Practice Essentials
Spinal dysraphism, or neural tube defect (NTD), is a broad term encompassing a heterogeneous group of congenital spinal anomalies that result from defective closure of the neural tube early in fetal life and anomalous development of the caudal cell mass. Spinal dysraphism consists of congenital malformations caused by maldevelopment of ectodermal, mesodermal, and neuroectodermal tissues and results in abnormal bony formations of the spine or spinal cord. Common manifestations are meningocele, myelomeningocele, lipomeningocele, lipomyelomenigocele, myeloschisis, and rachischisis. In cases of myelomeningocele, the spinal cord and its contents herniate through a congenital bony defect at the posterior elements of the spine; it constitutes about 80% of the cases of spinal dysraphism. [1]
Choosing the most appropriate modality for imaging congenital malformation of the spine (eg, spinal dysraphism/myelomeningocele) involves considering many factors. Imaging of the bony spine requires methods different from those used to image the spinal canal and its contents. The age of the patient and the required plane of imaging influence the choice of modality. The best way to image skeletal anomalies is by means of plain radiography, possibly combined with conventional tomography, though this modality has now been more or less replaced by CT.
Plain images may suffice from the orthopedic point of view, but they provide little information of the associated malformations of the spinal cord and its coverings. When spinal malformations are suspected, investigation of the spinal canal and its contents are best performed by MRI.
Skeletal scintigraphy with technetium-99m diphosphonates has high sensitivity but low specificity. Bone scintigraphy is a useful procedure in children with backache of unknown origin. Minor vertebral anomalies may show increased radionuclide uptake because of abnormal stresses and reactive changes. Further imaging may be restricted when an abnormality is localized.
In cases of spinal dysraphism, MRI provides more information than myelography or CT in defining spinal cord anatomy. Sagittal MRIs are superior to CT myelograms in demonstrating the lipoma-cord interface. Individual nerve roots are less well seen with MRI. [2, 3, 4]
In the evaluation of the spinal canal, ultrasonography is limited to the neonatal period, though a spinal defect covered with soft tissue may be imaged well into adult life. Fetal ultrasonography is increasingly used as a primary screening tool for NTDs, usually at about 18 weeks' gestational age. This trend reflects the increasing confidence in fetal ultrasonography. Ultrasonography helps in avoiding the calculated 1% risk of miscarriage associated with diagnostic amniocentesis. [5]
In myelocele or myelomeningocele/meningomyelocele, detailed imaging before closure is usually not required, but after repair or spontaneous reepithelialization, imaging may be performed to depict associated pathology, such as diastematomyelia or lipoma, at other levels and to evaluate intracranial abnormalities.
Deterioration in neurologic function later in the disease course or after surgery suggests a complication; such deterioration is an indication for imaging to look for a surgically remediable cause of the deterioration, such as retethering of the cord as a result of adhesions or an increase in size of an inclusion lipoma, epidermoid, or hydromyelia. Normally, after repair, the cord is low; it should not be tethered unless scars extend into the closure site.
(See the images below.)
Some forms of spinal dysraphism may cause progressive neurologic deterioration. The anatomic features common to the entire group is an anomaly in the midline structures of the back, especially the absence of some of the neural arches, and defects of the skin, filum terminale, nerves, and spinal cord (see the images below).
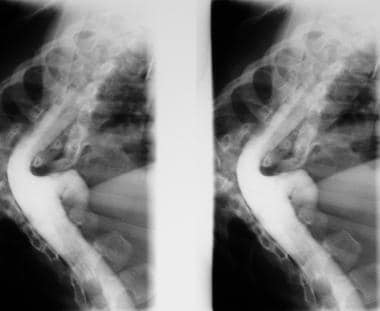 Myelograms in a 5-year-old patient show the dorsal region of the spine and an anterior thoracic meningocele. Note the gross dorsal kyphosis.
Myelograms in a 5-year-old patient show the dorsal region of the spine and an anterior thoracic meningocele. Note the gross dorsal kyphosis.
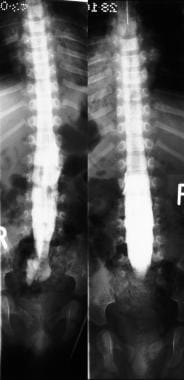 Myelograms in a 4-year-old patient show the lumbosacral region; a long, tethered cord; and diastematomyelia.
Myelograms in a 4-year-old patient show the lumbosacral region; a long, tethered cord; and diastematomyelia.
Spinal dysraphism occurs in closed forms and open forms. Open forms include myelocele, meningocele, and myelomeningocele. These open forms are often associated with hydrocephalus and Arnold-Chiari malformation type II and may be classified as spina bifida aperta. In 1886, Von Recklinghausen gave a detailed account of spina bifida cystica (aperta). Spina bifida is described in literature of the Middle Ages, although the condition was recognized even earlier. Indeed, the mythologic figure of the satyr may have its origins in the association of foot deformities with lumbar or lumbosacral hypertrichosis. [6]
The closed form of spina bifida is termed spina bifida occulta. Of the general population, 5-10% may have bony spina bifida occulta with intact overlying skin. Most of these cases are found incidentally.
Open NTD (ONTD) represents a serious congenital anomaly. If the neural tube fails to fuse at the skull, the result may be that of anencephalus or encephalocele. Open NTDs, such as meningomyelocele, occurs when the tube fails to fuse along the spine. Infants with NTDs frequently have additional serious neurologic, musculoskeletal, genitourinary, and bowel anomalies.
Spina bifida occulta is characterized by the variable absence of several neural arches and various cutaneous abnormalities, such as lipoma, hemangioma, cutis aplasia, dermal sinus, and hairy patch; it is often associated with a low-lying conus and other spinal cord anomalies. In infants, whenever the conus lies below the L2-3 interspace, cord tethering should be considered. The term tethered cord implies that the cord may be attached to the vertebral column or subcutaneous tissues by a thickened filum terminale, fibrous band, dermal sinus tract, diastematomyelia, or a lipoma (lipomyelomeningocele). Patients with spina bifida occulta may present with scoliosis in later years.
Approximately 95% of couples that have a fetus affected with ONTD have a negative family history for ONTDs. Most ONTDs are caused by factors relating to multifactorial inheritance, including genetic and environmental factors.
Limitations of techniques
The amount of radiation involved in plain radiography and CT of the spine is particularly important in the examination of infants, children, and young, fertile women. Plain radiography, as well as CT of the lower spine, delivers a high dose to the gonads, particularly in female patients.
Ultrasonography remains operator dependent; accurate diagnosis depends on the skill and experience of the operator and on the quality of the equipment.
Transaxial CT images may be difficult to interpret because of the complex anatomy of the vertebral bodies, the presence of segmentation anomalies, and the presence of spinal curvature abnormalities. With modern CT scanners, this limitation may not be such a disadvantage, inasmuch as sagittal and coronal reconstruction now provide exquisite images of the spine.
In parts of the developing world, MRI is not readily available. In addition, use of MRI may not be possible in patients with claustrophobia, and it is contraindicated for some patients with implanted devices. Children may require sedation.
