Practice Essentials
Spleen injuries are among the most frequent trauma-related injuries. Although protected under the bony rib cage, the spleen remains the most commonly affected organ in blunt injury and is affected in about 33% of patients with traumatic abdominal injuries. Blunt injuries can result from motor vehicle crashes, physical altercations, sporting events, and accidents involving bicycle handlebars. While penetrating trauma (eg, gunshot wounds, knife wounds) may involve the spleen, the incidence of injury is well below that of the small and large intestine. A third mechanism for splenic trauma is explosive-type injuries, such as occurs in warfare and civilian bombing. [1, 2, 3, 4]
The classic presentation of splenic injury includes hemodynamic instability, severe abdominal pain, and symptoms of peritonitis. Most patients with minor focal injury to the spleen have left upper quadrant abdominal tenderness. Patients may also have left shoulder tenderness resulting from subdiaphragmatic nerve root irritation with referred pain. In cases of free intraperitoneal blood, diffuse abdominal pain, peritoneal irritation, and rebound tenderness may be present. [5]
Hypotension in a patient with a suspected splenic injury can be a grave sign and a surgical emergency. It should prompt immediate evaluation and intervention or interventional radiology if a state of compensated shock can be maintained.
Missed splenic rupture or delayed diagnosis is associated with a 10-fold increase in mortality over the rate associated with prompt recognition of injury. Therefore, the radiologist must have a high index of suspicion and a low threshold for suggesting further evaluation with cross-sectional imaging.
A FAST exam should be instituted in hemodynamically unstable patients with splenic trauma to assess the degree of both trauma and bleeding. If the FAST exam is negative, it should be followed by a CT scan. If CT scanning is not available, then diagnostic peritoneal lavage (DPL) can be used. [6, 7, 2, 8, 3, 9]
If IV contrast is not used with CT, very significant splenic injuries may be missed by the lack of image optimization even with modern multi-detector CT scanning. CT findings may include hemoperitoneum, hypodensity, and contrast blush or extravasation. Repeat CT scans are not indicated in patients who are hemodynamically stable. [10, 11, 2, 3, 12, 13]
However, because of the expense and exposure to radiation, there have been efforts to minimize the use of CT. Children are at especially high risk of radiation-induced malignancies. Algorithms have been designed to help differentiate between high-risk and low-risk for intra-abdominal injury to guide abdominal CT usage and reduce radiation exposure. Models proposed combine examination, vital signs, radiographic findings, ultrasound, and laboratory values. [7, 14, 15, 16]
Nonoperative management is considered the gold standard for trauma patients with specific parameters. Studies have confirmed that the integration between CT in the early management of trauma and nonoperative management results in increased survival. [17, 6, 18, 19, 7]
American Association for the Surgery of Trauma (AAST) splenic injury scale
The American Association for the Surgery of Trauma (AAST) splenic injury scale is based on computed tomography (CT) findings, as follows. [20]
Grade I:
-
Subcapsular hematoma < 10% of surface area
-
Parenchymal laceration < 1 cm depth
-
Capsular tear
Grade II:
-
Subcapsular hematoma 10-50% of surface area
-
Intraparenchymal hematoma < 5 cm
-
Parenchymal laceration 1-3 cm in depth
Grade III:
-
Subcapsular hematoma >50% of surface area
-
Ruptured subcapsular or intraparenchymal hematoma ≥5 cm
-
Parenchymal laceration >3 cm in depth
Grade IV:
-
Any injury in the presence of a splenic vascular injury or active bleeding confined within splenic capsule
-
Parenchymal laceration involving segmental or hilar vessels producing >25% devascularization
Grade V:
-
Shattered spleen
-
Any injury in the presence of splenic vascular injury with active bleeding extending beyond the spleen into the peritoneum
World Society of Emergency Surgery (WSES) classification
The World Society of Emergency Surgery (WSES) classification divides spleen injuries into minor (I), moderate (II and III), and severe (IV) classes based on the AAST grades (see above), as follows. [5]
-
Minor spleen injuries: WSES class I includes hemodynamically stable AAST grade I–II blunt and penetrating lesions.
-
Moderate spleen injuries: WSES class II includes hemodynamically stable AAST grade III blunt and penetrating lesions; WSES class III includes hemodynamically stable AAST grade IV–V blunt and penetrating lesions.
-
Severe spleen injuries: WSES class IV includes hemodynamically unstable AAST grade I–V blunt and penetrating lesions.
The WSES guidelines include the following imaging recommendations [5] :
-
The choice of diagnostic technique at admission must be based on the hemodynamic status of the patient.
-
Extended focused assessment sonography for trauma (eFAST) is effective and rapid to detect free fluid.
-
CT scan with intravenous contrast is the gold standard in hemodynamically stable or stabilized trauma patients.
-
Doppler ultrasonography and contrast-enhanced ultrasonography are useful to evaluate splenic vascularization and in follow-up.
-
Injury grade on CT scan, extent of free fluid, and the presence of pseudoaneurysm (PSA) do not predict nonoperative management failure or the need of surgery.
Preferred examination
A FAST exam should be instituted in hemodynamically unstable patients with splenic trauma to assess the degree of both trauma and bleeding. If the FAST exam is negative, it should be followed by a CT scan. If CT scanning is not available, then diagnostic peritoneal lavage (DPL) can be used. [6, 7, 2, 8, 3, 9]
Although many plain radiographic imaging findings suggest spleen trauma injury, CT is the radiographic modality used at most institutions. CT scanning should be performed in conjunction with the intravenous administration of contrast material to maximize density differences between the splenic parenchyma and hematomas. In this fashion, CT provides the best evaluation of the spleen and the surrounding tissues. An additional advantage of CT is the ability to use it to image all of the abdominal organs simultaneously in excluding a secondary injury. (See the splenic trauma images below.)
If IV contrast is not used with CT, very significant splenic injuries may be missed by the lack of image optimization even with modern multi-detector CT scanning. CT findings may include hemoperitoneum, hypodensity, and contrast blush or extravasation. Repeat CT scans are not indicated in patients who are hemodynamically stable. [10, 11, 2, 3, 12, 13]
However, because of the expense and exposure to radiation, there have been efforts to minimize the use of CT. Children are at especially high risk of radiation-induced malignancies. Algorithms have been designed to help differentiate between high-risk and low-risk for intra-abdominal injury to guide abdominal CT usage and reduce radiation exposure. Models proposed combine examination, vital signs, radiographic findings, ultrasound, and laboratory values. [7, 14, 15, 16]
Focused abdominal sonographic technique (FAST), observing for the presence or absence of fluid in the peritoneal cavity, may be performed rapidly and safely in trauma patients. FAST is poor for delineating organ-specific anatomy with any reliability in the emergency setting. In addition, the learning and interpretation curve is rather steep when compared to diagnostic peritoneal lavage. In experienced hands, visualization of fluid in the right upper quadrant, the left upper quadrant, and the pelvis suggests solid organ injury (or mesenteric injury) and the possibility of splenic injury.
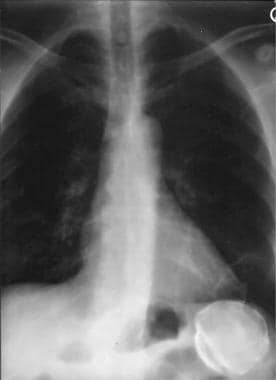 Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
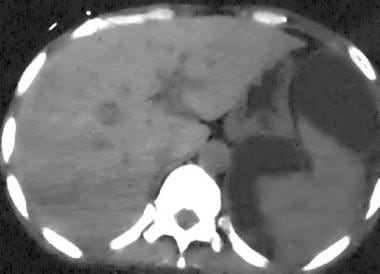
 Spleen, trauma. Contrast-enhanced arterial-phase CT scan of the abdomen shows a mottled appearance of the spleen. This finding should not be mistaken for splenic injury. Confirmation of a normal spleen can be shown by repeat imaging in a later phase of contrast enhancement. The spleen then appears homogeneously enhanced.
