 3 weeks after esophageal repair shows the relative narrowing at the anastomotic site (A). Reflux (arrow) is seen with an associated sliding hiatal hernia (R).
-
Stricture with food bolus. Frontal view from a barium swallow examination in a patient with a repaired EA shows a stricture at the anastomotic site, with a bolus of food proximal to the stricture.