Special concerns
Neural tube defects (NTDs) exact an enormous emotional and economic toll on families and health care systems in both developed and developing countries. The tragedy is that NTDs are preventable simply by having women take a folic acid supplement during the 2 months before they become pregnant. For all women, supplementation with folic acid, 0.4 mg daily before conception and for the first 3 months of pregnancy, reduces the risk of having a baby with spina bifida. Women considered to be at risk should take a higher dose (4 mg) of folic acid.
The etiology of spina bifida or anencephaly is multifactorial and includes genetic factors, environmental influences, and folic acid deficiency in the mothers. It is known that if a woman has had 1 baby with an NTD, the chance of having another affected baby is about 1 in 20. About one half of this risk is of anencephaly, and the other half is of spina bifida. A woman is at equal risk for either spina bifida or anencephaly in future pregnancies, regardless of which of these NTDs occurred in a previous pregnancy. She is also at risk for having a baby with an NTD if she has a close relative who has had a baby with either spina bifida or anencephaly or is taking certain drugs to control epilepsy. Ultrasonography is performed in high-risk pregnancies. If the sonograms do not clearly show an NTD, amniocentesis with alpha-fetoprotein (AFP) measurements is indicated.
Kleijer and associates measured amniotic fluid levels in 2180 patients judged to be at risk for fetal abnormality on the basis of previous history, family history, advanced maternal age, suspected fetal growth retardation, or the presence of hydramnios, and of 428 patients with a previous offspring who had an NTD, only 8 (1.9%) had a fetus with an NTD. In 12 patients examined before 20 weeks' gestation, pregnancy was terminated because of an increase in amniotic fluid AFP level. Eleven fetuses had NTDs. No false-negative results were observed in the 1927 patients who were tested before 20 weeks' gestation for whom the outcome of pregnancy was known. In patients tested after 20 weeks' gestation, the amniotic fluid AFP concentration was increased in 20 fetuses with anencephaly, in 9 fetuses with severe congenital malformations without NTDs, and in 1 apparently healthy fetus. [7]
Allen and associates performed amniotic fluid AFP assays and targeted sonography in 376 antenatal patients at risk for an NTD (high-risk group) and 2436 low-risk patients, [8] and they found that when both sonographic and AFP results were normal, the likelihood of a normal outcome was high in both groups (99.7% and 100%). In the low-risk group, the likelihood of an abnormal outcome in women with elevated AFP level and a normal sonogram was low (0 of 5). They found 10 NTDs in the high-risk group (7 open, 3 closed) and 3 in the low-risk group (all open). Two of the 3 closed defects were detected prenatally. The predictive value of an elevated AFP level for an abnormal fetus was higher in the high-risk group (6 of 6, 100%) than in the low-risk group (1 of 6, 17%).
Holschneider and associates analyzed the increased rate of antenatally diagnosed malformation and the accuracy of antenatal sonographic diagnosis and concluded that, because of the uncertainty of antenatal diagnosis and the uncertainty of many physiologic parameters, intrauterine surgical treatment could not be advocated. [9] They published a report concerning an increase in sonographic examinations at the First University Gynecological Hospital of Munich from 6000 studies in 1969 to more than 13,000 studies in 1982. In their study, a correct diagnosis was made in over 80% of cases. However, the diagnosis was incomplete in approximately 40% because of associated malformations of the GI tract, congenital heart disease, myelomeningocele in a correctly diagnosed hydrocephalus, and unrecognized cases. Since the time of their study, sonographic technology has improved. Nevertheless, fetal surgery is still in its infancy.
Measurement of amniotic fluid AChE levels confirms the diagnosis. Termination of the pregnancy is advised if results of this test are positive. The sensitivity of the entire screening process is estimated to be 86% for anencephaly and 78% for open spina bifida. The specificity is 99.99%.
The value of AChE qualitative analysis as a tool for the diagnosis of fetal NTD was investigated in a series of 2815 amniotic fluid samples. This test enabled successful identification of all 84 cases of ONTDs, 16 of which had been missed with ultrasonography. AChE electrophoresis made it possible to exclude 1 false-positive sonographic diagnosis of NTD and to differentiate meningocele from myelomeningocele. The authors' experience suggests that AChE electrophoresis should be performed in all cases, in place of amniotic fluid AFP estimations; in all cases of fetal abnormality detected with sonography, especially in fetal hydrocephaly; and for all pregnant women who have had a previous pregnancy with an NTD.
Special racial groups should be targeted in terms of prophylaxis and screening; such groups include people of Celtic origin (Irish), the European white population, and the North American Hispanic population (which has a risk more than 3-fold higher than that of non-Hispanic whites). Migration studies in the white migrant population showed a prevalence of NTDs that more closely corresponded to the risk of the place to which they had migrated, as opposed to the place of their origin. By contrast, descendants of the black and Asian migrant populations in Europe and North America have prevalences not substantially higher than those of their parent countries. These variations are consistent with the theory that NTDs are a phenotypically heterogeneous group of malformations with multifactorial inheritance in some cases and single gene defects in others.
Radiography
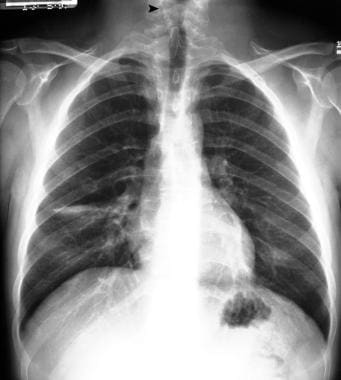
With spinal dysraphism, radiographs may show structural vertebral anomalies such as hemivertebra, butterfly vertebra, or incomplete fusion of posterior elements; it does not allow imaging of the spinal cord. Radiographs of the vertebrae provide information for early evaluation of infants born with myelomeningocele. Congenital spinal deformities need to be tracked closely. Paralytic spinal deformities require imaging based on clinical examination findings; these deformities should be followed up frequently during times of rapid growth. Plain radiographs of patients with myelomeningocele demonstrate incomplete fusion of posterior elements and increased interpedicular distance (see the images below).
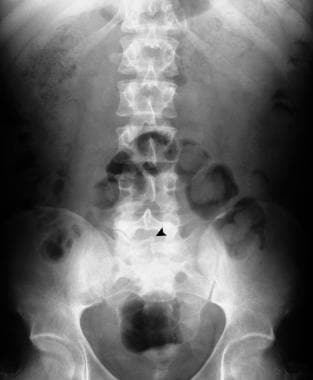 Plain abdominal radiograph in the same patient as in the previous image shows spina bifida occulta of S1.
Plain abdominal radiograph in the same patient as in the previous image shows spina bifida occulta of S1.
 Myelograms in a 5-year-old patient show the dorsal region of the spine and an anterior thoracic meningocele. Note the gross dorsal kyphosis.
Myelograms in a 5-year-old patient show the dorsal region of the spine and an anterior thoracic meningocele. Note the gross dorsal kyphosis.
 Myelograms in a 4-year-old patient show the lumbosacral region; a long, tethered cord; and diastematomyelia.
Myelograms in a 4-year-old patient show the lumbosacral region; a long, tethered cord; and diastematomyelia.