Spleen, trauma. Contrast-enhanced arterial-phase CT scan of the abdomen shows a mottled appearance of the spleen. This finding should not be mistaken for splenic injury. Confirmation of a normal spleen can be shown by repeat imaging in a later phase of contrast enhancement. The spleen then appears homogeneously enhanced.
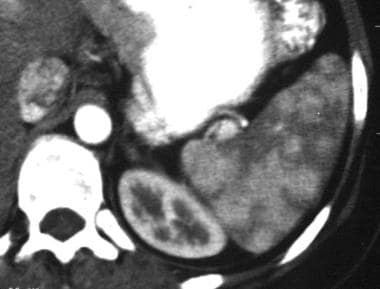
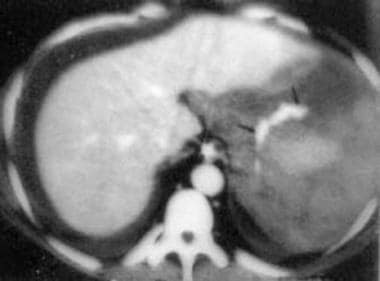 Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
Anatomy and etiology
The spleen is a functionally complex organ occupying the left upper quadrant (LUQ). It is an intraperitoneal organ weighing 75-100 g in the adult.
The spleen is the most vascular organ of the body, and approximately 350 L of blood passes through it per day. The spleen contains approximately 1 unit of blood at a given time. For this reason, splenic injury poses a potentially life-threatening situation. This risk is true especially because the spleen is the organ most commonly injured when thoracoabdominal trauma occurs, and splenic injuries represent approximately 25% of all blunt injuries to the abdominal viscera. Penetrating injuries also frequently involve the spleen along with other abdominal organs. The diagnosis of penetrating splenic injury is made easily because patients almost always are referred for surgery. [21, 22, 10, 23, 24, 25]
For the most part, the diagnosis of splenic rupture is not particularly challenging; however, radiologists should be aware of the possible processes that may simulate splenic injury.
Foreign material
Occasionally, iatrogenically introduced material can demonstrate the appearance of splenic rupture on CT. In a typical trauma center, a nasogastric tube is placed, and contrast material is orally administered prior to CT examination. Streak artifact and beam-hardening artifact from the nasogastric tube and oral contrast medium, respectively, can overlay the spleen and cause confusion. Beam-hardening artifact from the ribs and streak artifact from gastric air-fluid levels can also produce false-positive results. A combination of these effects, together with poor-quality scans secondary to increased patient size, is fairly typical in everyday practice.
Hematoma
To some degree, hemoperitoneum always accompanies splenic injury, with the exception of an intact subcapsular process. However, not all intra-abdominal fluid represents a hematoma (see the image below). The radiologist must be wary of assuming that splenic injury is the cause if fluid is appreciated in the abdomen or around the spleen. Most blunt splenic trauma is seen in children struck by a motor vehicle, in those involved in an activity-related fall, or in occupants of motor vehicles involved in accidents. The greatest potential for false-positive results occurs in patients injured in motor vehicle accidents primarily because they tend to be older and have a higher rate of preexisting medical conditions.
 Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
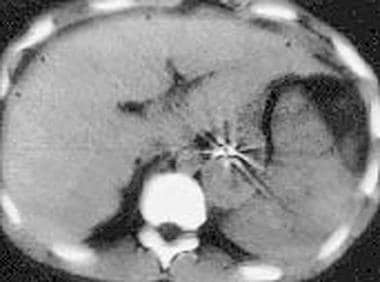
 Spleen, trauma. Contrast-enhanced CT scan of the abdomen in the equilibrium phase shows perisplenic fluid with mass effect on the spleen. The spleen appears compressed by the fluid, reminiscent of subcapsular fluid collections. In this patient, the fluid was secondary to pancreatic pseudocysts mimicking subcapsular hematomas.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen in the equilibrium phase shows perisplenic fluid with mass effect on the spleen. The spleen appears compressed by the fluid, reminiscent of subcapsular fluid collections. In this patient, the fluid was secondary to pancreatic pseudocysts mimicking subcapsular hematomas.
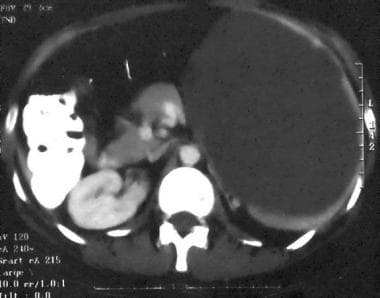
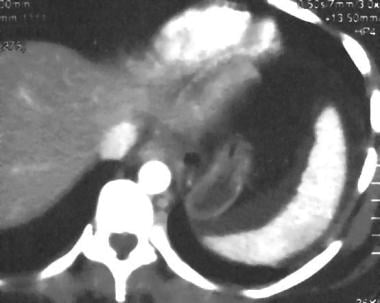
 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a perisplenic fluid collection with internal increased attenuation. The splenic border is displaced by mass effect. This was a subacute subcapsular hematoma. This is a grade I injury.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a perisplenic fluid collection with internal increased attenuation. The splenic border is displaced by mass effect. This was a subacute subcapsular hematoma. This is a grade I injury.
Fluid accumulation
Liver, pancreatic, renal, and left-sided colon disease can lead to accumulation of fluid in the LUQ and inferior to the spleen. Other potential causes of fluid accumulation should not be forgotten; these include undiagnosed abdominal malignancy with ascites and peritoneal dialysis. Although many of these situations seem unlikely, the opportunity to obtain pertinent information from the patient may not exist. In most motor vehicle accidents, multiple individuals are injured. Elderly individuals tolerate even minor trauma poorly, and their hemodynamic status is commonly out of proportion to their apparent injuries. In addition, many trauma patients are combative upon their arrival to the hospital secondary to drug and alcohol use. The net result is that patients are transported to the radiology department after sedation or intubation.
Extended focused assessment sonography for trauma (eFAST) has been found to be an effective and rapid initial assessment to detect free fluid in trauma patients. Two meta-analyses reported that eFAST is a useful bedside tool for ruling in intra-abdominal free fluid in the trauma setting. However, a negative eFAST exam does not rule out injuries and must be verified by a reference test such as CT. [26, 27]
 Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows perisplenic fluid without identification of a laceration in a patient who sustained blunt abdominal trauma. A large amount of pelvic fluid was seen, prompting laparotomy during which a small laceration was found; this is not evident on the scan.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows perisplenic fluid without identification of a laceration in a patient who sustained blunt abdominal trauma. A large amount of pelvic fluid was seen, prompting laparotomy during which a small laceration was found; this is not evident on the scan.
Cysts
Many processes can affect the spleen and simulate laceration or intrasplenic hematoma. A seemingly endless list of etiologies related to splenic cyst has been reported in the literature. Any one of these causes may be misdiagnosed as splenic injury, but these should not be accompanied by abdominal fluid or hemoperitoneum. Splenic abscesses caused by bacterial endocarditis, splenic infarction, and invasive procedures can simulate splenic injury, and they may be associated with perisplenic fluid.
Cystic lesions that mimic trauma can be classified by etiology as follows:
-
Congenital – Epidermoid (see the image below)
-
Vascular – Hematoma, posttraumatic cyst (80%), cystic infarct, and peliosis
-
Inflammatory – Pyogenic abscess; fungal microabscess due to Candida, Aspergillus, or Cryptococcus species; tuberculosis; infection by Mycobacterium avium complex, Pneumocystis carinii, or Echinococcus species; and pancreatic pseudocyst
-
Neoplastic – Cavernous hemangioma, angiosarcoma, lymphangioma, lymphoma, and metastasis (melanoma 50%)
Infarcts
Infarcts of the spleen can simulate the appearance of trauma. Classically, infarcts are well defined and wedge or triangular shaped. Infarcts extend from the outer margin with the apex pointing toward the splenic hilum. A thin rim of normal parenchyma can sometimes be seen along the outer margin. Although infarcts do not enhance, the outer rim may show enhancement along with the remainder of the spleen because of preserved capsular vessels. On sonograms and nonenhanced CT scans, infarcts can be confused with a laceration without perisplenic fluid.
