Practice Essentials
Subarachnoid hemorrhage (SAH) is a condition in which there is bleeding into the subarachnoid space around the brain and spinal cord, as shown in the images below. This space is normally filled with clear, colorless cerebrospinal fluid (CSF). The most common causes of subarachnoid hemorrhage are head trauma and rupture of an intracranial aneurysm. Atraumatic subarachnoid hemorrhage accompanied by the sudden onset of neurologic symptoms has been termed hemorrhagic stroke. Radiologic evaluation is essential for determining the prognosis and treatment of subarachnoid hemorrhage. Radiologic interventional procedures have become increasingly important for the management of this condition. [1, 2, 3, 4, 5, 6]
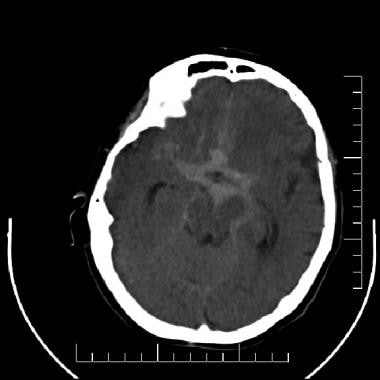 Subarachnoid hemorrhage (SAH). A nonenhanced computed tomography scan of the brain that demonstrates an extensive SAH filling the basilar cisterns in a patient with a ruptured intracranial aneurysm.
Subarachnoid hemorrhage (SAH). A nonenhanced computed tomography scan of the brain that demonstrates an extensive SAH filling the basilar cisterns in a patient with a ruptured intracranial aneurysm.
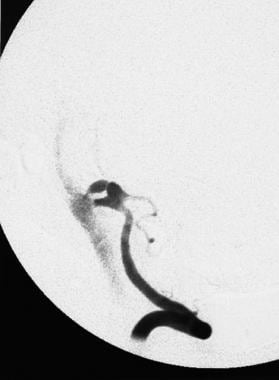 An angiogram showing the onset of an aneurysmal rupture, with extravasation of contrast material into the subarachnoid space from the anterosuperior aspect of a bilobed aneurysm in a posteroinferior cerebellar artery.
An angiogram showing the onset of an aneurysmal rupture, with extravasation of contrast material into the subarachnoid space from the anterosuperior aspect of a bilobed aneurysm in a posteroinferior cerebellar artery.
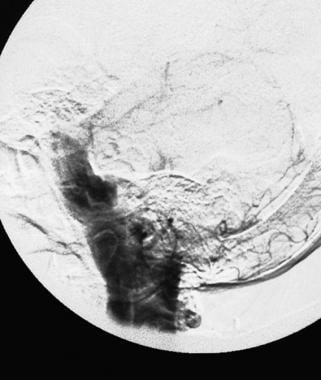 A late angiogram demonstrating contrast medium filling the posterior fossa subarachnoid spaces, including the ambient, prepontine, and perimedullary cisterns.
A late angiogram demonstrating contrast medium filling the posterior fossa subarachnoid spaces, including the ambient, prepontine, and perimedullary cisterns.