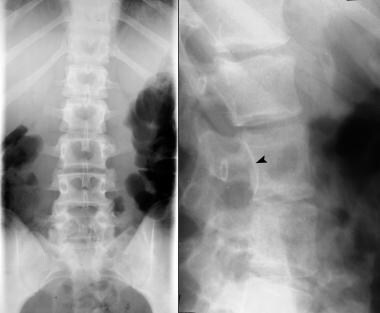
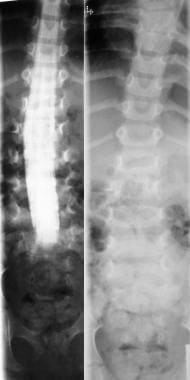 Right, plain radiograph of the lumbar spine shows diastematomyelia. Left, myelogram in the same patient shows a filling defect at the level of diastematomyelia.
Right, plain radiograph of the lumbar spine shows diastematomyelia. Left, myelogram in the same patient shows a filling defect at the level of diastematomyelia.
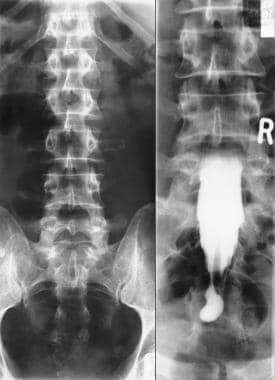 Left, plain anteroposterior (AP) radiograph of the lumbar spine shows spina bifida occulta. Right, myelogram of the same patient shows a thick tethered cord.
Left, plain anteroposterior (AP) radiograph of the lumbar spine shows spina bifida occulta. Right, myelogram of the same patient shows a thick tethered cord.
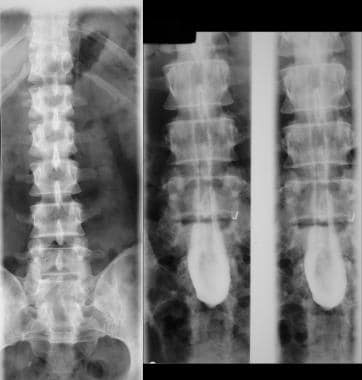 Left, anteroposterior (AP) plain radiograph of the lumbar spine shows a defect within the laminae of S1 and S2. Right, myelograms in the same patient show a markedly thickened, low tethered cord.
Left, anteroposterior (AP) plain radiograph of the lumbar spine shows a defect within the laminae of S1 and S2. Right, myelograms in the same patient show a markedly thickened, low tethered cord.
 Plain anteroposterior (AP) lumbar spinal radiograph in a 7-year-old patient shows a defect within the laminae of L4-5 and S1. Note the diastematomyelia.
Plain anteroposterior (AP) lumbar spinal radiograph in a 7-year-old patient shows a defect within the laminae of L4-5 and S1. Note the diastematomyelia.
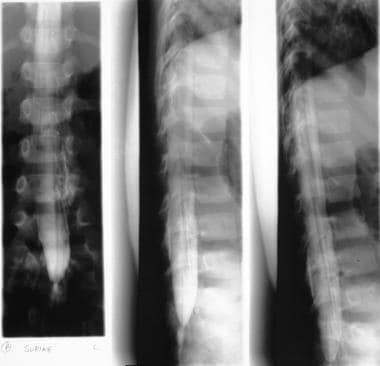 Myelograms in the same patient as in the previous image show a low, tethered cord. Note also the diastematomyelia.
Myelograms in the same patient as in the previous image show a low, tethered cord. Note also the diastematomyelia.
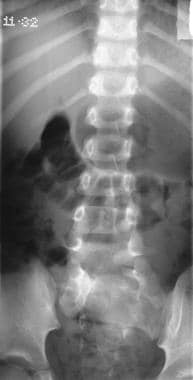
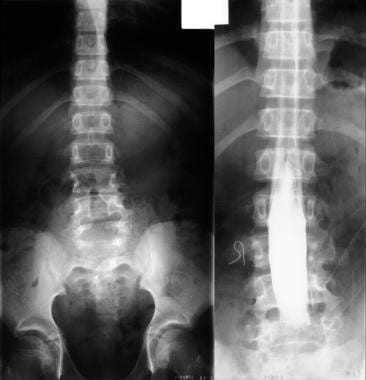 Left, plain radiograph of the lumbar spine shows bony defects in the laminae of L2 to S1. Right, myelogram shows a split cord.
Left, plain radiograph of the lumbar spine shows bony defects in the laminae of L2 to S1. Right, myelogram shows a split cord.
Tubbs and associates showed that a horizontal sacrum, as seen on plain radiographs after closure of a myelomeningocele, is an indicator of a tethered cord. [10] For most of the patients in their study, the lumbosacral angle was greater than that expected for patients with late and decreased ambulatory abilities. They also observed that the lumbosacral angle was often inappropriately increased. Many patients presented with symptoms indicative of a tethered cord. Tubbs et al postulated that, in this group of children, the tethered cord alters the lumbosacral angle and that the horizontal nature of the sacrum may predate the clinically appreciable symptoms of a tethered spinal cord.
Early accurate assessment and subsequent frequent reassessments of neurologic status are necessary with both anatomic and physiologic evaluation techniques in the investigation of the urinary system. Anatomic information may be obtained with a voiding cystogram that assesses the lower urinary tract, including bladder capacity and whether or not vesicoureteral reflux is present.
Posterior neural arch defects and associated interpedicular widening are seen in most patients with lumbosacral lipoma. Myelography may demonstrate contrast filling and associated meningocele in addition to dural ectasia and low-lying conus. Myelography shows the tethered spinal cord to be posteriorly located, sometimes tenting the dorsal thecal sac. The filum is thickened, and there is lack of cord movement in various positions.
Intraspinal lipomas may produce posterior scalloping of vertebral bodies and flattening of the pedicles. In cases of diastematomyelia, plain radiographs may demonstrate a bony spur. Frequently associated anomalies, such as spina bifida, interpedicular widening, hemivertebrae, and fusion of the vertebral bodies and laminae, are well depicted on plain radiographs.
Degree of confidence
The radiation dose from plain radiographs of the spine is a major limiting factor in examining infants, children, and young, fertile women. Plain radiography of the lower spine delivers a high dose to the gonads, particularly in female patients. Plain images may be sufficient for assessing myelomeningocele before early surgery to assess the extent of the bony defect, though this is not always required.
Although plain radiographs are sufficient from the orthopedic point of view, they provide little information of the associated malformations of the spinal cord and its coverings. When spinal malformations are suspected, investigation of the spinal canal and its contents are best performed by MRI. If MRI is unavailable or is contraindicated, myelography in combination with CT may be used.
Posterior scalloping of vertebral bodies and flattening of the pedicles are nonspecific findings; such findings may be present in cases of intraspinal tumors; neurofibromatosis; acromegaly; achondroplasia; communicating hydrocephalus; syringomyelia; and a number of congenital syndromes, including Ehlers-Danlos, Marfan, Hurler, Morquio, and osteogenesis imperfecta syndromes.
Computed Tomography
CT myelography demonstrates splitting of the cord and, in some cases, splitting of the meningeal sheath. In addition, other bony anomalies, such as an intervertebral septum, and aberrant fibroneural bands may be depicted. CT myelography allows better definition of cord expansion or deformity than that which can be achieved by conventional myelography. In the case of intrinsic cord tumors, repeat CT after 24 hours reveals intramedullary contrast enhancement if associated syringomyelia is present. MRI is the imaging procedure of choice.