Malignant tumors
Tumors of the spleen are uncommon overall. Most tumors of the spleen are related to lymphoma, which accounts for 70% of lesions. In addition, metastatic disease of the spleen is not uncommon, and melanoma, breast, lung, renal, and ovarian carcinomas are the leading primary cancers. These processes are hypoechoic on sonograms and hypoattenuating on CT scans, and they may simulate laceration or intraparenchymal blood. Metastatic disease can have associated ascites that simulates hemoperitoneum. Similar lesions in other organs and lymphadenopathy are usually present and exclude trauma. Lesion enhancement on contrast-enhanced CT scans is not demonstrated in blunt splenic trauma.
Benign tumors
The most common benign tumor of the spleen is the cavernous hemangioma. The tumor can be either hyperechoic or hypoechoic on sonograms and can simulate hematoma or noncoagulated blood, respectively. Hemangiomas appear hypoattenuating on CT scans, with variable contrast enhancement. Typically, the enhancement pattern is confined to the rim and fills in to become isoattenuating on delayed images. However, hemangiomas may not show contrast enhancement in a minority of patients. This finding is probably related to size of the lesion, with nonenhancement more commonly associated with smaller lesions.
Benign lesions can simulate parenchymal hematoma or small lacerations if near the periphery. The clue to correct diagnosis is the smooth distinct borders and rounded configuration of the hemangioma versus traumatic injury. Snowflake-like calcifications or phleboliths are uncommon, but they can differentiate tumor from trauma. Diffuse splenic hemangiomatosis is a condition in which the spleen is enlarged and replaced almost completely by hemangiomas. The striking appearance of the spleen can be dismissed as severe traumatic injury at first glance.
Splenic rupture
Nontraumatic splenic rupture is rare but has been associated with a number of disease processes. These entities may cause confusion, first because of their rarity and second because a traumatic etiology may be assumed. Careful inspection of the images usually leads to the correct diagnosis.
Sarcoidosis
Sarcoidosis is a disease of unknown etiology in which noncaseating granulomas form in tissues and organs of the body and have a predilection for the lymphatic system. The spleen is involved in 24-59% of patients with sarcoid but usually is asymptomatic. Those with marked involvement can develop abdominal symptoms. Severe cases can lead to hypersplenism and spontaneous rupture of uncertain etiology. [28] Most commonly, the spleen is diffusely affected, and the findings can mimic those of lymphoma. Splenomegaly is apparent in approximately one third of patients and frequently shows associated lymphadenopathy. Discrete hypoattenuating nodules are present on CT scans in approximately 15% of patients; these result from aggregated granulomas.
Amyloidosis
The spleen is involved in amyloidosis, which is a disease in which plasma cells deposit amyloid, a complex protein made mostly of polypeptide chains, throughout the tissues and organs. Amyloidosis can be primary or secondary, related to chronic inflammation (most notably rheumatoid arthritis), and occurs in association with multiple myeloma.
The spleen is affected in all forms of amyloidosis and appears diffuse and homogeneous in most patients. Diffuse decreased splenic attenuation with contrast enhancement can be seen on CT scans, but focal abnormalities, which can mimic laceration, are also possible. Spontaneous splenic rupture, believed to result from capsular weakening from amyloid deposition, has been reported. [29] Decreased attenuation in other involved organs (eg, liver and kidneys) can aid in distinguishing amyloidosis from a traumatic etiology. In addition, amyloid deposition in the retroperitoneal fat and mesentery leads to increased attenuation on CT scans.
Infection
Bartonella organisms are gram-negative bacilli originally considered to primarily infect patients with HIV infection. However, recent studies have demonstrated a Bartonella species to be the causative organism of catscratch disease. Therefore, the bacteria can infect immunocompetent individuals as well. Two primary infectious processes result from infection by Bartonella organisms; the form involving the liver and spleen is called bacillary peliosis hepatis.
Pathologically, the bacilli cause capillary dilation, resulting in numerable thin-walled, blood-filled cavities throughout the liver and spleen. Abdominal CT shows multiple low-attenuating lesions in the liver and spleen with associated lymphadenopathy and possible ascites. Lesions can coalesce to form multiloculated or septated lesions demonstrating variable contrast enhancement. Spontaneous splenic rupture has been reported in patients with bacillary peliosis hepatis. [30] A similar appearing process has been described related to the use of oral contraceptives, chemotherapeutic agents, and anabolic steroids, but the etiology of these processes remains obscure.
Secondary trauma
Any of the processes mentioned above may predispose the spleen to rupture, resulting in trivial degrees of trauma. A spleen enlarged by tumor masses or anemia may be injured with a simple fall on the sidewalk. A peripherally situated hemangioma or cyst may rupture with apparently insignificant trauma because of a weakened overlying capsule. These conditions are associated with hemoperitoneum or parenchymal hemorrhage and are virtually indistinguishable from simple splenic trauma.
Limitations of techniques
The limitations of CT scanning are few but possibly important. The most detrimental limitation to confident interpretation of a CT scan is motion artifact. The sensitivity for the detection of a splenic injury decreases precipitously if the patient cannot remain still on the scanning table. Adequate sedation is essential in such patients. Overall, the sensitivity and specificity of CT in the detection of splenic injury is close to 100% in the authors' experience. Any deficiency in detection is usually a result of misinterpretation of the information and not an absence of findings.
Intervention
Failure of conservative treatment involves grade III, IV, or V injuries more often than grade I and II injuries. In many studies, splenic artery embolization (SAE) has been described by using many different approaches. One primary point of discussion concerns the differences between main SAE, selective or superselective SAE, and embolization in a combination of sites. To the authors' knowledge, no studies have been performed to compare outcomes or complication rates based on the various levels of SAE. Theoretically, the infarction rate is expected to increase as embolization becomes more selective. Conversely, therapeutic failure is expected to increase with more proximal SAE. This presumption is based on collateral blood flow differences inherent with the particular level of embolization.
Sclafani et al [31] and Hagiwara et al [32] have described SAE techniques dependent on angiographic findings. The visualization of extrasplenic extravasation was treated with selective Gelfoam embolization or superselective gelatin sponge particle injection, respectively, followed by main SAE by means of coil occlusion. Main SAE alone was performed if intraparenchymal contrast-material extravasation was the only finding. Hagiwara et al [32] also selected an additional group of patients whose angiograms demonstrated vascular disruption without extravasation. This group also was treated by using main SAE alone. SAE was not performed if the angiogram showed only avascular areas or evidence of subcapsular hematoma without extravasation. The overall success rate in the 2 studies was greater than 90%.
The treatment of posttraumatic arteriovenous fistulas and pseudoaneurysms appears to require a different approach. Arteriovenous fistulas probably remain patent after main SAE, and they have been reported by Hagiwara et al. [32] Many investigators have reported the use of superselective coil embolization without main SAE to be successful in these patients. [33, 34, 35] Davis et al reported successful nonoperative treatment in 20 of 20 patients in whom pseudoaneurysms were embolized. All of the 6 patients in whom pseudoaneurysms could not be coiled for various reasons eventually required splenectomy. [33] Data attest to the need for definitive treatment if a pseudoaneurysm is documented angiographically.
Another interesting point elicited by Davis et al was the fact that 4 patients whose CT scans demonstrated contrast blush indicative of a pseudoaneurysm had negative findings on SA angiograms. [33] All 4 patients were treated successfully without surgery. The pseudoaneurysms may have thrombosed spontaneously, or the CT findings may have related to small areas of contained extravasation that caused tamponade. In either situation, data support a scenario in which positive CT findings should lead to angiographic evaluation. Negative angiographic findings are highly correlated with a good outcome in patients undergoing conservative treatment. Positive angiographic findings necessitate some form of intervention, be it angiographic or surgical.