Subarachnoid hemorrhage is defined as blood between the arachnoid membrane and the pia membrane. The incidence of subarachnoid hemorrhage in the United States is 10-14 per 100,000 individuals per year. Most cases of aneurysmal SAH occur in patients older than 50 years, and 30% of subarachnoid hemorrhages occur during sleep. Aneurysmal subarachnoid hemorrhage can be preceded by a sharp, severe headache a few weeks before rupture. [1, 7, 2]
Imaging modalities
Computed tomography (CT) scanning without intravenous contrast enhancement is the preferred initial diagnostic study, with cerebral angiography the next procedure of choice. [3, 8, 9]
Because of advances made with CT angiography and magnetic resonance angiography (MRA), institutions that have a high degree of expertise and experience with these noninvasive imaging modalities may choose to use them in addition to, or even instead of, catheter angiography. [3]
At most institutions in the United States, conventional angiography remains the standard for evaluating patients with subarachnoid hemorrhage. If a CT scan of the brain is negative and a strong clinical suggestion of subarachnoid hemorrhage exists, a CSF tap may be of value for confirming this diagnosis. If the CSF reveals no evidence of subarachnoid hemorrhage (ie, either overt hemorrhage or xanthochromia), cerebral angiography may not be indicated. [10]
For SAH caused by rupture of an intracranial aneurysmal vessel or arteriovenous malformation, emergency physicians have classically performed a noncontrast CT (NCCT), followed by a lumbar puncture. [11, 10] However, as CT technology has advanced, many studies have questioned the need for lumbar puncture and have advocated for noninvasive techniques, such as NCCT alone or NCCT with CT angiography. [4]
Nonenhanced CT scanning may fail to depict small subarachnoid hemorrhages, particularly if imaging is performed several days after the onset of bleeding. Furthermore, CT scans are degraded by patient motion. If a patient cannot cooperate because of an alteration in mental status, sedation may be necessary to obtain satisfactory diagnostic images.
Cerebral angiography is an invasive procedure with a small but significant risk of complication. Without the use of a special hemostasis device, at least 6 hours of bed rest is required after the procedure to prevent bleeding at the puncture site. Additionally, because of its small false-negative rate for aneurysm, cerebral angiography must be repeated after 1-2 weeks to further improve its diagnostic sensitivity.
The American Heart Association (AHA) guidelines for evaluation and diagnosis of subarachnoid hemorrhage emphasize the need to maintain a high level of suspicion for SAH in patients with an acute severe headache and recommend evaluation with head CT scanning followed by lumbar puncture (LP) if the CT scan is negative. [5]
The American College of Radiology (ACR) recommends cervicocerebral arteriography or CT angiography of the head with IV contrast for patients with findings of acute SAH on initial CT scan. [6]
Anatomy
Rupture of a saccular intracranial aneurysm causes approximately 80% of cases of nontraumatic subarachnoid hemorrhage. Intracranial aneurysms develop predominantly at vessel bifurcation or branching points. Saccular aneurysms are acquired lesions that rarely present before the third decade of life.
Most intracranial aneurysms occur at typical locations within or near the circle of Willis. The most common specific locations of intracranial aneurysms are at the middle cerebral artery bifurcation and along the anterior communicating artery. These 2 locations account for approximately 60% of all intracranial aneurysms. Other common sites of aneurysm formation in the anterior circulation are at the origins of the posterior communicating and ophthalmic arteries. Approximately 10-20% of aneurysms arise from the vertebral and basilar arteries.
The tip of the basilar artery is the most common location of aneurysm formation in the posterior circulation. The origins of the posterior inferior cerebellar arteries are also common sites of aneurysm formation. Arteriovenous malformations (AVMs) occur throughout the brain without predisposition for a particular anatomic area.
Neuroradiologic intervention
Approximately 10-30% of patients with subarachnoid hemorrhage die before reaching medical attention. For those reaching a hospital alive, mortality rates for nontraumatic subarachnoid hemorrhage have been reported in the 30-60% range. [12] In-hospital mortality has been shown to be lower at facilities with interventional neuroradiology. [13]
The endovascular treatment of intracranial aneurysms has evolved rapidly. The initial experience in the treatment of intracranial aneurysm with catheter-based techniques relied predominantly on parent-vessel occlusion by various mechanisms, including endovascular detachable balloons and coils. With widespread physician acceptance and approval of the Guglielmi detachable coil (GDC) by the US Food and Drug Administration (FDA), the emphasis of endovascular management has changed to aneurysm occlusion with the preservation of patency of the parent vessel. [14, 15]
Although the primary indication for GDC embolization of an intracranial aneurysm is for patients with surgically high-risk aneurysms, a growing body of evidence indicates that endovascular treatment should be considered as a primary option for aneurysm in certain anatomic locations. Specifically, patients with basilar tip aneurysms appear to have better outcomes with endovascular therapy than with open craniotomy and surgical aneurysm clipping. The technical expertise and experience of the local treating physicians may determine the optimal treatment for aneurysms at other locations.