Spinal lipomas, with their fatty tissue contents, are identifiable both on CT scans and on MRIs. On CT scans, fatty tissue has a strongly hypoattenuating appearance that may best be appreciated in comparison with CSF on soft tissue windows and in comparison with air on bone windows. On MRIs, fatty tissue is strongly hyperintense on images obtained with both short and long repetition times. Newer techniques of fat suppression, such as short-tau inversion recovery (STIR) imaging, may resolve any doubts. The 2 techniques are complementary: On the one hand, CT better shows osseous abnormalities associated with the lipomas; on the other, MRI is preferred because it allows better depiction of detail and contrast resolution of soft tissues (see the images below).
 Right, plain radiograph of the lumbar spine shows diastematomyelia. Left, myelogram in the same patient shows a filling defect at the level of diastematomyelia.
Right, plain radiograph of the lumbar spine shows diastematomyelia. Left, myelogram in the same patient shows a filling defect at the level of diastematomyelia.
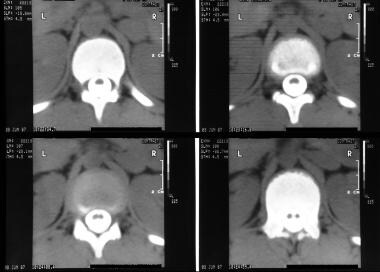
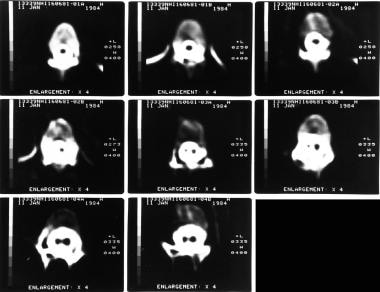 Axial CT scans through the lumbar spine with bone window setting in the same patient as in the previous images show a bony bar due to diastematomyelia.
Axial CT scans through the lumbar spine with bone window setting in the same patient as in the previous images show a bony bar due to diastematomyelia.
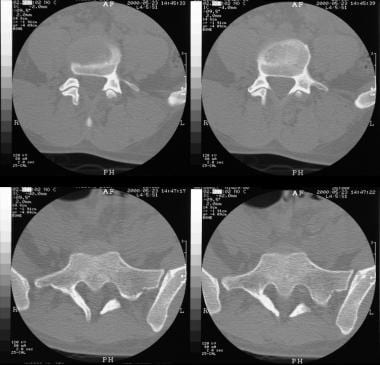 Axial CT scans through the lumbosacral junction shows absence of the posterior spinal elements at L5-S1. Note sclerosis of the laminae and the wide spinal canal.
Axial CT scans through the lumbosacral junction shows absence of the posterior spinal elements at L5-S1. Note sclerosis of the laminae and the wide spinal canal.
At CT and MRI, epidermoid cysts usually have attenuation and signal intensity values that roughly parallel those of CSF. Occasionally, the cyst contents may have slightly negative attenuation values on CT; signal intensity may be slightly greater than that of CSF with T1-weighted MRI. Unlike dermoids, epidermoid cysts do not usually have the low negative attenuation values (-60 to -90 HU) seen with true fatty tissue, though the actual characteristics of an epidermoid may vary with regard to its ratio of keratin to cholesterol. The squamous epithelial lining of an epidermoid cyst is usually too thin to be discerned on CT; calcification or enhancement is only rarely seen with the administration of contrast material.
In comparison, dermoid inclusion cysts may have a more complex imaging appearance, though they are still unilocular. Their imaging characteristics depend on their contents and lining. The walls in these cysts are thick because of dermal adnexa, and they may be radiologically visible on CT scans and MRIs. This thick lining may become calcified and may enhance with the use of contrast material. The lipid material in a dermoid has attenuation and signal intensity characteristics of fat on both CT scans and MRIs. Occasionally, dermoids do not have these radiologic features; in such cases, they may be difficult to distinguish from epidermoids.
The appearance of a unilocular, midline, cystic mass, especially one with attenuation and signal intensity characteristics similar to those of fat or with a fat-fluid level, is suggestive of a dermoid inclusion cyst. A suspected dermoid inclusion cyst should be carefully evaluated for any associated skeletal dysraphism, fibrous band, or sinus tract leading to the surface of the skin.
Accidental or iatrogenic rupture of a dermoid may be diagnosed when characteristic lipid droplets are seen in the subarachnoid spaces of the sulci and cisterns.
Magnetic Resonance Imaging
Mangels and associates showed that fetal MRI is an effective, noninvasive means of assessing of fetal CNS anatomy. [11] Its ability to resolve posterior fossa anatomy is superior to that of ultrasonography, though with reference to hydrocephalus and the level and nature of spinal anomalies, it may be equivalent or inferior to sonography (see the images below). [2, 3, 4, 12, 13, 14, 15, 16, 17]
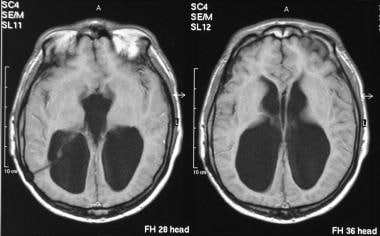
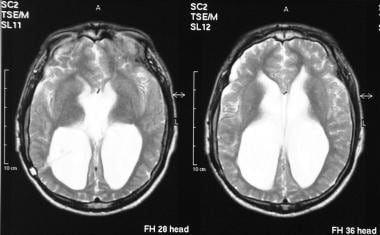 Axial T2-weighted MRIs of the brain (same patient as in the previous image) show gross ventriculomegaly.
Axial T2-weighted MRIs of the brain (same patient as in the previous image) show gross ventriculomegaly.
 T1-weighted coronal MRIs of the brain in the same patient as in the previous 2 images show a Chiari II malformation. Note the stretching of the brainstem, aqueduct, and fourth ventricle.
T1-weighted coronal MRIs of the brain in the same patient as in the previous 2 images show a Chiari II malformation. Note the stretching of the brainstem, aqueduct, and fourth ventricle.
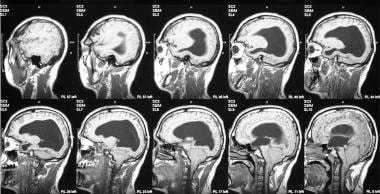
 T1- and T2-weighted sagittal MRIs of the cervical and dorsal spine in the same patient as in the previous 3 images show evidence of previous surgery for cervical meningocele. Note the associated congenital fusion of C5 and C6.
T1- and T2-weighted sagittal MRIs of the cervical and dorsal spine in the same patient as in the previous 3 images show evidence of previous surgery for cervical meningocele. Note the associated congenital fusion of C5 and C6.
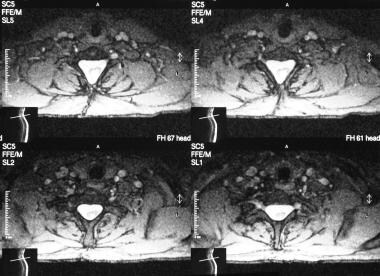 Axial T2-weighted MRIs of the cervical spine in the same patient as in the previous 4 images show a large spinal canal, evidence of previous surgery, and a split cord.
Axial T2-weighted MRIs of the cervical spine in the same patient as in the previous 4 images show a large spinal canal, evidence of previous surgery, and a split cord.