The complication rate of SAE appears to be sufficiently low that it is not a significant concern compared with that of splenectomy. Data by Mozes et al showed a 2.4% (3 of 126) mortality within the first 6 months, compared with an 8% (2 of 25) mortality associated with splenectomy. [36] Both deaths related to splenectomy were associated with postoperative pancreatitis. Of the 126 patients who underwent SAE, 3 (2.4%) developed pancreatitis after embolization, but they did not die. Pancreatitis likely was a result of embolization of important collateral pancreatic vessels from the SA. Splenic abscess formation occurred in 4 (3%) of 126 patients. The most common cause of morbidity after SAE was the formation of pleural effusion in 9.5% of patients (12 of 126).
The morbidity of SAE is correlated with the percentage of splenic tissue embolized. Statistics reported by Mozes et al were based on the embolization of no more than 60-70% of splenic tissue. [36] Others have confirmed the unacceptably high morbidity and mortality rates involved with excessive tissue embolization or attempted nonsurgical splenectomy. Morbidity rates as high as 79% [37] and mortality rates ranging from 12% [38] to 43% [37] have been reported in the literature.
The angiographer must be aware of these reports. SAE should probably not be offered as an option if the procedure is likely to result in excessive splenic tissue loss, because splenectomy is associated with lower relative risk. A patient who has significant or total splenic volume infarction after SAE requires close observation for the development of complications. When the data are considered, continuing with splenectomy when the patient is stabilized may be prudent if complete splenic infarction occurs after SAE.
Radiography
In most circumstances, chest and abdominal radiography is the initial step in the evaluation of patients with blunt torso trauma (see the image below). However, the effects of blunt trauma are frequently masked by more obvious associated injuries. In most patients, the symptoms of blunt splenic trauma are absent or subtle; this characteristic probably explains the higher mortality associated with blunt abdominal trauma than with penetrating injury.
 Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
Missed splenic rupture or delayed diagnosis is associated with a 10-fold increase in mortality over the rate associated with prompt recognition of injury. Therefore, the radiologist must have a high index of suspicion and a low threshold for suggesting further evaluation with cross-sectional imaging.
Plain radiographic findings are numerous but occasionally subtle, and awareness of the imaging possibilities is important in effectively evaluating the information. Plain radiographs demonstrate a wide variety of abnormal findings. The constitution of findings reflects whether the spleen has sustained capsular rupture. Normal findings on chest and abdominal radiographs do not exclude splenic injury.
Common findings
The most common finding associated with splenic injury is left lower rib fracture. Rib fractures signify that adequate force has been transmitted to the left upper quadrant (LUQ) to cause splenic pathology. Left lower rib fracture is present in 44% of patients with splenic rupture and necessitates further workup by abdominal CT.
The classic triad indicative of acute splenic rupture (ie, left hemidiaphragm elevation, left lower lobe atelectasis, and pleural effusion) is not commonly present and should not be regarded as a reliable sign. However, any patient with apparent left hemidiaphragm elevation following blunt abdominal trauma should be considered to have splenic injury until proven otherwise.
More reliable signs of LUQ injury are medial displacement of the gastric bubble and inferior displacement of the splenic flexure gas pattern. These findings are indicative of an LUQ mass and result from either subcapsular or perisplenic hematoma. LUQ hematoma, if sufficiently large, can displace the shadow of the inferior splenic margin caudally, simulating splenomegaly. Subcapsular hematoma can produce a similar appearance, and the appreciated mass has distinct borders. Associated displacement of the left renal shadow also may be evident.
The constitution of findings present when retroperitoneal hemorrhage or free intra-abdominal blood exists contrasts with those mentioned above. Little, if any, mass effect on LUQ organs is apparent. Splenic margins are obscured, but this finding is not specific. Retroperitoneal blood can obliterate the left renal outline and psoas muscle margin. Free blood pools exist dependently in the left paracolic gutter, displacing the descending colon gas pattern medially.
Larger amounts of abdominal hemorrhage can obliterate the flank stripe. The small bowel gas pattern can be displaced out of the pelvis by collecting hemorrhage, and a midpelvic opacity with sharp convex lateral borders may result. The bladder margin is enhanced and demarcated by a thin lucency outlining the dome and representing extraperitoneal fat.
Splenic hematoma
Chronic splenic hematoma appears different and is more problematic because it is accompanied by a long list of differential diagnoses. The usual course of a subcapsular or parenchymal hematoma is to contract; liquefy; and, usually, resorb.
Occasionally, cystic degeneration of an intrasplenic hematoma results in formation of a false cyst. Approximately 80% of splenic cysts are estimated to be posttraumatic in origin. Approximately 80% of these are termed hemorrhagic cysts, and the other 20% are termed serous cysts and probably represent hemorrhagic cysts in which the blood has been resorbed totally.
Cysts
Thin, regular, annular calcification develops in the fibrous lining of approximately 30% of cysts. [39] Cysts are symmetric and unilocular, and their calcified lining has a smooth inner and outer margin. A single, large, annular, splenic calcification most likely represents a residual traumatic cyst in areas not endemic for Echinococcus organisms.
The imaging characteristics of traumatic cysts are not distinctive. The most common cause of calcified splenic cysts worldwide is infection by Echinococcus granulosus, but the organism is rare outside its normal geographic distribution. E granulosus affects the liver more commonly than other organs, with a minority of patients having concurrent splenic disease.
Isolated splenic involvement is uncommon. When it occurs, multiple lesions are present; however, calcification almost always is restricted to a single cyst. [40] Cysts tend to have a smooth outer wall, but calcification can be irregular. Often, part of the wall appears flattened or indented, and intracystic opacities are common. Collapse of the hydatid cyst can occur with infolding of the walls. Splitting of the wall calcification represents separation of the endocyst and pericyst linings. A single cyst in an enlarged left lobe of the liver may simulate splenic pathology. Careful observation reveals mass effect directed laterally, in contrast to the medial displacement appreciated with a splenic mass.
An epidermoid cyst of the spleen is a congenital abnormality that results from infolding or entrapment of peritoneal mesothelium within the spleen. Cysts are slow growing and usually appear in those aged 10-30 years. Cysts are unilocular and solitary 80% of the time, with an average size of 10 cm. Curvilinear wall calcification occurs in 9-25% of cysts, and appearance is identical to a traumatic splenic cyst.
Calcified cysts of surrounding organs in the LUQ can simulate chronic splenic injury. Simple renal cysts have a peak incidence in patients older than 30 years. Renal cysts are present in 3-5% of all individuals at autopsy and account for 62% of all renal masses. Although only 1% of renal cysts show marginal calcification, a calcified renal cyst is not an uncommon finding, considering the large number that exist. Adrenal cysts usually are solitary, and 50% are larger than 5 cm. They most commonly appear in those aged 20-60 years, with a slight female predominance. Rimlike nodular calcification is present in 51-69% of adrenal cysts. Pancreatic pseudocysts and mesenteric cysts are possible but rarely calcify.
Aneurysms
Splenic artery (SA) aneurysm is the number one visceral aneurysm. The etiology can be atherosclerotic disease, pancreatitis, or previous trauma. The incidence in females is higher than that in males, and it is highest in women of childbearing age who have had 2 or more pregnancies. Although usually asymptomatic, LUQ pain or fullness is a complaint in 50% of patients.
As many as 66% of aneurysms develop calcification, which is annular but characteristically thicker and more irregular than that seen in splenic cysts. Associated SA calcification may be present if atherosclerotic disease is the cause. Plain radiographs can show a diagnostic interruption in the calcification where the aneurysm originates in the SA. Complex multiloculated configurations also are possible and are distinct from traumatic splenic cysts.