Improvements in small-vessel angioplasty balloon catheters and promising initial therapeutic results have led to increased use of intracranial angioplasty for the treatment of subarachnoid hemorrhage–induced vasospasm. In general, intracranial angioplasty may be performed in the internal carotid, proximal middle or anterior cerebral, and vertebral and basilar arteries. Selective intra-arterial papaverine infusion has also been used in the treatment of intracranial vasospasm. [3]
Ottawa Subarachnoid Hemorrhage Clinical Decision Rule
The Ottawa Subarachnoid Hemorrhage Clinical Decision Rule is a clinical tool designed for use in the emergency department to detect the 5-6% of cases of SAH that may be missed. Multiple studies have validated the initial finding of 100% sensitivity. However, specificity is low across studies (7.5%-22%). [16] The American College of Radiology (ACR) cautions that this tool is meant to identify SAH that might otherwise be missed but is not intended to independently diagnose SAH. [17]
The Ottawa SAH rule includes the following 6 clinical criteria that were deemed a high risk for SAH [18] :
-
Age ≥40 yr
-
Neck pain/stiffness
-
Loss of consciousness
-
Onset during exertion
-
Thunderclap headache (instantly peaking pain)
-
Limited neck flexion
If one or more of these clinical features are present, the patient must undergo computed tomography (CT).
Differential diagnosis and other problems to be considered
The differential diagnosis includes cerebral aneurysm, cerebral arteriovenous malformation, stroke, reversible cerebral vsoconstriction syndrome (RCVS). [17]
Traumatic subarachnoid hemorrhage must be distinguished from spontaneous subarachnoid hemorrhage. Cerebral angiography may sometimes be avoided if it can be confidently established that the hemorrhage is caused by trauma. This distinction can be difficult to make, because the traumatic event may not have been witnessed and the patient may be unable to provide a reliable history. There is often a question as to whether a spontaneous subarachnoid hemorrhage has caused a traumatic event or the trauma caused the hemorrhage. When in doubt, it is usually best to obtain a cerebral angiogram to exclude an underlying aneurysm or vascular malformation; such angiograms can sometimes be limited to the location of the hemorrhage, if no pathology is detected.
Complete and proper written informed consent must be obtained before diagnostic and interventional procedures are performed. [19] The informed consent form should specifically include the possibility of stroke with diagnostic cerebral angiography and neurovascular interventional procedures. [20]
Radiography
In unresponsive or unreliable patients requiring magnetic resonance imaging (MRI), plain radiographs of the skull and orbits may be used to exclude the presence of aneurysm clips or intraorbital foreign bodies. Plain radiographs may also be useful for evaluating facial or cervical spinal fractures to assess the probability of traumatic subarachnoid hemorrhage (SAH) versus spontaneous SAH.
Some neuroradiologists use skull radiographs in their routine follow-up care of patients with aneurysm treated with coil embolization. Interval follow-up skull radiographs can be compared with baseline studies to check for coil compaction.
The degree of confidence is high for the purposes stated above; however, plain radiography offers no reliable findings for detecting SAH.
Guglielmi detachable coils (GDC) have been observed to shift in position during the subsequent thrombosis of large and medium-sized aneurysms. This finding does not necessarily indicate that the aneurysm is patent. Cerebral angiography should be performed when any change in coil position is observed on postembolization follow-up studies.
Computed Tomography
On CT scans, subarachnoid hemorrhage (SAH) appears as a high-attenuating, amorphous substance that fills the normally dark, CSF-filled subarachnoid spaces around the brain, as shown in the images below. The normally black subarachnoid cisterns and sulci may appear white in acute hemorrhage. These findings are most evident in the largest subarachnoid spaces, such as the suprasellar cistern and Sylvian fissures.
 Subarachnoid hemorrhage (SAH). A nonenhanced computed tomography scan of the brain that demonstrates an extensive SAH filling the basilar cisterns in a patient with a ruptured intracranial aneurysm.