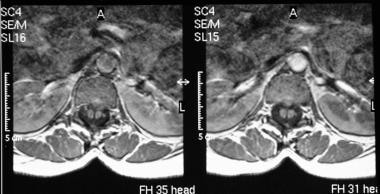 Axial MRIs in the same patient as in the previous image shows a hypointense bar, which is in an anteroposterior location because of diastematomyelia that splits the cord.
Axial MRIs in the same patient as in the previous image shows a hypointense bar, which is in an anteroposterior location because of diastematomyelia that splits the cord.
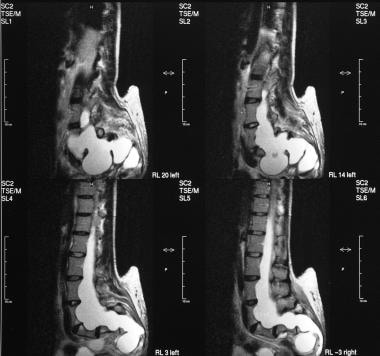
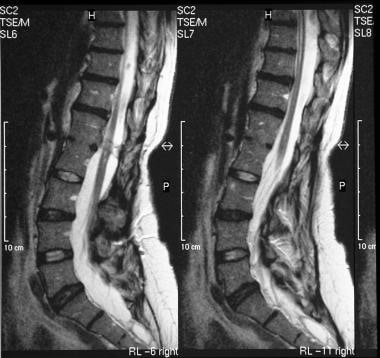
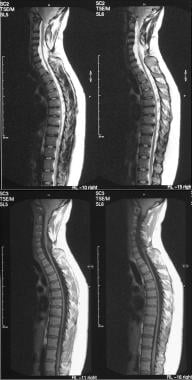
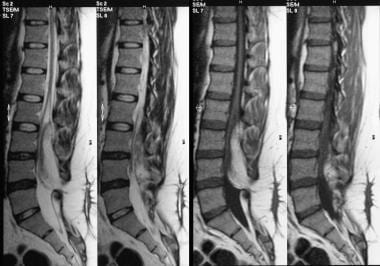 T1- and T2-weighted sagittal MRIs of the lumbar spine show an extradural spinal lipoma communicating with the subcutaneous fat.
T1- and T2-weighted sagittal MRIs of the lumbar spine show an extradural spinal lipoma communicating with the subcutaneous fat.
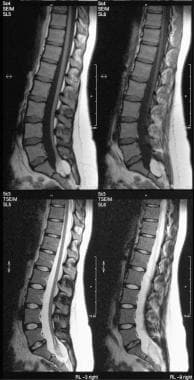 T1- and T2-weighted sagittal MRIs of the lumbar spine show an intradural sacral lipoma. Note the scalloping of the posterior sacral vertebral bodies and the syrinx.
T1- and T2-weighted sagittal MRIs of the lumbar spine show an intradural sacral lipoma. Note the scalloping of the posterior sacral vertebral bodies and the syrinx.
Ultrasonography alone can accurately diagnose most neural tube lesions, but MRI as an adjuvant study is helpful in assessing the degree of hindbrain herniation and in evaluating fetal brain and spinal cord anatomy when ultrasonography is limited. [18]
MRI of the spine is seldom required before surgery. MRI findings include absent posterior bony elements at the site of the defect. The soft tissue sac containing CSF may be obvious, and the conus is invariably low, coursing dorsally within a capacious dural sac. Inclusion dermal tissue is generally evident as a rounded mass of variable signal intensity that is often greater than that of the surrounding CSF. Postoperative scarring may obliterate the CSF space, and obliteration of the CSF distal to the site of repair suggests that additional scarring is responsible for the tethering. Scarring is generally hypointense on images obtained with all sequences, and it may be enhancing after the administration of gadolinium-based contrast material.
To evaluate CNS abnormalities associated with myelomeningocele, prenatal MRI using half-Fourier acquisition single-shot turbo spin-echo (HASTE) imaging has been shown to permit the diagnosis and understanding of the gross anatomy of myelomeningocele and associated hindbrain herniation and ventriculomegaly; however, postnatal heavily T2-weighted imaging (hT2WI) has been shown to be superior for evaluating detailed anatomy. [19]
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Chiari type II malformations
Chiari type II malformations are noted in nearly all cases of myelomeningocele. [5, 4] These malformations are best assessed with MRI; in patients younger than 6 months, cranial ultrasonography is as effective at MRI. The small posterior fossa, low insertion of the tentorium, and downward displacement of cerebellar tissue and medulla through a widened foramen magnum are obvious. When the medullary migration is not vertical, the characteristic cervicomedullary kink is noted.
The presence of Chiari II malformation is one of the eligibility criteria for fetal surgery, with improvement in Chiari II malformation considered an objective indicator of successful repair of prenatal spina bifida. In a meta-analysis, confirmation of Chiari II improvement was 85.9% (CI 66.3-94.9%). Regarding ventricular size, in fetuses greater than 26 weeks’ gestation, the detection rate for reduced ventriculomegaly postoperatively was 6.3% (CI 1.6-22.2%) and for increased ventriculomegaly was 76.8% (CI 63.1-86.4%) postoperatively. [20]
Syringomyelia
Syringomyelia of the cervical cord or syringobulbia and progressive dilatation of the fourth ventricle may account for worsening neurologic deficit. Additional findings in Chiari II malformation include stenogyria, partial agenesis of corpus callosum, large massa intermedia, and a beaked tectum. Rarely, a Chiari I malformation is associated with myelomeningoceles in which only the tonsils herniate below the foramen magnum.
Tethered cord
A tethered cord manifests itself as a low conus with associated spinal lesions. By the age of 2 months, a conus below L2-L3 is considered abnormal. Axial T1-weighted images are most accurate in determining the conus level. In symptomatic patients, a conus below L2-L3 may be regarded as abnormal; other supporting evidence, such as dorsal displacement of the cord in association with obliteration of the surrounding CSF spaces, may support the clinical suspicion. A low cord invariably occurs with a myelomeningocele; retethering may occur after repair. [3]
Neural arch defects
Posterior neural arch defects and an increased interpedicular distance are often associated with a lumbosacral lipoma. T1-weighted images have high sensitivity in the detection of lipomas because of the short relaxation time of fat. The fat content may also be confirmed by using fat-saturation techniques.
Thickened filum
The diagnosis of a thickened filum is made when the filum measures more than 2 mm at the L5-S1 disk space. In infants and young children, the filum is not discretely visualized on multiple axial images below the tip of the conus. Thus, visualization of the filum in this age group should be considered abnormal even if the thickness is less than 2 mm; in such cases, further investigation is warranted.
Diastematomyelia
In diastematomyelia, MRI is used to evaluate the extent of cord clefting; conus of low position; scoliosis; other bony anomalies; and syringohydromyelia, which is commonly associated with diastematomyelia. The bony spur is well seen on T1-weighted MRIs when fatty marrow is present. On T2-weighted images, less well-developed spurs are seen as areas of low signal intensity that split the thecal sac, which appears as an area of high signal intensity.