Subcapsular hematoma
Subcapsular hematoma is a common result of splenic trauma and demonstrates imaging characteristics different from those of parenchymal pathology. As the hematoma resolves, fine marginal calcification of the cavity can develop. Depending on the projection, the calcified cavity can appear linear or discoid. The degree of mass effect depends on the size of the regressing hematoma.
Many pathologic entities can have similar findings, most notably sickle cell disease. A chronic splenic infarct may develop calcification similar to that of subcapsular hematoma. A large hematoma viewed en face can be misinterpreted as a contracted hyperopaque spleen of sickle cell disease. In sickle cell disease or thalassemia, the spleen remains enlarged despite calcification or increased density, confusing the differential. In fact, any process leading to splenic infarction or fibrosis can cause calcification and simulate chronic subcapsular hematoma.
Splenic infarction
Causes of splenic infarction include the following:
-
Embolic - Endocarditis, atherosclerotic plaque, cardiac thrombus, and metastasis (gastric and/or pancreatic)
-
Local thrombosis - Sickle cell disease, myelofibrosis, myelolymphoproliferative disorders (chronic myelogenous leukemia type 1), polycythemia vera, and Gaucher disease
-
Vasculitis - Polyarteritis nodosa, systemic lupus erythematosus, and drug abuse
-
Pancreatitis
-
SA aneurysm
-
Splenic torsion
Computed Tomography
At most institutions, CT is the modality of choice for evaluation of blunt abdominal trauma. [41, 42, 43, 11] Overall, sensitivity and specificity are high for detection of splenic trauma. Intravenous contrast material is necessary for complete evaluation because areas of hematoma are frequently isoattenuating relative to the parenchyma on nonenhanced images. [6, 3, 12, 13] If IV contrast is not used with CT, very significant splenic injuries may be missed by the lack of image optimization even with modern multi-detector CT scanning. CT findings may include hemoperitoneum, hypodensity, and contrast blush or extravasation. Repeat CT scans are not indicated in patients who are hemodynamically stable. [10, 11, 2, 3, 12, 13]
However, because of the expense and exposure to radiation, there have been efforts to minimize the use of CT. Children are at especially high risk of radiation-induced malignancies. Algorithms have been designed to help differentiate between high-risk and low-risk for intra-abdominal injury to guide abdominal CT usage and reduce radiation exposure. Models proposed combine examination, vital signs, radiographic findings, ultrasound, and laboratory values. [7, 14, 15, 16]
(CT images of splenic trauma are provided below.)
 Spleen, trauma. Contrast-enhanced arterial-phase CT scan of the abdomen shows a mottled appearance of the spleen. This finding should not be mistaken for splenic injury. Confirmation of a normal spleen can be shown by repeat imaging in a later phase of contrast enhancement. The spleen then appears homogeneously enhanced.
Spleen, trauma. Contrast-enhanced arterial-phase CT scan of the abdomen shows a mottled appearance of the spleen. This finding should not be mistaken for splenic injury. Confirmation of a normal spleen can be shown by repeat imaging in a later phase of contrast enhancement. The spleen then appears homogeneously enhanced.
 Spleen, trauma. Contrast-enhanced CT scan of the abdomen in the equilibrium phase shows perisplenic fluid with mass effect on the spleen. The spleen appears compressed by the fluid, reminiscent of subcapsular fluid collections. In this patient, the fluid was secondary to pancreatic pseudocysts mimicking subcapsular hematomas.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen in the equilibrium phase shows perisplenic fluid with mass effect on the spleen. The spleen appears compressed by the fluid, reminiscent of subcapsular fluid collections. In this patient, the fluid was secondary to pancreatic pseudocysts mimicking subcapsular hematomas.
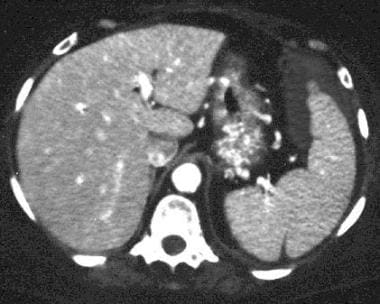 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows some perisplenic fluid in the anterior aspect. A small well-defined irregularity is noted in the splenic wall posteriorly. This was a congenital splenic cleft in a patient with perisplenic fluid secondary to nonsplenic injury.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows some perisplenic fluid in the anterior aspect. A small well-defined irregularity is noted in the splenic wall posteriorly. This was a congenital splenic cleft in a patient with perisplenic fluid secondary to nonsplenic injury.
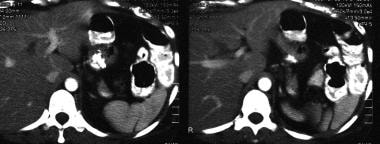 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows congenital splenic clefts with perisplenic fluid secondary to metastatic ovarian carcinoma. This mimics a splenic injury.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows congenital splenic clefts with perisplenic fluid secondary to metastatic ovarian carcinoma. This mimics a splenic injury.
 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows perisplenic fluid without identification of a laceration in a patient who sustained blunt abdominal trauma. A large amount of pelvic fluid was seen, prompting laparotomy during which a small laceration was found; this is not evident on the scan.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows perisplenic fluid without identification of a laceration in a patient who sustained blunt abdominal trauma. A large amount of pelvic fluid was seen, prompting laparotomy during which a small laceration was found; this is not evident on the scan.
The spleen demonstrates a multitude of normal variations in shape. Lobulations are common, and clefts between lobulations can be as deep as 2-3 cm. Clefts can mimic lacerations but should appear more smoothly contoured and sharply marginated than lacerations.
Accessory splenic tissue occurs in 10-30% of individuals and develops in multiple sites in approximately 10%. The most common location is in the hilar region, followed by the suspensory ligaments, particularly the gastrosplenic ligament. Accessory tissue can mimic fragmentation, but margins are smooth and better defined than they are in lacerations.
Blunt splenic trauma can result in subcapsular hematoma, intraparenchymal hematoma, laceration, or fragmentation with autosplenectomy. Laceration appears as an irregular hypodense area of nonenhancement, with somewhat indistinct borders compared to the margin in an unlacerated spleen.
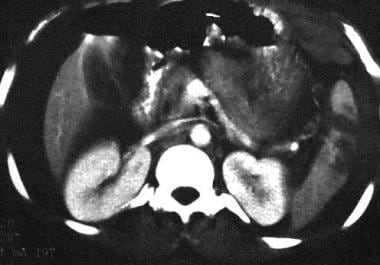
Subcapsular hematomas are regularly shaped, crescentic, hypoattenuating collections closely applied to the perceived splenic margin. The margins are usually sharp in distinction to a perisplenic clot. Underlying deformity or indentation of the parenchyma is appreciated.
Intraparenchymal hematoma is a broader, more irregular, hypoattenuating area with mass effect and enlargement of the spleen. A parenchymal hematoma, which is contained within the spleen, should have a discernible rim of surrounding splenic tissue on contrast-enhanced images. On nonenhanced images, the hematoma can appear hypoattenuating, isoattenuating, or hyperattenuating compared with the parenchyma.
Grading system
Many grading systems for splenic injury have been formulated, with the earliest by Buntain et al. [44] The Organ Injury Scaling Committee of the American Association for the Surgery of Trauma has devised a universal grading systems, as explained below. [45]
Grade I injuries include the following (see the image below):
-
Subcapsular hematoma of less than 10% of surface area
Grade II injuries include the following (see the image below):
-
Subcapsular hematoma of 10-50% of surface area
-
Intraparenchymal hematoma of less than 5 cm in diameter
Grade III injuries include the following (see the images below):
-
Subcapsular hematoma of greater than 50% of surface area or expanding and ruptured subcapsular or parenchymal hematoma
-
Intraparenchymal hematoma of greater than 5 cm or expanding
Grade IV injuries include laceration involving segmental or hilar vessels, with devascularization of more than 25% of the spleen (see the images below).