Subarachnoid hemorrhage (SAH). A nonenhanced computed tomography scan of the brain that demonstrates an extensive SAH filling the basilar cisterns in a patient with a ruptured intracranial aneurysm.
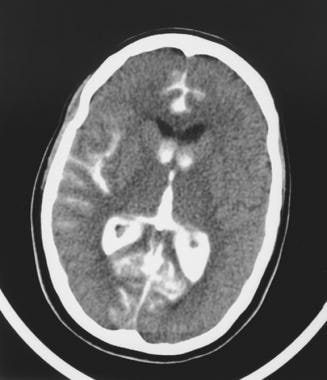 A computed tomography scan obtained after angiography of a rupturing bilobed aneurysm of the posteroanterior cerebellar artery. This image shows a subarachnoid hemorrhage and contrast medium filling the right sylvian fissure, the interhemispheric fissure, and the lateral and third ventricles.
A computed tomography scan obtained after angiography of a rupturing bilobed aneurysm of the posteroanterior cerebellar artery. This image shows a subarachnoid hemorrhage and contrast medium filling the right sylvian fissure, the interhemispheric fissure, and the lateral and third ventricles.
Over the cerebral hemispheres, SAH is exhibited by the filling in of normally low-attenuating (black) sulci with high-attenuating (white) subarachnoid blood. SAH is most conspicuous within 2-3 days of the onset of acute bleeding. Acute SAH is typically 50-60 Hounsfield units (HU). The protein content of the hemoglobin molecule is predominantly responsible for the attenuating effect of blood; therefore, the absolute measurement in HU varies somewhat with the hematocrit value.
When CT scanning is performed several days or weeks after the initial bleed, the findings are more subtle. The initial high-attenuation of blood and clot tend to decrease, and these appear as intermediate gray. These findings can be isointense relative to normal brain parenchyma. If the patient presents during this subacute period, evidence of SAH includes decreased visualization of the normally hypoattenuating fluid within the sulci and basal cisterns and enlargement of the ventricles caused by communicating hydrocephalus.
In addition to detecting SAH, CT scanning is useful for localizing the source of bleeding. This is particularly important in cases of multiple intracranial aneurysms, which occur in 20% of patients. Localization of SAH on CT scans correlates with the location of the ruptured aneurysm. The presence of blood in the anterior interhemispheric fissure or the adjacent frontal lobe suggests rupture of an anterior communicating artery aneurysm. Sylvian fissure clot correlates with an ipsilateral middle cerebral artery aneurysm. Blood predominantly localized in the posterior fossa suggests bleeding from a posterior circulation aneurysm.
The ability to discern the location of an aneurysm rupture is limited by the fact that many patients with SAH have a diffuse distribution of blood in the subarachnoid spaces and basal cisterns on CT scans. The effect of gravity has been suggested as a possible cause for misleading patterns of blood distribution. Published studies report a wide variation in the accuracy of CT scanning in localizing the bleeding source.
In addition to the diagnosis of SAH and the localization of the bleeding site, CT scanning also allows for some degree of prognostication, particularly in the probability of the development of vasospasm. Fisher originally demonstrated that the amount of blood and the presence of localized clots in the subarachnoid space are correlated with a higher incidence of delayed symptomatic arterial spasm; this correlation has since been well validated. [21]
A specialized CT scanning technique involving the inhalation of xenon gas allows for the quantitative determination of regional cerebral blood flow, which can be of value in monitoring the severity and effect of cerebral vasospasm. [3, 22]
Degree of confidence
Nonenhanced CT scanning of the brain is the study of choice for the initial evaluation of patients with potential SAH. The sensitivity is 93-100% in patients presenting with SAH within 24 hours of symptom onset. Conversely, the detection of SAH on CT scanning has a 0-7% false-negative rate during this period. As the time from the onset of the bleeding episode increases, the sensitivity of CT scanning decreases. At 5 days, the sensitivity is approximately 85%; at 1 week, it is approximately 50%.