Dermoids and epidermoids
Dermoids and epidermoids account for about 1-2% of all spinal tumors in patients of all ages; however, they account for about 10% of tumors in patients younger than 15 years. They may be associated with a dermal sinus or occur in isolation. When not associated with dermal sinuses, they may occur with progressive compressive myelopathy or with chemical meningitis of acute onset; such compression is caused by the rupture of the cyst and the spread of cholesterol crystals in the CSF. The thoracic lumbar and sacral spine are affected; there is a slight increase in incidence in the craniocaudal direction. CT and MRI characteristics are similar to those seen in epidermoids at other sites. Diffusion MRI performed after an intrathecal injection of contrast medium is a promising alternative to CT for detecting the lesion and for delineating its limits.
Lipomas
Lipomas in the dura occur mainly in the caudal portion of the spine. Those in the region of the filum terminale are associated with congenital spinal anomalies. The lipoma is hyperintense on T1-weighted MRIs. Spinal lipomas are usually found in the extradural space in the thoracic region. The radiologic and MRI signal intensity characteristics are similar to those of intradural lipomas.
Degree of confidence
MRI is an excellent imaging modality for visualizing the spinal cord of patients of all ages; it is the imaging modality of choice for defining complex spinal dysraphism. CT myelography is now used infrequently.
Brophy and associates performed MRIs in 42 patients with spinal dysraphism, [21] and in 22 of the 42 patients, CT, pyelography, or surgical findings corroborated the MRI findings. There were 3 false-positive MRI findings of hydromyelia; there were no false-negative results. Scoliosis and a progressive neurologic deficit were the primary indications. Spinal cord anomalies included hydromyelia, diastematomyelia, lipoma, thickened filum terminal, and spinal cord atrophy. All patients but one had an Arnold-Chiari malformation.
Sutton and associates compared MRIs of lipomyelomeningocele and tethered cord with operative findings in 25 patients who were diagnosed with lipomyelomeningocele, tethered cord, or both. In 8 patients, postoperative MRIs were compared with the preoperative studies; of those 8 patients, 5 were in stable condition. This review revealed 1 false-negative MRI result and 4 MRIs on which the relationship of the lipoma to the conus or filum was not demonstrated accurately. In 6 patients, incidental intramedullary cystic lesions at the conus were identified on MRIs. All 8 postoperative MRIs (obtained at 1 mo to 2 yr) demonstrated no change in the level of the conus, as compared with the preoperative study. [22, 23, 24]
A study 119 fetal MRIs found limited accuracy for the identification of type II split cord malformations. In the study, 16 malformations were identified on fetal MRI, but only 5 (31%) were confirmed on postnatal imaging. The authors postulated that clumped nerve roots clumped nerve roots from the ventral neural placode or a prominent anterior median fissure of the spinal cord were misdiagnosed as a split cord malformation on the fetal MRI. [25]
MRI is an accurate screening modality in the initial diagnosis of occult spinal dysraphism. MRI was not useful in the postoperative evaluation of lipomyelomeningocele and tethered cord because the caudal, posterior displacement of the conus was unchanged in all studies.
Normal nerve roots of the cauda equina may simulate low conus on sagittal MRIs. MRI accurately depicts the extent of syringohydromyelia, which may clinically mimic a tethered cord. Despite surgical repair, postoperative ascent of the conus medullaris is seldom seen on subsequent MRIs.
Ultrasonography
It is possible to perform spinal ultrasonography in the newborn, owing to the lack of ossification of the predominantly cartilaginous posterior elements of the spine. Spinal ultrasonography is usually not possible after the age of 6 months except in cases of a persistent posterior spinal defect; in such cases, ultrasonography may be performed at any age (see the images below). [26, 27]
In the neonate, the cord appears as a tubular hypoechoic structure with hyperechoic walls. The central canal is hyperechoic. The cord position is dependent. The subarachnoid space around the cord is echo poor. In the neonate, the conus medullaris is smooth and tapering and lies above the middle of L2; the range varies from D10 to L2-L3. The cauda equina is seen as echogenic lines surrounding a hyperechoic filum terminale with dependent positioning.
The normal filum terminale is 1.0-1.5 mm in diameter. The vertebral bodies are seen as echogenic segmental structures lying anterior to the spinal cord. In the normal infant, the cord lies one third to one half the distance between the anterior and posterior walls of the spinal canal. There is normal pulsatile movement of the cord. When the cord is viewed axially, it appears round to oval and is surrounded by the fluid-filled subarachnoid space. The cord is fixed within the spinal canal by the dentate ligaments, which pass laterally from the cord. Below L2, the echogenic nerve roots are identified with a vertical or oblique orientation.
The spinal cord may be depicted throughout its length, allowing visualization of the conus and free movement of the nerve roots. An absence of normal transmitted pulsations and a lack of free movement of nerve roots on sonograms suggest a tethered cord. In cases in which there is a low tethered cord, the conus is low and the spinal cord is displaced dorsally. There is lack of normal cord pulsatility, and the filum terminale is thickened to over 2 mm. The thickened filum terminale may be fibrous or lipomatous. An abnormal cord may lie in a dorsal position rather than being dependent. The clinical significance of a low cord without tethering is unknown.
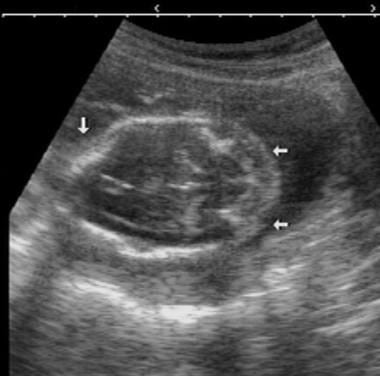
In a newborn term infant, the normal conus usually lies above the level of the mid L2. In cases in which there is a skin-covered back mass, the contents of the mass may be characterized. Axial spinal sonograms readily show the 2 hemicords and the echogenic spur in cases of diastematomyelia. In cases of diastematomyelia, sonography may show the spur. A dorsal dermal sinus may be depicted as an echogenic tract deep to a hole in the skin.
It may be difficult to be confident in the sonographic findings if the tract communicates with the spinal canal. However, a low-lying cord suggests tethering by intraspinal extension of the sinus; sonograms may show abnormal echogenicity at the depth of the tract, suggesting lipoma or dermoid. Alternatively, images may show matted nerve roots, caused by arachnoiditis. No conus may be identified if the cord terminates in a lipoma.
Anterior sacral meningocele
Anterior sacral meningocele may occur as an isolated anomaly or in association with neurofibromatosis or Marfan syndrome. Images show herniation of the dural sac containing CSF through a defect through the anterior surface of the sacrum or coccyx. Patients may be asymptomatic or present with a pelvic mass or bowel or bladder dysfunction. Ultrasonography shows a unilocular or multicystic pelvic mass anterior to the sacrum. A plain radiograph may confirm an anterior sacral defect. MRI may demonstrate the CSF content, which communicates with the spinal canal. Associated anomalies such as cord tethering and lipomata should be sought.