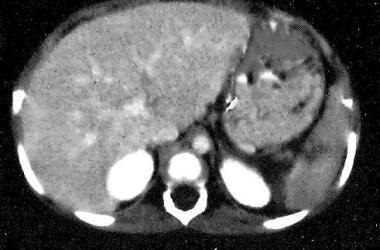 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a small hilar laceration. This is a grade III-IV injury.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a small hilar laceration. This is a grade III-IV injury.
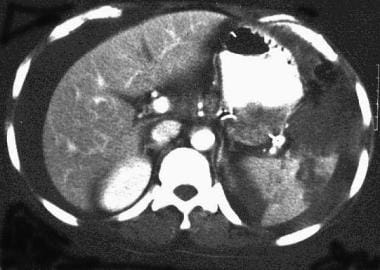 Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a complex laceration extending to the hilum. This is a grade IV injury.
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a complex laceration extending to the hilum. This is a grade IV injury.
Grade V injuries include a shattered spleen or hilar vascular injury (see the image below).
 Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
Hemoperitoneum
Hemoperitoneum almost always accompanies splenic injury. Uncommonly, a perisplenic clot is present without evidence for capsular disruption, which has been reported in approximately 9% of patients and is termed the sentinel clot. [46] The sentinel clot is a sensitive sign of visceral injury and should prompt careful inspection of the images for an etiology; however, a small laceration can be compressed by perisplenic clot, rendering it invisible.
Intraperitoneal blood
The CT appearance of intraperitoneal blood depends on the age and physical state of the clot. Immediately after hemorrhage, intraperitoneal blood has the same attenuation as circulating blood of 20-30 HU. However, attenuation values less than 20 HU are a frequent finding in the acute setting. [47] The proposed reason for this is that blood, being a strong peritoneal irritant, causes a local inflammatory response with transudation of fluid across the peritoneum.
Transudate fluid mixes with and dilutes the blood before coagulation begins, decreasing the attenuation. Within hours, a clot forms. Attenuation increases as hemoglobin concentrates, and values in the range of 50-75 HU are seen. Densely clotted blood may have attenuation values upwards of 100 HU. Clot lysis begins within 48-72 hours, and attenuation decreases to fluid values. Lysis proceeds more rapidly for intraperitoneal hematomas than visceral hematomas secondary to abdominal and respiratory motion and bowel peristalsis. [48]
After a few weeks, most hematomas have attenuation values approaching those of water—namely, 0-20 HU. The scenario just described is a simplified version based on a single episode of hemorrhage. In reality, hemoperitoneum can have a complex appearance as a result of recurrent hemorrhage and irregular resorption. Blood may exist in many different stages at the time of imaging if hemorrhage has been intermittent. Fresh blood that is confined to a localized space or that has been relatively undisturbed may separate, with plasma layered on top of precipitated red blood cells causing the hematocrit effect.
Hemoperitoneum does not indicate whether active hemorrhage is present. Repeat imaging, as clinically warranted, can aid in detecting ongoing hemorrhage. Increasing hematoma size or changes in character contrary to the expected sequence are indications of continued hemorrhage. In most instances, hemoperitoneum significantly resolves within 1 week. In one study, intra-abdominal hematoma with a stable appearance 3-7 days after injury was suggestive of continued hemorrhage. Depending on the physical state of the existing hematoma, fresh blood appears either relatively hypoattenuating or hyperattenuating.
Extrasplenic extravasation
On contrast-enhanced CT, extrasplenic extravasation of contrast material is rarely seen. When extravasation occurs, patients most likely have hemodynamic instability and proceed to laparotomy. However, an intraparenchymal vascular blush may appear as single or multiple well-defined areas of contrast material collection when a bolus injection is performed. Hyperattenuating areas represent localized areas of contrast material extravasation from pseudoaneurysms or arteriovenous fistulas. [35, 49]
Pseudoaneurysm
Pseudoaneurysm formation is reportedly a delayed finding in 10% of patients with splenic injury. [33] The presence of a pseudoaneurysm is a strong predictor of nonoperative failure. Davis et al found that of patients in whom conservative treatment failed, CT scans in 67% demonstrated a contrast blush. Note that 74% of pseudoaneurysms were not documented on the initial CT scan; this observation provides strong support for repeat examination in patients who receive conservative treatment. [33]
Additional scoring systems
Many authors have attempted to develop grading systems and delineate specific findings to predict the need for laparotomy and assess the success of conservative treatment. Resciniti et al proposed a CT scoring system to address the need. [50]
The region CT scoring system is as follows:
Splenic parenchyma:
-
Intact - 0
-
Laceration (thin, linear defect) - 1
-
Fracture (thick, irregular defect) - 2
-
Shattered - 3
Splenic capsule:
-
Intact - 0
-
Perisplenic fluid present - 1
Abdominal fluid:
-
No fluid - 0
-
Any fluid except perisplenic - 1
Pelvic fluid:
-
No fluid - 0
-
Any pelvic fluid - 1
In adult patients with a total CT score of less than 2.5, nonsurgical treatment was successful in all patients. A score of 2.5 or more is correlated with a 46% likelihood of successful nonsurgical treatment. In one study, all pediatric patients younger than 17 years had successful conservative treatment without delayed complications irrespective of the score. [50]
A subsequent study elucidated potential errors of the scoring system, particularly in discriminating subcapsular from perisplenic fluid and accounting for interobserver variability. However, 13 of 15 patients treated nonsurgically who had a score of less than 2.5 had favorable outcomes. [51]
Despite the criticisms, overall conservative treatment failed in only 2 (10%) of 21 patients. This result represents a significant improvement over the 22-75% failure rates reported in the literature. Conversely, 8 unnecessary laparotomies would have been performed if the grading system had been used. This would have been an 89% increase. Obviously, the scoring system created by Resciniti is imperfect, and further evaluation is needed. [50, 51]
Initial reports describing conservative treatment for blunt splenic trauma found that patient age greater than 55 years is an indicator of poor outcome. Perhaps the addition of patient age to the CT scoring system would improve results. [52, 53, 54, 55, 56]
Degree of confidence
In the authors' experience, the overall sensitivity and specificity of CT in the detection of splenic injury is close to 100%.
One pitfall of the use of contrast material is that bolus injection with early imaging can produce heterogeneous splenic enhancement, because red and white pulps demonstrate differing blood flow rates. The characteristic tiger-stripe pattern results. The pattern should not be confused with traumatic change and can be remedied by delayed imaging or slower injection rates.
An overall decrease in splenic enhancement compared to that of the liver can occur in patients with hypotension and can be misinterpreted as vascular pedicle injury. This phenomenon is believed to result from adrenergic effects on blood flow. [57]
In a study to determine the value of MRI in characterizing spleen lesions from blunt abdominal trauma in patients for whom CT results were indeterminate, MRI performed better (5 of 25 lesions) than CT (2 of 25 lesions) in depicting thin subcapsular hematomas. [42]
Ultrasonography
The primary goal of splenic ultrasonography in the setting of blunt abdominal trauma is to detect the presence of blood in the left upper quadrant (LUQ). [58]
Acute blood is hypoechoic and can be almost anechoic. Differentiating subcapsular from perisplenic blood is difficult, but a few clues are available. For example, a smooth crescentic collection conforming to the splenic margin probably should be considered subcapsular, whereas extracapsular blood is usually shaped more irregularly. Although mass effect is produced in both cases, subcapsular blood is more likely to distort splenic shape. In addition, the membrane overlying subcapsular collections is thin and uncommonly depicted; therefore, the absence of this finding does not suggest either diagnosis.
Within hours, coagulation of hemorrhage occurs. Echogenicity increases as the thrombus condenses. Mature hematomas demonstrate echogenicity equal to or slightly greater than parenchyma and retain this appearance for approximately 48 hours until lysis begins. The echogenic phase usually corresponds to the time when imaging is performed in most acute circumstances. As lysis proceeds, the hematoma returns to fluid echogenicity, and the pathology is again more apparent.