If a high clinical concern for SAH exists and the CT brain scan is negative, a lumbar puncture (LP) is indicated. Although LP is considered to have a higher sensitivity than that of CT scanning, its specificity is lower. Additionally, LP is an invasive procedure. [3]
In patients with a negative noncontrast CT and xanthochromia detected on lumbar puncture, a CT angiography (CTA) can be performed to evaluate for a saccular aneurysm. Although some institutions will perform catheter angiography after a negative CTA on clinical grounds, multiple studies have reported that CTA is adequate to exclude aneurysms in cases with no visible blood on CT. [23, 24]
Various normally attenuating extra-axial structures may be misinterpreted as SAH, and these can lead to false-positive results. The falx cerebri, tentorium cerebelli, and intracranial blood can be confused for small amounts of SAH. Streak artifacts from bone at the skull base and partial volume-averaging artifacts may also lead to a false diagnosis of SAH. False-negative studies may occur from errors in interpretation or from failure of the technology itself. Perceptual errors aside, several conditions may lead to the inability of CT scanning to detect a SAH. [3]
An interval of days to weeks between the bleeding episode and the CT scan allows for the breakdown and resorption of some or all of the hemoglobin from the subarachnoid space, decreasing the contrast between the SAH and CSF. Similarly, small amounts of SAH may be masked by dilution by the CSF and/or CT volume averaging with CSF. Motion artifacts occurring in the scans of agitated or confused patients can lead to either false-positive or false-negative SAH diagnoses.
"Pseudosubarachnoid hemorrhage" can occur when high-density areas are seen in the cisterns and cortical sulci in the setting of severe brain edema. [25] Although the mechanism is not clear, it may be related to a combination of decreased attenuation of the brain parenchyma and distention of the superficial vessels secondary to elevated intracranial pressure. Yuzawa et al suggested that pseudosubarachnoid hemorrhage can be differentiated from true subarachnoid hemorrhage by a lower attenuation (pseudosubarachnoid hemorrhage is < 43 HU) and an absence of intraventricular high attenuation (patients with subarachnoid hemorrhage, however, may not have intraventricular blood). [26]
In a study of patients with aneurysmal SAH, CT evidence of SAH was present but went unrecognized in 4% (18 of 452 cases), according to the final radiology report in cases of presumed CT-negative aSAH. [27]
Magnetic Resonance Imaging
Fluid-attenuated inversion recovery (FLAIR) is the most sensitive MRI pulse sequence for the detection of subarachnoid hemorrhage (SAH). On FLAIR images, SAH appears as high signal-intensity (white) in normally low signal-intensity (black) CSF spaces. In cases of SAH, FLAIR and CT scanning have similar findings. T2- and T2*-weighted images can potentially demonstrate SAH as low signal-intensity in normally high signal-intensity subarachnoid spaces. On T1-weighted images, acute SAH may appear as intermediate-intensity or high-intensity signal in the subarachnoid space. [28]
MRA may be useful for evaluating aneurysms and other vascular lesions that cause SAH. The low sensitivity for aneurysms smaller than 5 mm, the inability to evaluate small aneurysm contour irregularities, and difficulty in obtaining high-quality images in patients who are agitated or confused limits the utility of MRI in the diagnosis of acute SAH. [3]
A study of 49 patients with aneurysmal SAH found that T2* was highly predictive of the location of the initial hemorrhage (positive predictive value, 95%), especially in the Sylvian cisterns (positive predictive value, 100%) and the anterior interhemispheric fissure (positive predictive value, 90%). [29]
Degree of confidence
In vivo and in vitro studies suggest that FLAIR MRI is as sensitive as, or more sensitive than, CT scanning in the evaluation of acute SAH; however, compared with lumbar puncture, FLAIR MRI cannot exclude SAH. [28] Relative to CT scanning, MRI is often more valuable in the subacute phase of SAH, in which the density of hemorrhage on CT scans decreases. In patients with equivocal findings on CT scanning or angiography or in those patients who cannot undergo CT scanning or conventional angiography, MRI and/or MRA may provide clinically useful information.