Meningocele and myelomeningocele
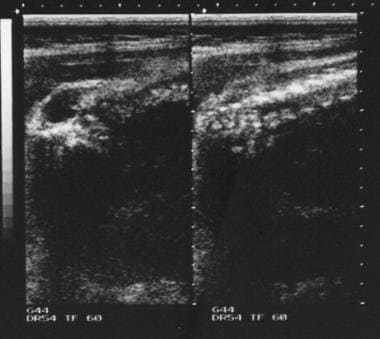
Meningocele and myelomeningocele represent the most common forms of spinal dysraphism; they arise as a result of a localized failure of fusion of the dorsal neural fold. A meningocele represents a herniation of distended meninges only, whereas in a myelomeningocele, part of the spinal cord and or nerve roots lie within the herniated sac. Ultrasonography shows an anechoic lobulated sac that is continuous with a low-lying tethered cord. [5]
Myelocystocele
Myelocystocele is a variant of a meningocele in which the central spinal canal dilates and herniates through a posterior spinal defect, appearing as a subcutaneous mass. Hydromyelia is invariably associated. On sonograms, the CSF-filled central canal appears to funnel into the larger anechoic subcutaneous cystic mass.
Spinal lipomas
Spinal lipomas may be classified into 3 groups: (1) lipomyelocele/lipomyelomeningocele (representing 84% of spinal lipomas), (2) fibrolipoma of the filum terminale (representing 12%), and (3) intradural lipoma (representing 4%). Spinal lipomas in association with spinal dysraphism have been reported in 20-50% of patients. Spinal lipomas are commonly located in the cervical and dorsal spine; they may be large enough to cause spinal cord compression. Lipomas are highly echogenic and are easily identified on sonograms.
Myelocele
Myelocele is an exposed open spinal defect that usually occurs in the lumbar region. Surgery is usually performed on an urgent basis. Sonography is typically avoided because of the risk of infection.
Dermal sinus
A dermal sinus may appear as an echogenic sinus tract deep to a hole in the skin. With sonography, it may be difficult to be confident in the diagnosis if the tract communicates with the spinal canal. However, a low-lying cord suggests tethering by intraspinal extension of the sinus; sonograms may show abnormal echogenicity at the depth of the tract, suggesting lipoma or epidermoid or dermoid, or they may show matted nerve roots, which are caused by arachnoiditis.
Diastematomyelia
In cases of diastematomyelia, axial spinal sonograms readily depict the 2 hemicords and the echogenic spur.
Spina bifida
Ultrasonographic evaluation for spina bifida should include both spinal and cranial imaging. Findings in cases of spina bifida include widening of the posterolateral spinal ossification centers, an absence of the continuity of the skin over the spine, and a bulging sac past the dorsal skin line. Suggestive cranial findings include the presence of ventriculomegaly; small head size; an elongated cerebellum with obliteration of cisterna magna from a Chiari II malformation (the banana sign); and scalloping of the frontal skull region (the lemon sign). [28]
Lemon sign
The lemon sign is best obtained superior to the plane used to measure the biparietal diameter (BPD) at the level of lateral ventricles. The lemon sign usually resolves by the third trimester because of increasing ossification of the calvarium. Therefore, this sign is sensitive for an NTD only in the second trimester. The absence of a lemon sign at 14-24 weeks' gestation makes spina bifida unlikely. [28]
Chiari II malformation
In Chiari II malformation, the cerebellum takes on a crescent or banana shape, and the cisterna magna becomes obliterated. In the second trimester, this banana sign is both sensitive and specific for ONTDs without the false-positive rate of the lemon sign. In the third trimester, the obliterated cisterna magma may be easier to see; it may be more helpful than the banana sign. Hydrocephalus is obvious on antenatal sonograms. Hydrocephalus is associated with Chiari II malformation in as many as 90% of these cases; conversely, one third of fetuses with hydrocephalus have a spinal defect.
Degree of confidence
Ultrasonography is a useful initial screening test for infant suspected of having a spinal abnormality. Spinal ultrasonography is a useful technique in the detection of the various types of spinal dysraphism, spinal tumors, arteriovenous malformations, and spinal trauma, particularly in the neonate.
The sensitivity of ultrasonography in the detection of spinal dysraphism in the neonate has been reported be equal to that of MRI; it has the added advantage of not requiring sedation or general anesthesia. Moreover, pulsating artifact and CSF flow does not affect the sensitivity of ultrasonography.
The examination is performed by use of a 7.5- to 10-MHz linear probe in both the sagittal and axial planes along the whole of the spine, preferably with the patient in a prone position. In many centers around the world, fetal sonography is used as a primary screening tool for NTDs, usually at approximately 18 weeks' gestational age. This trend reflects the increasing confidence in fetal ultrasonography.
Ultrasonography helps avoid the calculated 1% risk of miscarriage-associated diagnostic amniocentesis. Cesarean delivery before rupture of the amniotic membranes and onset of labor reduces the degree of neurologic deficit in fetuses with myelomeningocele.
Ultrasonography improves the ability to detect the defect and determine its level; this is helpful in identifying mothers who are most likely to benefit from an elective cesarean delivery. However, ultrasonography remains operator dependent; accurate diagnosis depends on the skill and experience of the operator and the quality of the equipment.
False positives/negatives
The false-positive rate for the banana sign has been reported to be 0%.
The lemon sign consists of findings of scalloping of the fetal frontal bones in axial view at the level of BPD. When seen in cases of meningomyelocele, it is usually associated with an Arnold-Chiari type II malformation at an abnormal cerebellar position. The positive predictive value of the lemon sign in a low-risk population is 6%. The lemon sign is also seen in the normal fetus; the incidence is 0.66-1.3%. No associated intracranial abnormality is apparent. The ventricle is of normal size. The cerebellum and the spine are normal. An encephalocele is often associated with a Chiari type III malformation. Most of these are occipital. In rare cases, they occur in the frontal region; in such cases, findings may mimic a lemon sign.
Ultrasonography is not sensitive enough to enable reliable and accurate detection of the level of the spinal defect. After confirmation of fetal myelomeningocele, clinicians at most tertiary care centers perform weekly ultrasonographic examinations to observe the growth and development of the fetus.
A bifid sacrum artifact is a skewed representation of normal anatomy and should not be interpreted as a true anomaly. It is produced by a steeply angled parasagittal scanning plane that intersects normal structures. Sonograms of the distal spine may be misleading and deceptive. The normal spine is constructed of multifaceted anatomic structures that change in appearance and relative position throughout gestation. The complex structures may be seriously misinterpreted if scanning planes are skewed or are rotated off axis.
A false-positive sonographic diagnosis of spina bifida in a fetus with triploidy has been reported. A patient with elevated maternal serum AFP level presented for a fetal ultrasonographic examination. Findings on the scan included a lemon sign, a banana sign, an effaced cisterna magna, and splayed lumbar vertebrae. After termination of the pregnancy, no spinal abnormality was detected on autopsy. Radiographs of the fetal spine demonstrated narrowing in the thoracic spine. The karyotype of the fetus was 69,XXY.
Various cutaneous stigmata and congenital anomalies are accepted as sufficient reasons to perform lumbar ultrasonography as a screening tool to rule out occult spinal dysraphism. Chern et al [29] studied 1,273 infants who underwent lumbar ultrasonography screening at a major pediatric tertiary referral center. The presence of cutaneous stigmata and/or congenital anomalies and lumbar ultrasonography results were reviewed for all patients. The indications for the referral included a sacral dimple, hairy patch, cutaneous hemangioma, deviated gluteal fold, skin tag, and skin discoloration. A total of 173 patients presented with congenital anomalies, such as imperforate anus and tracheoesophageal fistula/esophageal atresia, most of which were detected prenatally by fetal ultrasonography. A total of 17 infants underwent surgical exploration.