Parenchymal abnormalities commonly are subtle. Lacerations appear as hypoechoic regions, which can be irregular or linear in configuration. A splenic infarct has a similar appearance, but it is usually better defined. Infarcts are wedge shaped, with the apex toward the hilum, compared to traumatic injury in which a more complex distribution is seen. Subtlety of parenchymal injury probably relates to associated local hemorrhage. Any trapped blood soon coagulates, becoming isoechoic with the surrounding tissue.
Focused abdominal sonographic technique (FAST)
FAST, observing for the presence or absence of fluid in the peritoneal cavity, may be performed rapidly and safely in trauma patients. FAST is poor for delineating organ-specific anatomy with any reliability in the emergency setting. In addition, the learning and interpretation curve is rather steep when compared to DPL. In experienced hands, visualization of fluid in the right upper quadrant, the left upper quadrant, and the pelvis suggests solid organ injury (or mesenteric injury) and the possibility of splenic injury. [27, 8, 9]
Degree of confidence
Increasing support has emerged for using ultrasonography in the initial assessment of blunt abdominal trauma. The focused assessment for the sonographic examination of the trauma patient test has been developed. [59] The evaluation surveys for fluid in the pericardium and peritoneum. Dependent areas of the abdomen are imaged (ie, the hepatorenal space [right upper quadrant, or RUQ]; splenorenal space, LUQ; and pelvis, rectovesical or rectouterine space). Cadaver studies have shown that as little as 100 mL of fluid can be discerned. [60]
In assessing patients with abdominal trauma, CT scanning has been the standard, but ultrasonography has a high sensitivity for detecting free fluid in the peritoneum. Cagini et al used contrast-enhanced ultrasound (CEUS) to improve the accuracy of ultrasound in diagnosing parenchymal lesions in the polytrauma patient. Although CEUS is not the first method of diagnosis, it is useful for following up on abdominal traumatic injuries in young people. [61]
A study aimed at correlating the presence of abdominal fluid with visceral injury seen on sonograms revealed fluid in the RUQ in 71% of the 69 patients confirmed to have isolated blunt splenic injury; fluid in the LUQ in 33%; and fluid in the pelvis in 30%. [62] Although individual patient data were not supplied, a significant number of patients had fluid in the RUQ without any appreciated in the LUQ. Obviously, blood must have been present in the LUQ at some point after the initial injury. Although the authors concluded that ultrasonography was sensitive for detection of blunt visceral injury overall, the study revealed its insensitivity in recognizing splenic injury. Surely, something is amiss if the LUQ was interpreted as being fluid-free in 69 patients with documented blunt splenic trauma.
A number of possible reasons may explain the discrepancy. First, blood in the LUQ is, on average, older than blood elsewhere in the abdomen. Hemorrhage initially pools in the perisplenic area and spills over into the remainder of the abdomen as a larger amount accumulates. With lower grades of injury or slower bleeding rates, the blood coagulates and fills the potential space. On subsequent episodes of hemorrhage, fresh blood may be displaced from the LUQ secondary to mass effect from the thrombus. Ultrasonography of the LUQ does not show any free fluid. Conversely, the hemorrhage may be subdiaphragmatic and difficult to image. The spleen is displaced inferiorly, and no fluid is appreciated in the splenorenal space.
Second, the echogenicity of an acute clot is equal to or greater than that of the splenic parenchyma. A clot may be inseparable from the adjacent spleen. The injury is misdiagnosed as splenomegaly or not appreciated at all. The clot may also be mistaken for overlying bowel or artifact from a poor acoustic window.
False positives/negatives
Yoshii et al found 90% specificity for detecting splenic injury irrespective of the presence of fluid. Of the 9 false-negative results, 3 were classified as major injuries requiring celiotomy. Compared with the 96% or better sensitivity of CT and the superiority of CT in determining injury extent, the authors concluded that CT should be the preferred test in hemodynamically stable patients able to cooperate for the examination. The sensitivity, accuracy, and negative predictive value of ultrasonography were comparable to those of diagnostic peritoneal lavage. [63]
Nuclear Imaging
The use of nuclear radiology to assess trauma has decreased significantly because of dedicated trauma centers, new treatment protocols, and improved imaging technology. The use of nuclear imaging is relegated to evaluation of chronic injury or injuries with delayed presentation. The role of nuclear imaging with respect to splenic trauma is limited to distinguishing findings in the differential diagnosis when the presentation or clinical history does not equivocally indicate trauma.
The importance of obtaining multiple views cannot be stressed enough. Imaging must include both anterior and posterior projections, with multiple oblique images because small defects may be discernible on only one image or a few images.
Splenic trauma may appear as simple displacement, reduced size, focal defects, a band of decreased activity, or loss of splenic contours.
Currently, the trend is toward splenic salvage, as opposed to splenectomy, whenever possible. In a patient who has undergone splenorrhaphy or has received conservative treatment, physical activity is limited significantly for 8-12 weeks. Some institutions image the spleen before releasing the restrictions to document adequate fracture healing.
The liver-spleen scan is a viable alternative to CT for follow-up studies of splenic injury. Intravenous contrast material is not required, and the whole-body radiation dose of 0.0002 Gy/mCi (range, 3-5 mCi) is less than that of conventional CT. Scans demonstrate resolution of the defect in uptake, although a small area of decreased activity may persist secondary to scar formation.
Degree of confidence
In splenic imaging, technetium-99m sulfur colloid scanning remains the test of choice. Acute splenic injury can be diagnosed with a high degree of sensitivity, but the specificity is less than desirable.
Small subcapsular hematomas or contusions may be missed. Splenic abscesses, infarcts, cysts, and benign or malignant masses present with the same findings and are indistinguishable from trauma.
False-positive findings may result from variations in the normal shape of the spleen.
Angiography
A thorough knowledge of the splenic artery (SA) is needed to analyze traumatic damage correctly and offer therapeutic options. Splenic trauma can produce a wide variety of angiographic findings, either directly or indirectly (see the images below).
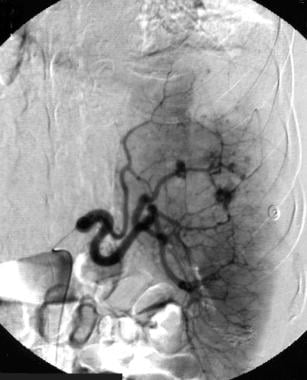 Spleen, trauma. Arteriogram obtained with a main splenic artery catheter injection shows multiple areas of parenchymal contrast agent extravasation.
Spleen, trauma. Arteriogram obtained with a main splenic artery catheter injection shows multiple areas of parenchymal contrast agent extravasation.
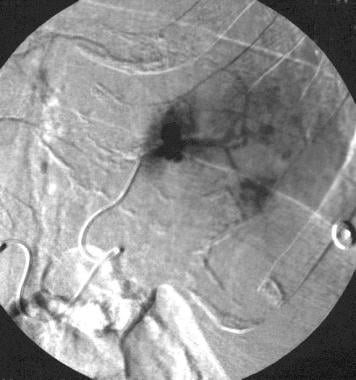 Spleen, trauma. Selective splenic arteriogram shows traumatic pseudoaneurysms with extravasation in the upper pole.
Spleen, trauma. Selective splenic arteriogram shows traumatic pseudoaneurysms with extravasation in the upper pole.
 Spleen, trauma. Arteriogram obtained with a main splenic artery injection after superselective coil embolization of pseudoaneurysms. Irregular contrast opacification is still present within an avascular area; it possibly represents another area of vascular injury.
Spleen, trauma. Arteriogram obtained with a main splenic artery injection after superselective coil embolization of pseudoaneurysms. Irregular contrast opacification is still present within an avascular area; it possibly represents another area of vascular injury.