Magnetic field inhomogeneity can lead to artifactual increase in signal intensity in sulci over the cerebral convexities on FLAIR images, which can mimic SAH. CSF flow artifacts can mimic the appearance of SAH on either T1- or T2-weighted images. Intracranial thrombus can appear similar in signal to flowing blood on time-of-flight (TOF) gradient-echo (GRE) MRA. In uncooperative patients, motion artifacts may produce images that can lead to either false-positive or false-negative interpretations.
Hyperintensity in the subarachnoid space on FLAIR images can also be secondary to other pathologies, such as meningitis or meningeal carcinomatosis. [28] It is important to know whether recent contrast-enhanced MRIs have been performed, as delayed leakage of gadolinium into the subarachnoid space can result in hyperintense signal on FLAIR images. This has been reported to result from contrast studies performed 24-48 hours before MRI scanning in patients with intact renal function and an intact blood-brain barrier. However, this is typically associated with abnormalities that can alter perfusion and disrupt the blood-brain barrier (eg, acute ischemic stroke and after carotid artery and balloon stenting), as well as found in patients with renal failure or who are receiving high doses of gadolinium. [30] Substantial increases in subarachnoid FLAIR signal have also been reported in patients receiving 100% supplemental oxygen. [28]
Ultrasonography
Echoencephalography is useful for diagnosing germinal matrix and intraventricular hemorrhage in the newborn; however, ultrasonography has no direct role in the diagnosis of subarachnoid hemorrhage (SAH) in the adult patient. Conversely, transcranial Doppler ultrasonography has become increasingly used in the diagnosis and management of vasospasm in patients with SAH.
Serial transcranial Doppler ultrasonographic examinations accurately detect the presence of vasospasm and allow for the maximization of medical therapy for vasospasm before the patient becomes symptomatic. Increases in flow velocity correspond to cross-sectional diameter decreases in vessel lumen resulting from vasospasm. The more severe the vasospasm, the higher the flow velocity.
Flow is most easily measured in the middle cerebral arteries, which have been found to have flow velocities normally in the 30-80 cm/s range. Elevation to 120 cm/s indicates moderate vasospasm, and elevation to 200 cm/s indicates severe vasospasm. Single abnormal measurements are much less reliable then serial examinations that performed to establish patient baseline velocities before the development of vasospasm.
The sensitivity of transcranial Doppler ultrasonographic imaging for the detection of vasospasm has been reported to be 85-90%. Because not all vasospasm is necessarily symptomatic, the finding must be correlated with a clinical neurologic examination to determine the appropriate therapy.
Elevated intracranial vascular flow from an arteriovenous malformation or fistula may cause high flow velocities in the absence of vasospasm. Similarly, elevation of cardiac output from any cause generally leads to a systemic increase in flow velocities. Doppler ultrasonographic measurements are highly dependent on technical factors. Inaccuracy in the angle of insonation may result in artifactual elevation or reduction in the reported velocity measurements.
Nuclear Imaging
Like ultrasonography, nuclear medicine studies are not useful in the initial diagnosis of subarachnoid hemorrhage (SAH), but they can play a role in the diagnosis of related vasospasm. The nuclear medicine study technique that is most used for this purpose is single-photon emission computed tomography (SPECT) scanning with the radiopharmaceutical technetium-99m (99mTc) hexamethylpropyleneamine oxime (HMPAO).
SPECT scanning allows for the evaluation of qualitative or semiquantitative regional blood flow. The information provided is complementary to the transcranial Doppler ultrasonographic findings. SPECT scanning demonstrates perfusion of the brain tissue, whereas transcranial Doppler ultrasonography provides information about the flow in medium and large intracranial arteries. These 2 data sets are independent variables.