In the Chern et al study, occult spinal dysraphism was diagnosed in 7 infants in the cutaneous stigmata group and in 10 infants in the group with congenital abnormalities. None of the cutaneous stigmata as recorded was indicative of occult spinal dysraphism. The authors concluded that cutaneous markers are not useful markers for predicting occult spinal dysraphism. The vast majority of findings on lumbar ultrasonography studies performed under these circumstances will be negative. [29]
McGovern and associates conducted a study of the link between the number of clinical markers for spinal dysraphism and its presence on spinal ultrasound—in particular, the relationship between the presence of an isolated sacral dimple and spinal dysraphism. The authors concluded spinal ultrasound performed on the basis of multiple clinical indications is 6 times more likely to detect spinal dysraphism than imaging performed for isolated abnormalities or risk factors. The authors found the presence sacral dimple a poor marker for occult spinal pathology. [30]
Nuclear Imaging
CSF flow dynamics may be assessed by injecting radionuclides into the subarachnoid space via a lumber puncture; alternatively, they may be administered directly via a ventricular injection. The technique of radionuclide cisternography with radioiodine-labeled serum albumen was first introduced by DiChiro in 1964; it has been used extensively to investigate CSF dynamics.
The introduction of CT and MRI has resulted in a reduction in the use of radionuclide cisternography. Radionuclide techniques are used in conjunction with CT, MRI, or both to assess CSF circulation in cases of hydrocephalus, to determine CSF shunt patency, and to identify CSF leaks.
With the widespread application of various CSF shunts in the treatment of hydrocephalus, a need has arisen to assess shunt patency in cases of shunt malfunction. Methods include the assessment of responses to digital compression, the injection of contrast media, radionuclide methods, ultrasonographic flow measurements, and indirect infusion methods.
Indium-111 diethylenetriamine pentaacetic acid (DTPA) and technetium-99m DTPA were used successfully in the evaluation of CSF shunt malfunction; they were found to be particularly useful for children who presented with nonspecific clinical features, such as headaches, malaise, vomiting, and irritability. In conjunction with the use of CT, MRI, or both, a normal shunt study may prevent unnecessary surgical intervention.
Radionuclide may be injected directly into the shunt reservoir under strict aseptic conditions to assess shunt patency. In patients with a normally functioning shunt, the tracer passes freely into the peritoneum via the shunt tube when a ventriculoperitoneal shunt is present. In patients with a partially blocked shunt or a completely blocked shunt, the tracer is not seen in the peritoneum and passes up the subarachnoid space into the basal cisterns; reflux into the ventricles may occur.
The following flow patterns have been described in cases of shunt malfunction:
-
No flow detectable with tracer retained at the site of reservoir
-
No ventricular reflux into the ventricles
-
No tracer in the peritoneum or blood-pool activity, indicating a distal limb obstruction
-
Breaks in column but activity in the peritoneum, indicating a proximal limb blockage
-
Extravasation of tracer at connector of reservoir or valve
-
Loculation of tracer at the end of the distal limb of the shunt
-
Tracking of the tracer along shunt pathway due to extravasation
Degree of confidence
Radionuclide methods allow for the study of CSF flow dynamics without altering them, because only a small volume of the radionuclide is injected into the CSF space. Use of radionuclide cisternographic methods has declined as a result of increasing reliance on CT and MRI. However, these are used primarily to image anatomy, whereas radionuclide methods provide information about function. Special sequences and gated MRI may enable assessment of CSF flow and may replace radionuclide methods.
Depending on the type of shunt used (ventriculoperitoneal, ventriculoatrial, or ventriculopleural), reflux into the ventricles is often seen even in patients with patent shunts. If ventricular reflux does not occur, it cannot always be inferred that the ventricular limb of the shunt is occluded. This is particularly true with ventriculopleural shunts, in which negative pressure in the pleural cavity draws the CSF through the tube reducing ventricular reflux.
In small babies with a large ventricle, CSF flow through the shunt may be slower than in older patients. This finding is said to be related to the larger volume of the ventricles and a comparatively low intraventricular pressure.
-
Antenatal ultrasonogram shows a lumbar meningocele.
-
Antenatal ultrasonogram shows a lemon sign and a banana sign.
-
Posteroanterior (PA) chest radiograph shows defects of the laminae of the lower cervical spine.
-
Plain abdominal radiograph in the same patient as in the previous image shows spina bifida occulta of S1.
-
Axial T1-weighted MRIs of the brain show gross ventriculomegaly.
-
Axial T2-weighted MRIs of the brain (same patient as in the previous image) show gross ventriculomegaly.
-
T1-weighted coronal MRIs of the brain in the same patient as in the previous 2 images show a Chiari II malformation. Note the stretching of the brainstem, aqueduct, and fourth ventricle.
-
T1- and T2-weighted sagittal MRIs of the cervical and dorsal spine in the same patient as in the previous 3 images show evidence of previous surgery for cervical meningocele. Note the associated congenital fusion of C5 and C6.
-
Axial T2-weighted MRIs of the cervical spine in the same patient as in the previous 4 images show a large spinal canal, evidence of previous surgery, and a split cord.
-
Myelograms in a 5-year-old patient show the dorsal region of the spine and an anterior thoracic meningocele. Note the gross dorsal kyphosis.
-
Myelograms in a 4-year-old patient show the lumbosacral region; a long, tethered cord; and diastematomyelia.
-
Sagittal MRIs of the lumbar spine show diastematomyelia. Note the congenital fusion of L1 and L2.
-
Axial MRIs in the same patient as in the previous image shows a hypointense bar, which is in an anteroposterior location because of diastematomyelia that splits the cord.
-
Right, plain radiograph of the lumbar spine shows diastematomyelia. Left, myelogram in the same patient shows a filling defect at the level of diastematomyelia.
-
Axial CT scans through the lumbar spine with bone window setting in the same patient as in the previous images show a bony bar due to diastematomyelia.
-
Left, plain anteroposterior (AP) radiograph of the lumbar spine shows spina bifida occulta. Right, myelogram of the same patient shows a thick tethered cord.
-
Left, anteroposterior (AP) plain radiograph of the lumbar spine shows a defect within the laminae of S1 and S2. Right, myelograms in the same patient show a markedly thickened, low tethered cord.
-
Plain anteroposterior (AP) lumbar spinal radiograph in a 7-year-old patient shows a defect within the laminae of L4-5 and S1. Note the diastematomyelia.
-
Myelograms in the same patient as in the previous image show a low, tethered cord. Note also the diastematomyelia.
-
Left, plain radiograph of the lumbar spine shows bony defects in the laminae of L2 to S1. Right, myelogram shows a split cord.
-
Axial CT scans through the upper lumbar spine show a split cord.
-
T1- and T2-weighted sagittal MRIs of the lumbar spine show an extradural spinal lipoma communicating with the subcutaneous fat.
-
T1- and T2-weighted sagittal MRIs of the lumbar spine show an intradural sacral lipoma. Note the scalloping of the posterior sacral vertebral bodies and the syrinx.
-
Axial CT scans through the lumbosacral junction shows absence of the posterior spinal elements at L5-S1. Note sclerosis of the laminae and the wide spinal canal.
-
Plain radiographs show posterior scalloping.
-
T2-weighted sagittal MRIs of the sacrum show an anterior sacral meningocele.