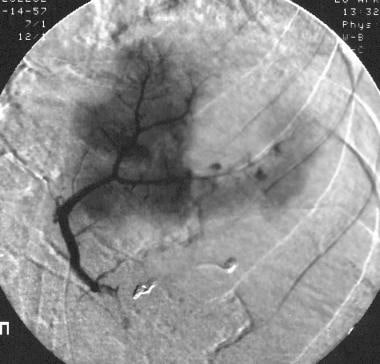 Spleen, trauma. Arteriogram obtained with a superselective splenic artery injection in the upper pole confirms a second zone of vascular disruption with contrast agent extravasation.
Spleen, trauma. Arteriogram obtained with a superselective splenic artery injection in the upper pole confirms a second zone of vascular disruption with contrast agent extravasation.
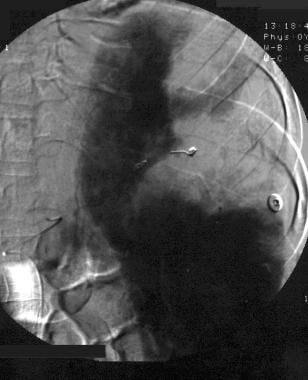 Spleen, trauma. Angiogram obtained after superselective coil embolization of the upper pole artery shows adequate treatment without extravasation.
Spleen, trauma. Angiogram obtained after superselective coil embolization of the upper pole artery shows adequate treatment without extravasation.
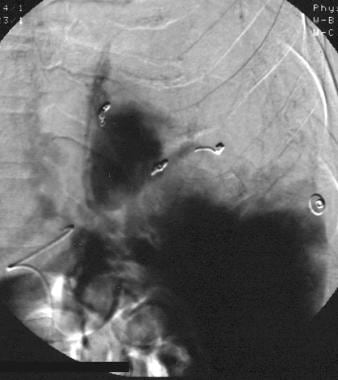 Spleen, trauma. Final arteriographic image from a main splenic artery catheter injection after selective/superselective coil embolization. Approximately 50% of the spleen has been devascularized. No residual arterial vascular injury or extravasation is present. The patient recovered uneventfully.
Spleen, trauma. Final arteriographic image from a main splenic artery catheter injection after selective/superselective coil embolization. Approximately 50% of the spleen has been devascularized. No residual arterial vascular injury or extravasation is present. The patient recovered uneventfully.
Indirect signs include displacement of the spleen from the abdominal wall and avascular parenchymal areas from hematoma. Peripheral parenchymal defects may represent subcapsular hematomas, but care must be taken not to confuse traumatic change with developmental notching or lobulation. Mass effect can be identified with traumatic injury.
Compression of the vascular pattern along the defect margin is characteristic of subcapsular hematoma. Defects from chronic change, such as infarct, should have clear margins that are more regular in contour without mass effect. Parenchymal hematoma usually demonstrates hazy borders with splaying of the surrounding vessels. Hematoma age affects the degree to which these characteristics are visualized.
Parenchymal irregularity or mottling may result from localized edema of a contusion without apparent vessel abnormalities. The most reliable angiographic sign of splenic trauma is contrast-material extravasation, either parenchymal or extrasplenic. At times, extravasation may be observed only after the administration of vasopressin or epinephrine. These medications enhance detection of vascular injury by increasing precapillary arteriolar resistance. Abrupt cutoff of vessels, vessel wall irregularity, pseudoaneurysms, and early filling of splenic veins are findings of traumatic injury.
Angiography has been used to elucidate risk factors for delayed complications of splenic injury. The literature reports an approximate 5% incidence of delayed hemorrhage more than 4 days after injury. [64]
Classically, rupture of a subcapsular hematoma had been implicated as the cause of delayed bleeding; however, it has been found that subcapsular hematoma may not be a predictor of delayed splenic rupture and that lysis of hematoma at the injury site may be the most likely cause. Angiographic criteria have been proposed in an attempt to determine which injuries pose the greatest risk of delayed hemorrhage. Many authors have proposed grading systems of splenic injury, but these systems are somewhat confusing and complicated.
Splenic fragmentation or major arterial injury signifies impending life-threatening complications in most patients, and they require immediate surgical intervention. The absence of contrast-material extravasation from the parenchymal vessels was correlated with successful conservative treatment in one study. [31] Active extravasation, even intraparenchymal extravasation, can cause tamponade and cease spontaneously, but it is correlated with delayed capsular rupture. Large avascular areas, defined as those involving more than 25% of the spleen, is correlated with a poor outcome. An avascular area of less than 25% is associated with a good outcome.
A large avascular area requires injury to either a more central, larger vessel or multiple regional peripheral vessels, as opposed to a smaller avascular area. A central vessel has greater blood flow and higher systolic pressure, both of which increase the likelihood of rupture as the hematoma matures and undergoes lysis. When small vessel disruption is suspected, an increased risk of delayed hemorrhage between the large and small avascular areas may be related to statistical chance when a large number of vascular branches are injured.
-
Spleen, trauma. Chest radiograph shows a peripherally calcified mass in the left upper quadrant under the diaphragm. The mass represents a calcified splenic hematoma.
-
Spleen, trauma. Contrast-enhanced arterial-phase CT scan of the abdomen shows a mottled appearance of the spleen. This finding should not be mistaken for splenic injury. Confirmation of a normal spleen can be shown by repeat imaging in a later phase of contrast enhancement. The spleen then appears homogeneously enhanced.
-
Spleen, trauma. Contrast-enhanced CT scan shows a localized area of dense contrast collection in the splenic hilum, with a massive amount of surrounding fluid/blood. Findings here are indicative of active extravasation of contrast in a patient with traumatic autosplenectomy. This is a grade V injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen in the equilibrium phase shows perisplenic fluid with mass effect on the spleen. The spleen appears compressed by the fluid, reminiscent of subcapsular fluid collections. In this patient, the fluid was secondary to pancreatic pseudocysts mimicking subcapsular hematomas.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a perisplenic fluid collection with internal increased attenuation. The splenic border is displaced by mass effect. This was a subacute subcapsular hematoma. This is a grade I injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows some perisplenic fluid in the anterior aspect. A small well-defined irregularity is noted in the splenic wall posteriorly. This was a congenital splenic cleft in a patient with perisplenic fluid secondary to nonsplenic injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows congenital splenic clefts with perisplenic fluid secondary to metastatic ovarian carcinoma. This mimics a splenic injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows perisplenic fluid without identification of a laceration in a patient who sustained blunt abdominal trauma. A large amount of pelvic fluid was seen, prompting laparotomy during which a small laceration was found; this is not evident on the scan.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a massive fluid collection in the upper abdomen. This was a chronic subcapsular splenic hematoma and a grade III injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a complex lower pole splenic laceration. This is a grade II injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a small hilar laceration. This is a grade III-IV injury.
-
Spleen, trauma. Contrast-enhanced CT scan of the abdomen shows a complex laceration extending to the hilum. This is a grade IV injury.
-
Spleen, trauma. Arteriogram obtained with a main splenic artery catheter injection shows multiple areas of parenchymal contrast agent extravasation.
-
Spleen, trauma. Selective splenic arteriogram shows traumatic pseudoaneurysms with extravasation in the upper pole.
-
Spleen, trauma. Arteriogram obtained with a main splenic artery injection after superselective coil embolization of pseudoaneurysms. Irregular contrast opacification is still present within an avascular area; it possibly represents another area of vascular injury.
-
Spleen, trauma. Arteriogram obtained with a superselective splenic artery injection in the upper pole confirms a second zone of vascular disruption with contrast agent extravasation.
-
Spleen, trauma. Angiogram obtained after superselective coil embolization of the upper pole artery shows adequate treatment without extravasation.
-
Spleen, trauma. Final arteriographic image from a main splenic artery catheter injection after selective/superselective coil embolization. Approximately 50% of the spleen has been devascularized. No residual arterial vascular injury or extravasation is present. The patient recovered uneventfully.