Perfusion of brain tissue can be maintained through collateral circulation and autoregulation despite the presence of severe vasospasm in a proximal artery. Conversely, perfusion can be persistently diminished in areas of infarction or small vessel ischemia despite the successful angioplasty of a proximal intracranial artery. [31]
The results of 99mTc HMPAO SPECT scanning are semiquantitative and qualitative in that the cerebellum is generally considered as a control value for normal perfusion. Generalized cerebral hypoperfusion may go unrecognized, but this pattern is not typical of vasospasm. Because early vasospasm is frequently asymptomatic, correlation with serial clinical examination is crucial to establish the appropriate therapy. Clinical use of this study is hampered by the qualitative nature of the results. Absolute negative results are uncommon, and positive results may not be clinically relevant.
Space-occupying lesions such as cerebral hematoma can cause perfusion defects on SPECT perfusion imaging. These should be obvious when correlated with conventional CT scan images. Hydrocephalus can lead to a global decrease in cerebral perfusion, which, because of the lack of absolute quantification of the cerebral blood flow, could go unrecognized.
Angiography
Cerebral angiography is considered the standard imaging technique for the detection of intracranial aneurysms, arteriovenous malformations (AVMs), and fistulae, as shown in the images below. Aneurysms are detected as focal areas of outpouching or dilatation of the arterial wall. These frequently occur at arterial branching points in characteristic locations within or near the circle of Willis. [5, 6, 17, 24, 11, 32, 33, 34, 35, 36]
Cerebral angiography should include anteroposterior (AP), lateral, and one or more oblique views of both carotid and vertebral artery contrast injection studies. A submentovertical view is sometimes useful in demonstrating the neck of a middle cerebral artery bifurcation aneurysm or anterior communicating artery aneurysm.
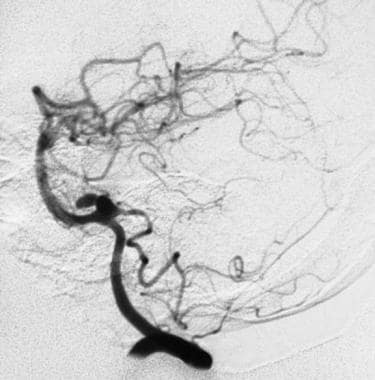 An angiogram showing a bilobed aneurysm of a posteroinferior cerebellar artery immediately before rupturing.
An angiogram showing a bilobed aneurysm of a posteroinferior cerebellar artery immediately before rupturing.
 An angiogram showing the onset of an aneurysmal rupture, with extravasation of contrast material into the subarachnoid space from the anterosuperior aspect of a bilobed aneurysm in a posteroinferior cerebellar artery.
An angiogram showing the onset of an aneurysmal rupture, with extravasation of contrast material into the subarachnoid space from the anterosuperior aspect of a bilobed aneurysm in a posteroinferior cerebellar artery.
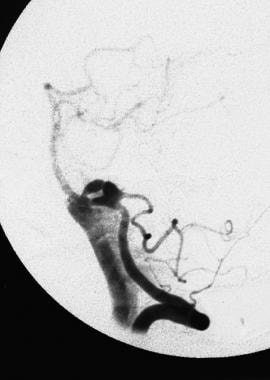 A later-phase angiogram of a rupturing bilobed aneurysm of a posteroinferior cerebellar artery shows progressive opacification of the subarachnoid space in the posterior fossa.
A later-phase angiogram of a rupturing bilobed aneurysm of a posteroinferior cerebellar artery shows progressive opacification of the subarachnoid space in the posterior fossa.
 A late angiogram demonstrating contrast medium filling the posterior fossa subarachnoid spaces, including the ambient, prepontine, and perimedullary cisterns.
A late angiogram demonstrating contrast medium filling the posterior fossa subarachnoid spaces, including the ambient, prepontine, and perimedullary cisterns.
Compression of the contralateral carotid artery should be performed during carotid cerebral angiography to demonstrate the anterior communicating artery if it does not fill spontaneously during one of the internal carotid artery injections. Carotid artery compression should be done on a segment of the common carotid artery without atherosclerotic plaque, if possible. One vertebral arteriogram is occasionally omitted by some angiographers if there is abundant reflux proximal to the origin of the posterior inferior cerebellar artery of that vertebral artery when the contralateral vertebral artery is injected with contrast medium. This technique does not, however, depict all aneurysms. The most reliable method of aneurysm detection is the invariable selective injection of contrast medium into both common or internal carotid arteries and both vertebral arteries.
Cerebral angiography reliably demonstrates the presence or absence of an intracranial aneurysm or an AVM, and it establishes the number and locations of aneurysms. Morphologic information, such as aneurysm size and shape, helps to determine which aneurysm has bled in a patient with multiple aneurysms. Specifically, the presence of a lobulation, teat, or daughter aneurysm is highly suggestive that the aneurysm is the one that has bled. In the absence of any distinguishing aneurysm shape features or hemorrhage localization by a CT scan, the largest aneurysm is the most likely to have bled. Features such as aneurysm location, shape, neck size, and neck-to-maximal diameter ratio are crucial in determining whether the aneurysm is better treated with open craniotomy or with an endovascular technique. [3]
According to published guidelines, diagnosis of an aneurysm by CT angiography or confirmation of negative CT angiography should be followed by cerebral angiography or digital subtraction angiography (DSA). [1, 7, 2, 35, 36]
Degree of confidence
Cerebral angiography provides a high degree of accuracy. A small false-negative rate does occur, probably in the range of 1-2%. A repeat cerebral arteriogram at 10-14 days is indicated if the initial angiogram does not demonstrate the cause of a subarachnoid hemorrhage (SAH). In a small number of patients, a follow-up angiogram will detect an aneurysm that was not demonstrated on the initial study.
According to study findings by Bechan et al, repeat 3D rotational angiography is necessary. In 25% of patients with aneurysmal SAH, repeat 3D rotational angiography demonstrated a ruptured aneurysm after initial 3D rotational angiography findings were negative. [32, 33, 34]
Bilateral selective external and internal carotid artery angiograms can be performed to exclude a dural arteriovenous fistula, which is a rare cause of SAH. Bilateral vertebral arteriograms of the neck (and, if necessary, selective thyrocervical trunk and/or careful injections of the right superior intercostal artery) demonstrate the arterial and venous circulation of the cervical spinal cord. In rare cases, they show a spinal vascular malformation or neoplasm, such as hemangioblastoma, as the cause of SAH.
Cervical spinal MRI and/or MRA may indicate the necessity of an additional arteriographic study. If thorough arteriographic studies do not demonstrate a specific cause for an SAH, a presumptive diagnosis of idiopathic perimesencephalic hemorrhage is sometimes made. [3]
The reason that some aneurysms are not initially diagnosed by angiography and are only detected on subsequent follow-up angiograms is not always evident. Vasospasm is believed to be the most common cause. Arteriographic double densities simulating aneurysm or apparent areas of vessel wall bulge or outpouching may be caused by arterial tortuosity and atherosclerosis or overlap of adjacent arteries on standard angiogram views. This can usually be determined by comparing all angiographic views and, if necessary, obtaining additional oblique arteriographic views.
-
Subarachnoid hemorrhage (SAH). A nonenhanced computed tomography scan of the brain that demonstrates an extensive SAH filling the basilar cisterns in a patient with a ruptured intracranial aneurysm.
-
An angiogram showing a bilobed aneurysm of a posteroinferior cerebellar artery immediately before rupturing.
-
An angiogram showing the onset of an aneurysmal rupture, with extravasation of contrast material into the subarachnoid space from the anterosuperior aspect of a bilobed aneurysm in a posteroinferior cerebellar artery.
-
A later-phase angiogram of a rupturing bilobed aneurysm of a posteroinferior cerebellar artery shows progressive opacification of the subarachnoid space in the posterior fossa.
-
A late angiogram demonstrating contrast medium filling the posterior fossa subarachnoid spaces, including the ambient, prepontine, and perimedullary cisterns.
-
A computed tomography scan obtained after angiography of a rupturing bilobed aneurysm of the posteroanterior cerebellar artery. This image shows a subarachnoid hemorrhage and contrast medium filling the right sylvian fissure, the interhemispheric fissure, and the lateral and third ventricles.